Abstract
DNA methylation contributes to the chromatin conformation that represses transcription of human papillomavirus type16 (HPV-16), which is prevalent in the etiology of cervical carcinoma. In an effort to clarify the role of this phenomenon in the regulation and carcinogenicity of HPV-16, 115 clinical samples were studied to establish the methylation patterns of the 19 CpG dinucleotides within the long control region and part of the L1 gene by bisulfite modification, PCR amplification, DNA cloning, and sequencing. We observed major heterogeneities between clones from different samples as well as between clones from individual samples. The methylation frequency of CpGs was measured at 14.5%. In addition, 0.21 and 0.23%, respectively, of the CpA and CpT sites, indicators of de novo methylation, were methylated. Methylation frequencies exceeded 30% in the CpGs overlapping with the L1 gene and were about 10% for most other positions. A CpG site located in the linker between two nucleosomes positioned over the enhancer and promoter of HPV-16 had minimal methylation. This region forms part of the HPV replication origin and is close to binding sites of master-regulators of transcription during epithelial differentiation. Methylation of most sites was highest in carcinomas, possibly due to tandem repetition and chromosomal integration of HPV-16 DNA. Methylation was lowest in dysplasia, likely reflecting the transcriptional activity in these infections. Our data document the efficient targeting of HPV genomes by the epithelial methylation machinery, possibly as a cellular defense mechanism, and suggest involvement of methylation in HPV oncogene expression and the early-late switch.
The spread of human papillomaviruses (HPVs) into mucosal and cutaneous epithelia can result in either a clinically asymptomatic infection not associated with pathological changes or the development of neoplastic disease. While the host immune system likely plays a role in the elimination of the virus, it is unclear which factors determine whether an infection leads to long-term persistence in the absence of symptoms or to neoplastic progression. The understanding of these alternative outcomes is important in the case of the 18 high-risk HPV types that are most frequently associated with cervical carcinomas. Population-based data suggest that high-risk HPV infections occur frequently, relative to the number of carcinomas that result. Cervical cancer cases generally number fewer than some hundreds of cases per 100,000 women, while the incidence rate of becoming infected at least once in a lifetime is believed to significantly exceed 50% (6, 35, 46, 49). Further research is needed to discriminate whether there are two different stages of infection, namely, asymptomatic productive infection and latency. In the latter case, the virus would be maintained not only without changes to the host cell but also without production of viral particles. While the former may represent immunological delays or failures, the latter may serve as a model for malignancies that evolve late in life.
The regulation of HPV oncogene transcription as a major modulator of productive HPV infections is a focus of debate. It has been reported that HPV oncogene transcription is only active in differentiating epithelial cells but is repressed in undifferentiated cells by the factors CDP and YY1, which alter, in collaboration with histone deacetylases (HDACs) chromatin conformation in ways unfavorable to transcription (1, 26, 39). This mechanism was identified in vitro by transcriptional studies and is supported by immunocytochemical data of HPV-infected tissue in situ (37). A second example is data that glucocorticoids and progesterones increase HPV oncogene transcription (11, 29). These molecular mechanisms may explain the increased risk of cervical carcinogenesis by multiparity, use of antiovulants (35), and possibly stress. Yet another mechanism is based on the observation that transcriptional stimulation results from the insertion of HPV genomes into cellular chromosomes during cancer progression. This event interrupts a negative feedback loop (36, 43) and stimulates transcription depending upon nuclear matrix attachment regions (38).
DNA methylation provides an additional means to regulate HPV transcription, since the repression of cellular and viral gene expression can occur by the introduction of methyl groups to cytosine residues (5, 14, 19, 20, 27, 31, 48). Methylated cytosines (meCpGs) are maintained in a stable form when they occur in the palindrome CpG (23). meCpGs can result in displacement of transcription factors (44) but more often lead to changes of the chromatin configuration under the influence of HDACs (21). This mechanism resembles the modifications triggered by complexes between HDACs and the transcription factors CDP and YY1, since HDACs can form complexes either directly with the DNA methylases or with the repressors of the MeCP2 family, which recognize and bind meCpGs (5, 8, 21).
Reports more than 20 years ago on HPV-1 and the cottontail rabbit papillomavirus suggested that papillomaviruses are targeted by cellular DNA methylation, although limited analytical power, namely methylation-sensitive restriction enzymes, was accepted as the state of the art of that time (9, 13, 40, 47). Research on the methylation of HPVs was extended recently to the high-risk HPV-16 (2, 22) by bisulfite modification and DNA sequencing. Ongoing pilot studies confirmed the occurrence of DNA methylation in HPV-6, -11, -18, and -31 (1A; our unpublished data). The consequence of CpG methylation is repression of HPV transcription as shown by transfection of in vitro methylated HPV DNA (34) and by in vivo studies with the cell lines CaSki and SiHa (2, 45). Our research presently concentrates on HPV-16, the most prevalent HPV type in cervical carcinoma (6). The observations cited above provide evidence for three mechanisms, which may occur concomitantly, namely for methylation (i) of HPV-16 genomes in episomal form in cells in asymptomatic smears, (ii) of HPV-16 genomes that form tandem arrays in cancer lesions, and (iii) de novo in a somatic tissue, namely mucosal epithelium.
The goal of the present study was to further investigate the methylation patterns of the CpG dinucleotides contained within the long control region (LCR) and L1 region of the HPV-16 genome in a large collection of clinical samples from the human cervix. We wanted to determine whether a thoroughly studied segment of the HPV-16 genome follows defined methylation patterns and whether certain CpG sites are targeted more or less frequently, depending upon the state of the cervical infection. In addition, we wanted to estimate the amount of de novo methylation in an HPV-infected cell population by measuring methylation in dinucleotides other than CpGs, namely, CpAs, CpTs, and CpCs. Each of these issues was addressed by the sequencing of multiple HPV-16 genomes from individual samples to further characterize the heterogeneity or homogeneity of methylation within each lesion, while also addressing the relationship between the observed patterns as they relate to the presence or absence of clinical disease.
MATERIALS AND METHODS
Origin of samples.
CaSki cells have served for 2 decades as a paradigm of a cervical carcinoma-derived cell line with 500 chromosomally recombined HPV-16 genomes. All clinical samples were archival. The carcinoma samples were fresh frozen, stored at −70°C, and histologically confirmed before and after DNA extraction. The samples were obtained from three sources: (i) from patients examined and treated by two of us (B.H. and K.L.) in Oslo, Norway (21 samples from asymptomatic patients, 17 samples from patients with cervical intraepithelial neoplasia [CIN] grade I to III lesions, and 29 samples from patients with squamous carcinomas); (ii) from patients examined during regular gynecological diagnosis in several different hospitals in Monterrey, Mexico, as discussed in a recent publication (10) (30 samples from asymptomatic patients and 13 samples from patients with cervical carcinomas); and (iii) from patients seen at the Cancer Center of the University of California, Irvine (5 samples from patients with cervical carcinomas).
DNA preparations.
All DNA preparations were done with QIAGEN DNA purification kits to assure removal of protein contaminations.
Bisulfite modifications.
For bisulfite treatment (16), 50 to 1,000 ng of sample DNA supplemented with 1 μg of salmon sperm DNA in a total volume of 18 μl in water was denatured with 2 μl of 3 M NaOH and incubated at 37°C. After being denatured, 278 μl of 4.8 M sodium bisulfite and 2 μl 100 mM hydroquinone were added and incubated in a thermal cycler for 20 cycles each at 55°C for 15 min and 95°C for 30 s. The modified DNA was desalted by the QIAquick PCR purification protocol. The modified DNA was desulfonated by adding 5.5 μl of 3 M NaOH and 5 μg of glycogen and incubated at 37°C for 15 min. The DNA was precipitated with 5.6 μl of sodium acetate and 150 μl of 100% ethanol. The pellet was washed with 70% ethanol and dissolved in 30 to 50 μl of TE buffer (10 mM Tris-HCl [pH 8], 1 mM EDTA).
PCRs, primers, TA cloning, and DNA sequencing.
The modified DNA was amplified in the form of three amplicons: part of the L1 gene and the 5′ LCR with the primers 16msp3F (positions 7049 to 7078; AAGTAGGATTGAAGGTTAAATTAAAATTTA) and 16msp3r (positions 7590 to 7560; AACAAACAATACAAATCAAAAAAACAAAAA), the HPV-16 enhancer with the primers 16msp4F (positions 7465 to 7493; TATGTTTTTTGGTATAAAATGTGTTTTT) and 16msp7R (positions 7732 to 7703; TAAATTAATTAAAACAAACCAAAAATATAT), and the HPV-16 promoter with the primers 16msp5F (positions 7748 to 7777; TAAGGTTTAAATTTTTAAGGTTAATTAAAT) and 16msp8R (positions 115 to 86; ATCCTAAAACATTACAATTCTCTTTTAATA). The sequences of the primers were designed according to the genomic sequence of HPV-16 (24), assuming conversion of all cytosine residues into uracils. PCR was carried out in a 25-μl volume containing 0.2 mM of each of the four deoxynucleoside triphosphates, 10 pmol of the primers, 2 mM MgCl2, and 1 U of AmpliTaq Gold (Perkin-Elmer). The PCR started at 94°C for 1 min, followed by 40 amplification cycles (denaturing at 94°C for 10 s, annealing at 58°C for 30 s, and extension at 68°C for 1 min) with final extension at 68°C for 7 min. The presence of PCR products was verified by agarose gel electrophoresis, and confirmed amplicons were cloned with the TOPO TA cloning kit for sequencing (Invitrogen). Cloned DNAs were sequenced by Big Dye Terminator Cycle Sequencing, version 3.1 (Applied Biosystems).
Statistical analysis.
Descriptive and tabular statistics were used to explore these data. Graphical representations of the CpG methylation frequency were estimated from the data. For some sites, DNA could not be amplified, and data were treated as missing for these analysis. The frequency of methylation of each CpG, CpA, and CpT site was compared among three diagnostic outcome groups: squamous cell carcinoma, CIN, and specimens from asymptomatically infected women (no identifiable lesion [NIL]). Fisher's exact test was used to test the hypotheses that the methylation frequencies for cancers and CINs were each greater than the methylation frequency of specimens from asymptomatically infected women. Additionally, Student's t Test was used to test the hypotheses that the observed methylation frequency at each site in each diagnostic outcome category was statistically significantly greater than zero. A total of 95 statistical comparisons were performed, and we adjusted the level of significance for our statistical tests by using Bonferroni's adjustment for multiple comparisons. The frequency of methylation on the many CpA and CpT sites examined was small; thus, P values of less than 0.05 are reported, even though they may not meet the most stringent test limits of multiple comparisons.
RESULTS
Study design.
We approached this study intending to expand upon the manner in which CpG methylation of HPV-16 DNA had previously been investigated. Our original observations were based upon bisulfite modified DNA from cell lines and clinical samples, which were then amplified with primers specific for the DNA in which the cytosine residues had been converted to uracil groups, followed by direct sequencing of the amplification product (2). At the beginning of our present study, we made observations with DNA from CaSki cells, as well as with clinical samples (data not shown), that sequencing reactions frequently showed overlapping C and T signals, which could not be suppressed by changing the temperature or the length of the bisulfite reaction. We concluded that cell populations from the same source contained mixtures of HPV-16 genomes with CpGs or meCpGs in the same position. Therefore, we altered our protocol, cloned the amplicons in Escherichia coli plasmids, and randomly selected for sequencing five independent E. coli colonies derived from each sample. This was done to identify divergently methylated HPV-16 genomes, assuming there may be similar amounts of methylated and unmethylated molecules. We acknowledge that it is impossible to analyze mixtures of methylated and unmethylated molecules in samples where one DNA type vastly outnumbers another.
The HPV-16 genome is a circular double-stranded DNA with a size of 7,906 bp. An 850-bp segment between the 3′ end of the L1 gene and the 5′ end of the E6 oncogene is called the LCR, since it contains most of the cis-responsive elements that regulate transcription, which occurs unidirectionally toward the E6 gene (3). The principal transcriptional start site is called p97 or the E6 promoter and is located 5 bp upstream of the ATG of the E6 gene. We decided to standardize the analysis by amplifying and sequencing a genomic segment between the positions 7079 and 85, which includes 19 CpGs. Three of these 19 CpGs were derived from the 3′ end of the L1 gene (positions 7091, 7136, and 7145), five from the 5′ segment of the LCR (positions 7270 to 7461), which makes as yet incompletely understood contributions to the transcriptional process (39, 42), six from the transcriptional enhancer (positions 7535 to 7862), and five from the E6 promoter (positions 31 to 58). We selected this segment in order to detect possible correlations of methylation targets with known functional units of the HPV-16 genome.
It is well known that bisulfite modification not only alters cytosine residues but introduces nicks in DNA. As a consequence, it is impossible to PCR amplify large genomic segments with sizes exceeding a few hundred base pairs with high efficiency. Because of this limitation, we had to dissect the 913-bp region that we wished to analyze into three amplicons, spanning positions 7091 to 7461, 7535 to 7695, and 7862 to 58. In other words, while we aimed to gather methylation information from five HPV-16 DNA molecules from each sample, data for each of these three segments represent the methylation status of different, noncontiguous molecules.
During the analysis of clinical samples, we regularly included as negative and positive controls DNA from SiHa and CaSki cells. The only HPV-16 genome in SiHa cells has no meCpGs throughout the enhancer and promoter region. Previously published findings based on direct sequencing of the amplification product showed that most of the 500 endogenous HPV-16 genomes in CaSki cells appeared to be completely methylated throughout this region (2).
To standardize the analysis of the clinical samples in this study, we aimed to analyze five bisulfite-modified, PCR-amplified, and bacterially cloned HPV-16 segments from 51 samples from patients with asymptomatic infections (NIL), 17 samples from patients with low- and high-grade CINs, and 47 samples from patients with from cervical carcinomas for each of the 19 CpGs in the L1-LCR segment described above. This strategy aimed to give information about a total of 10,925 CpGs. Unfortunately, some of our samples did not contain sufficient DNA to complete this study; in these cases (51 out of 115 samples), we had to eliminate the analysis of the L1 and 5′ LCR segment. As a result, our study reports the methylation status of only 8,885 CpG residues: 6,080 representing information about all 19 CpG residues in 320 clones, and 2,805 representing the methylation status of 11 CpGs in 255 clones from the enhancer and promoter.
It was not possible to confirm the physical state of HPV genomes in this study. It is generally accepted, however, that HPV genomes are episomal in latent infection and early dysplasia (CIN grade I), two of the pathological groupings we studied, while they are most often chromosomally integrated in carcinomas (12).
Heterogeneous DNA methylation patterns in the HPV-16 genomes of the CaSki cell line.
In a previous analysis involving one of us (2) of HPV-16 DNA in CaSki cells, amplicons were directly sequenced after bisulfite modification and PCR amplification, and the data obtained by this strategy suggested that all CpGs overlapping with the enhancer and promoter (positions 7535 to 58) were completely methylated in most viral copies (2). Here, we report a reanalysis based on sequences of individual clones of modified and PCR-amplified DNA, which also extended the analyzed segment in the 5′ direction. In contrast to the published data, Fig. 1 shows that none of 15 clones was completely methylated, but each clone contained between 1 and 5 (out of 19) unmethylated CpGs. None of five clones was methylated in position 7270 (which had not been previously analyzed), and methylation was infrequent at positions 7535, 7554, and 7862. Based upon these findings, we conclude that the 500 HPV-16 genomes of CaSki cells are not homogenously methylated but that there are differences either among tandemly repeated intrachromosomal copies or between copies inserted in different chromosomal locations (45). We reexamined this assumption by directly sequencing the bisulfite-modified and PCR-amplified products from several independent analyses of CaSki DNA. In these experiments, we observed overlapping C and T peaks at positions 7270, 7535, 7554, and 7862 in all sequence readouts, with the C peak slightly exceeding the T peak. This confirms the heterogeneous methylation of HPV-16 molecules in CaSki and the fortuitous selection of an excess of unmethylated molecules (Fig. 1).
FIG. 1.
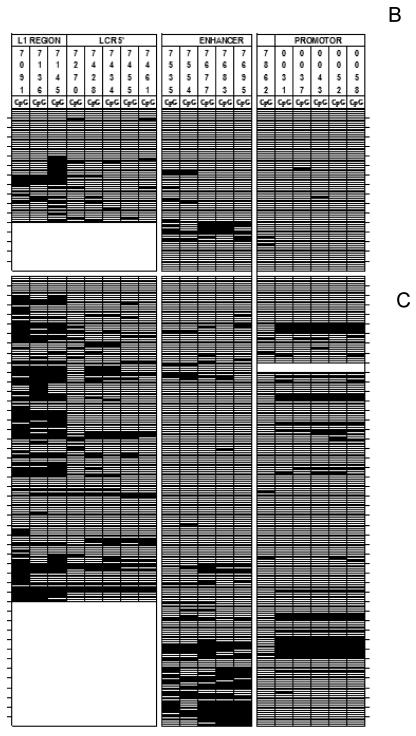
Heterogeneity of CpG methylation in HPV-16 genomes derived from CaSki cells. Each vertical set of rectangles represents one of 19 specific CpG dinucleotides, and the number on the top of the bar represents the position of this CpG in the genome of HPV-16. Each horizontal set of rectangles represents a 913-bp segment of the HPV-16 genome, covering the 3′ end of the L1 gene and the complete LCR. Unmethylated CpGs are indicated by white rectangles, and methylated CpGs are indicated by black rectangles. The two vertical white separators indicate the borders between amplicons and discontinuities between supposedly different HPV-16 molecules. The upper part of the figure represents three specifically positioned nucleosomes (39), which may overlap with regions of hypermethylation.
Different CpGs are altered with different frequencies.
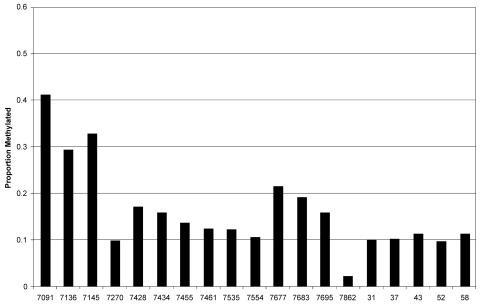
Figure 2 depicts the methylation and lack of methylation for each of the 8,885 CpG sites evaluated in this study. Altogether, we found 1,292 methylated CpGs, i.e., 14.5% of all CpGs were methylated. Figure 3 shows the relative frequency of methylation of the 19 sites. Approximately half (51 of 115) of the specimens between positions 7091 and 7461 could not be amplified. Thus, for this region, the reduced size of our sample may have limited our power to detect statistically significant relationships across sites and across diagnostic outcome groups. Nonetheless, these data point to systematic differences of probabilities of each site to become methylated. For example, 29 to 41% of the CpGs at the positions 7091, 7136, and 7145 were methylated (the only CpGs within a gene, the late gene L1), while methylation was only around 10% for most other sites. There are three regions where methylation frequencies reached minima, namely the positions 7270, 7554, and (most pronounced, with a frequency of only 3%) position 7862. Remarkably, these three regions coincide with the same three unmethylated positions detected in the LCRs of HPV-16 genomes in CaSki cells.
FIG. 2.
Heterogeneity of CpG methylation in HPV-16 genomes derived from clinical specimens. The figure visualizes the methylation of 19 CpG dinucleotides in the HPV-16 genomes of five independent clones, each derived from 1 of 115 clinical samples, a database of the methylation status of 8,885 CpG dinucleotides. (A) asymptomatic patients; (B) CIN; (C) cervical cancer. White horizontal rectangles represent an unmethylated CpG, black rectangles represent meCpGs. Each set of five horizontal lines represents clones derived from one patient. A horizontal line does not indicate a contiguous 913-bp amplicon but is derived from three different HPV-16 genomes, as indicated by two white vertical bars. These discontinuities were a technical necessity, since the destruction of bisulfite-modified DNA did not allow us to generate the analyzed 913-bp fragment in one contiguous amplicon. White indentations in this figure identify clones that could be only partially analyzed due to the small amount of sample DNA. Due to the compression of this figure, samples from Mexico, Norway, and the United States samples could not be visually distinguished. The Mexican cohort contained 70% genomic AA variants and only 30% E variants (10), while all Norwegian and United States samples were E variants. We could not detect any obvious distinction between the methylation patterns of these cohorts and variants.
FIG. 3.
Methylation frequencies of 19 CpG nucleotides in the HPV-16 genomes of five independent clones each derived from one of 115 clinical samples. For the overlap of the genomic positions with the L1 gene, 5′LCR, enhancer, and promoter, see Fig. 1.
For a subset of these samples, we performed control experiments and directly sequenced the amplification product after bisulfite sequencing. All of these controls confirmed that the individual DNA molecules that were sequenced after plasmid cloning correlated with the ratios that existed in situ: whenever we found hypomethylated or unmethylated clones, we also observed hypomethylation by direct sequencing, and hypermethylated clones correlated with highly methylated directly sequenced DNA. While it is obvious that five samples represent a complex mixture of molecules with a low statistical power, they are nevertheless a good representation of the diversity in situ.
Correlation of CpG DNA methylation patterns with pathology of the infected cell population.
The bar diagram shown in Fig. 4 separates the methylation frequencies shown in Fig. 3 by diagnostic outcome and visualizes CpG methylation in relation to the pathological status of the HPV-16 infection, i.e., asymptomatic infections (NIL), CIN lesions, and carcinomas. For cancer specimens, the proportion that showed methylation of CpGs was 4% at 7862 and ranged between 12 and 53% for the remaining CpG sites. The proportion of all CpGs that was methylated was significantly greater than zero (i.e., for CpG sites at positions 7091 to 7695 and 31 to 58 by Student's t test (P <) 0.0001). An exception was at position 7862, where methylation occurred between 2 and 4% of test specimens; we found no difference between CINs and NILs or between carcinomas and NILs (i.e., by Fisher's exact test; P = 1.0 and P = 0.6, respectively). CIN lesions showed the lowest methylation frequency of the specimens tested, and methylation frequencies were more often intermediate in asymptomatic patients. For example, for CIN specimens, we were able to detect methylation frequently at position 7145 (i.e., 33% by Student's t test; P < 0.0001). At all other sites, the frequency ranged from 0 to 17%, but none proved to be statistically significant. However, for specimens acquired from asymptomatically infected women, methylation frequencies greater than zero were detected at most CpGs (i.e., at positions 7091, 7136, 7428, 7434, 7535 to 7695, and 31 to 43 and position 58, ranging from 6% at position 37 to 33% at position 7091 by Student's t test (P <) 0.0001).
FIG. 4.
Methylation frequencies of 19 CpG nucleotides in the HPV-16 genomes of five independent clones each derived from one of 115 clinical samples separated by pathological properties. Grey bars, samples from asymptomatic patients (NIL); black bars, samples from patients with low- and high-grade CIN; white bars, samples from patients with cervical cancer.
For carcinomas, 42% of specimens showed methylation at CpG sites 7136 and 7145. In comparison, only 13 and 9% of sites at 7136 and 7145 were methylated in specimens from asymptomatically infected women (i.e., for each comparison, by Fisher's exact test; P < 0.00002). Additionally, at site 7145, 33% of CIN specimens showed methylation compared to 9% of specimens from asymptomatically infected women (P = 0.0005). Last, compared to that in specimens from asymptomatically infected women, methylation in cancer samples was significantly elevated at CpG positions 31, 37, 52, and 58 (using Fisher's exact test, for position 31, 17 versus 6%, P < 0.0004; for position 37, 17 versus 6%; for position 52, 18 versus 4%; for position 58, 18 versus 7% [for each comparison at positions 37, 52, and 58, P < 0.0001]; and for position 43, 17 versus 7%, P < 0.002).
The minima at positions 7270 and 7535 and 7554 (7535-7554) were pronounced in all three groups, while the minimum at position 7862 was clear only for specimens from patients with carcinomas and asymptomatic patients. We found no statistically significant difference in the methylation frequencies for these sites between carcinomas, CINs, and specimens from asymptomatically infected women by Fisher's exact test. Methylation at position 7862 was also low in the case of CIN lesions, but it did not form a dip, as there is a nearly complete lack of methylation in all promoter positions 3′ of 7862, and we found no statistically greater frequency of methylation at this site when pathology groups were compared. Thus, in nearly all positions, methylation was highest in carcinoma lesions and lowest among CIN lesions (Fig. 4).
meCpA and meCpT dinucleotides identify de novo methylation of HPV-16 DNA in cervical epithelia.
In the methylated state of the palindromic sequence CpG, the cytosine residues of the upper and lower strands are normally both methylated. The two replication products of a meCpG-expressing DNA are hemimethylated, and methylation of the unmethylated cytosine is restored by the maintenance DNA methylase DNMT1. Toward de novo methylation (44A, 47A), DNMT3a and DNMT3b are considered responsible for targeting unmethylated cytosine residues, while their contributions to maintenance methylation have recently been demonstrated (5, 14, 20). While this occurs preferentially at CpGs, changes at CpA and CpT dinucleotides can occur as well, although with less efficiency than with CpGs (14, 30). CpC methylation is known to be very rare. The methylation state of meCpAs and meCpTs is not maintained during replication, as one of the replication products does not carry a record of the methylation, and the other strand is diluted in multiple rounds of replication. As a consequence, the detection of meCpA and meCpT residues is proof that the sequenced DNA molecule or one of the immediate precursors had become methylated, and it does not constitute a record of a long-term maintenance of methylation. A record of these three methylated dinucleotides in HPV-16 genomes therefore amounts to a documentation of active de novo methylation in the cell populations carrying these HPV-16 genomes.
Toward measuring CpA, CpT, and CpC methylation, we investigated the same nucleotide sequence output of all 115 samples in quintuplicate, that had led to the data shown in Fig. 2. We detected 58 meCpAs and 49 meCpTs residues, among a total of 26,785 and 20,845, respectively, and no meCpC dinucleotide at all. We also found six additional meCpGs in CpG positions diverging from the HPV-16 reference sequence, apparently genomic variants of HPV-16 (10). The total number of meCpAs and meCpTs in the 115 samples is incomplete, however, since the three amplicons, originally designed only to detect CpGs, do not overlap in genomic regions that did not contain CpGs and were therefore excluded from the original strategy.
Figure 5 maps the distribution of meCpAs and meCpTs within the three amplicons across the L1-LCR segment. The numbers along the x axis of Fig. 5 indicate the genomic positions (24) of cytosine residues that are part of a CpA or CpT dinucleotide, while the y axis represents the frequency of finding a methylated C at this position. The raw data that led to this figure can be obtained upon request, as a representation in form of a figure or a table would be unwieldy and uninformative to most readers. Inspection of Fig. 5 suggests clusters of these two methylated dinucleotides between positions 7268 to 7327 and 7840 to 24. These two regions overlap with minima of CpG methylation and may suggest that de novo methylation is more efficient in nucleosomal linkers than in the nucleosomes. Our analyses suggested methylation was detectable frequently at positions 7268 and 7317, and less frequently at positions 7840, 7857, 7876, 7882, 7886, 7903, 2, 13, 73, and 77 (i.e., testing the hypothesis that the mean frequency of cytosine methylation was greater than the null, by Student's t test and P = 0.0015, P = 0.0045, P = 0.0015, P = 0.0046, P = 0.0015, P = 0.0009, P < 0.0001, P = 0.0009, P = 0.0046, P = 0.0003, P = 0.0026 and P = 0.0046, respectively). Also, we detected cytosine methylation statistically less precisely at dinucleotide positions 7301, 7327, 7554, 7602, 7689, 7829, 7829, 7841 to 7845, 7874, and 5 (i.e., P = 0.01 for each). Thus, using the most stringent level of significance, our analyses detected methylation at the maxima at positions 7886 and 13.
FIG. 5.
Methylation frequencies of CpA and CpT nucleotides in the HPV-16 genomes of five independent clones, each derived from 1 of 115 clinical samples. The figure represents 107 meCpA and meCpT dinucleotides in the same genomic segment that was studied for CpG methylation. The x axis of this figure indicates the positions of cytosine residues that are part of CpA or CpT dinucleotides in the LCR between genomic positions 7080 and 79, while the y axis represents the frequency of finding a methylated C at this position. Four sites with maximal methylation are highlighted by a number at the top of the column, indicating the genomic position of the cytosine residue.
Although sparse, when data were stratified by pathology outcomes, our analyses suggested that methylation at particular positions was detectable with statistical significance. For women with CIN, CpA was methylated at position 7268 (i.e., P = 0.0038). For women evidencing NIL, these analyses suggested that CpT was methylated at position 7840 and CpAs were methylated at positions 7857, 7876, 7882, and 7886 (i.e., P = 0.014, P = 0.014, P = 0.0079, P = 0.014, P = 0.0014, respectively). For samples from women with carcinomas, we were unable to precisely detect nucleotide regions where cytosines were methylated on CpA and CpT.
DISCUSSION
HPV-16 CpG methylation: preferred and protected sites.
We have reported a detailed methylation study of a 913-bp segment of the genome of HPV-16 encompassing part of the L1 gene and the complete LCR in the cell line CaSki and 115 patient samples. Altogether, we investigated the frequencies of methylation and position of CpGs among a total of 9,090 potential target sites (the sum of the clinical samples and CaSki clones). Our data confirm that HPV-16 genomes are efficiently targeted by the epithelial CpG methylation machinery and raise questions as to the mechanism of this reaction and the biological consequences for the epithelial cell and/or the HPV-16 life cycle.
We did not detect an HPV-16 L1-LCR segment that was completely methylated. A past study, involving one of us (2), observed among 15 clinical samples 5 that seemed to be completely methylated in this segment, but the present data are more representative of HPV-16 genomes in situ, as the past findings were based on a shorter segment and on direct sequencing, which may have scored mixtures of HPV-16 DNAs that were methylated or unmethylated in the same CpG position as completely methylated.
We measured a lot of “noise” in HPV-16 DNA methylation, i.e., there were no sites that were always or never methylated nor were there conserved stretches of contiguous CpG methylation in the majority of molecules. Methylation does not appear very often at isolated CpGs, but frequently on three to eight or even more flanking CpGs, reminiscent of spread of the modification along a DNA. In spite of these reservations, we observed decreased methylation in three regions, the sites 7270 and 7862, and to a lesser degree the flanking sites 7535 and 7554. It is noteworthy to interpret these positions in the context of known cis-responsive elements and chromatin structures of the HPV-16 LCR. The segment 3′ to position 7862, which is often heavily methylated, contains all promoter elements, i.e., the promoter activator Sp1, whose binding is not influenced by DNA methylation (17), the viral factor E2, which is displaced by methylation, and the TATA box. This segment is organized in form of a specifically positioned nucleosome, whose acetylation affects the promoter (38). Position 7862 is located 5′ of this nucleosome and coincides with the viral replication origin and a silencer regulated by YY1 and CDP, and the 3′ flank of the viral enhancer, activated by AP-1. This region serves as a superregulator, as YY1 and CDP with their associated HDAC activities and AP-1 with a histone acetylase activity repress and activate HPV transcription in different epithelial layers by influencing the promoter nucleosome and a second nucleosome encompassing the viral enhancer (1, 3, 26). Methylation and demethylation of CpG at position 7862 may add another layer of cross talk to this regulatory region. In addition, position 7862 is part of an E2 binding site that activates HPV replication and has to remain demethylated to permit another round of replication. DNA methylated at position 7862 would be replication incompetent and eliminated. The fact that position 7862 is close to an Sp1 site conserved among HPVs (43) recalls the ability of Sp1 to suppress methylation from some adjacent CpG elements (7). The properties of this DNA segment may establish a molecular basis for the poorly defined state of latency of HPV infections, i.e., the presence of HPV DNA in the absence of symptoms, as repression of the enhancer and promoter and accessibility of the replication origin could lead to maintenance replication of the virus without expression of transformation functions.
The enhancer is covered by a second nucleosome (Fig. 1); 5′ of the enhancer, we found a nucleosomal linker (39), which includes positions 7535-7554. DNase I sensitivity studies suggested additional specifically positioned nucleosomes 5′ of this region, which were separated by another linker around position 7270. These arguments would lead to a proposal that HPV-16 DNA tends to be hypermethylated in nucleosomally organized segments and undermethylated in nucleosomal linkers. This sounds counterintuitive but may have functional significance, since nucleosomally incorporated DNA is not protected from, but rather still efficiently targeted by, CpG maintenance methylation (28). The overlap of specifically positioned nucleosomes with hypermethylated regions is indicated in Fig. 1.
MeCpA and meCpT residues point to de novo methylation in epithelial cells, and our observations confirm that HPV genomes are efficiently targeted in undifferentiated cells in cell culture. While we measured only a total rate of about 0.4% of methylation of these two dinucleotides, the real frequency of de novo methylation is likely much higher, since our approach scored CpGs only in the maintenance methylation data set (Fig. 3), but not in the de novo methylation data set (Fig. 5), although CpGs are more efficiently methylated de novo than the other two dinucleotides. The occurrence of de novo methylation in a somatic tissue is little studied (44A, 47A), in contrast to embryos and cancer cells (19, 32). But this is not completely surprising, as DNMT3a and DNMT3b activities have been detected in a variety of normal somatic cells. Strangely, meCpAs and meCpTs appeared to occur frequently in regions of CpG undermethylation, and we speculate that maintenance methylation is particularly efficient within nucleosomes and de novo methylation in nucleosomal linkers. While this latter observation is not statistically significant for most sites based on our data set, it is of interest that 14 of 22 meCpA and meCpT residues observed by Kim et al. (22) in cell cultures containing HPV-16 occurred in the nucleosomal linkers flanking the positions 7535-7554 and 7862.
Correlations between HPV-16 DNA methylation and pathology.
We found methylation of HPV-16 DNA in three different clinical contexts: (i) in asymptomatic infections (NIL), (ii) in low- and high-grade CIN, and (iii) in carcinoma, although with differing frequencies.
The methylation of presumably episomal HPV DNA in asymptomatic patients is surprising. We are not aware of a molecular scenario that would be comparable to the persistent maintenance of HPV in asymptomatic infections, with the exception of the methylation of a CpG island in the EBNA promoters of Epstein-Barr virus. While this alteration occurs in asymptomatic B cells as well as during carcinogenic progression, it was interpreted to support the tumorigenic process by suppression of EBNA antigens (33). It is an open question whether our data point to an epithelial defense mechanism against heterologous DNA or a viral adaptation to be maintained in an epithelium without causing a neoplasia. As argued above, we propose that access to the viral replication origin with concomitant suppression of transcription may be an adaptation to latency, a molecular state and scientific term, which is not yet formally recognized for HPVs as it is for herpesviruses. Kim et al. (22) reported an excess of methylated HPV-16 genomes in undifferentiated cells in culture and a stable relationship between methylated HPV genomes and maintenance of the undifferentiated state may be an embodiment of latency.
The low methylation levels of HPV genomes in CIN lesions, where HPVs normally replicate episomally, may stem from the expansion of the transcriptionally active cell population, as expected from the initiation of the neoplastic process and an increase of the virus genomes (37). This observation can probably be best compared with the transcriptionally highly active productive life cycle of polyomaviruses and adenoviruses.
The heavy methylation in cancers can be interpreted as confirmation of the genome defense hypothesis based on the frequent methylation of chromosomally integrated retroviruses, transgenes (4), and transfected adenovirus genomes and their chromosomal targets (15, 41). It is generally known that HPV-16 occurs in carcinomas most often in the chromosomally integrated state (12, 36). This does not mean that carcinomas do not contain transcriptionally active HPV genomes, since the example of the cell line CaSki (2) shows that carcinomas often contain HPV genomes in integrated tandem arrays, which are efficient methylation targets, while some of these genomes remain unmethylated and therefore actively expressing the HPV oncogenes (2, 45). We caution against the hope that methylation inhibitors, resulting in demethylation and therefore activation of repressed tumor suppressor genes, are beneficial for all kinds of carcinomas, as they would actually be tumor promoters in HPV-activated lesions (18). While the increased methylation of the L1 gene in our samples may point to a role of methylation in the early-late switch, one may also consider that exonic CpGs have a higher propensity to becoming methylated and may be nuclei from where CpG methylation spreads to promoter regions (25).
Our database leads to hypotheses that we will study experimentally. An obvious route is to address the question of whether HPV DNA is methylated in capsids, and if not, by what kinetics it establishes methylation after infection of raft cultures. In preliminary studies of HPV-11, no methylated HPV-11 genomes were found in viral particles, but a significant fraction of methylated HPV-11 DNA was found in newly infected human transplants (H.-U. Bernard, N. Christensen, and M. Kalantari, unpublished data). In raft cultures with episomal HPVs, one can study the establishment of methylation patterns after integration into chromosomal DNA. Laser capture microdissection may allow localization of the correlation of methylation patterns with histological sites and pathological features and analysis, by quantitative RNA studies, of correlations between transcription and methylation patterns. These considerations and our observation that HPV methylation is taxonomically widespread suggest that HPV methylation is a rich system for basic as well as clinical research approaches.
Acknowledgments
This research was supported by NIH grant ROI CA-91964 and by funds from the Chao Family Comprehensive Cancer Center of the University of California—Irvine to H.-U.B., by a postdoctoral fellowship of the Cancer Center of the University of California—Irvine to M.K., and by a Collaborative Research Grant from UC MEXUS-CONACYT to I.E.C.-M, H.B.-S, and H.-U.B.
We also acknowledge technical support by John Huh and the services provided by Hanne Skomedal, Tor Molden, and Irene Kraus of NorChip, Oslo, Norway, in typing of HPV in cervical cancers.
REFERENCES
- 1.Ai, W., E. Toussaint, and A. Roman. 1999. CCAAT displacement protein binds to and negatively regulates human papillomavirus type 6 E6, E7, and E1 promoters. J. Virol. 73:4220-4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Badal, S., V. Badal, I. E. Calleja-Macias, M. Kalantari, L. S. H. Chuang, B. F. L. Li, and H. U. Bernard. 2004. The human papillomavirus-18 genome is efficiently targeted by cellular DNA methylation. Virology 324:483-492. [DOI] [PubMed] [Google Scholar]
- 2.Badal, V., L. S. H. Chuang, E. H.-H. Tan, S. Badal, L. L. Villa, C. M. Wheeler, B. F. L. Li, and H.-U. Bernard. 2003. CpG methylation of human papillomavirus-16 DNA in cervical cancer cell lines and in clinical specimens: genomic hypomethylation correlates with carcinogenic progression. J. Virol. 77:6227-6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard, H. U. 2002. Gene expression of genital human papillomaviruses and potential antiviral approaches. Antivir. Ther. 7:219-237. [PubMed] [Google Scholar]
- 4.Bestor, T. H., and B. Tycko. 1996. Creation of genomic methylation patterns. Nat. Genet. 12:363-367. [DOI] [PubMed] [Google Scholar]
- 5.Bird, A. P. 1992. The essentials of DNA methylation. Cell 70:5-8. [DOI] [PubMed] [Google Scholar]
- 6.Bosch, F. X., M. M. Manos, N. Munoz, M. Sherman, A. M. Jansen, J. Peto, M. H. Schiffman, V. Moreno, R. Kurman, K. V. Shah, et al. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 87:796-802. [DOI] [PubMed] [Google Scholar]
- 7.Brandeis, M., D. Frank, I. Keshet, Z. Siegfried, M. Mendelsohn, A. Nemes, V. Temper, A. Raxin, and H. Cedar. 1994. Sp1 elements protect a CpG island from de novo methylation. Nature 371:435-438. [DOI] [PubMed] [Google Scholar]
- 8.Burgers, W. A., F. Fuks, and T. Kouzarides. 2002. DNA methyltransferases get connected to chromatin. Trends Genet. 18:275-277. [DOI] [PubMed] [Google Scholar]
- 9.Burnett, T. S., and J. P. Sleeman. 1984. Uneven distribution of methylation sites within the human papillomavirus la genome: possible relevance to viral gene expression. Nucleic Acids Res. 12:8847-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.I. E. Calleja-Macias, M. Kalantari, J. Huh, R. Ortiz-Lopez, A. Rojas-Martinez, J. F. Gonzalez-Guerrero, A.-L. Williamson, B. Hagmar, D. J. Wiley, L. Villarreal, H.-U. Bernard, and H. A. Barrera-Saldaña. 2004. High prevalence of specific variants of human papillomavirus-16, 18, 31, and 35 in a Mexican population and relationship to European, African, and Native American variants. Virology 319:315-323. [DOI] [PubMed] [Google Scholar]
- 11.Chan, W. K., G. Klock, and H.-U. Bernard. 1989. Progesterone and glucocorticoid response elements occur in the long control regions of several human papillomaviruses involved in anogenital neoplasia. J. Virol. 63:3261-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel, B., G. Mukherjee, L. Seshadri, E. Vallikad, and S. Krishna. 1995. Changes in the physical state and expression of human papillomavirus type 16 in the progression of cervical intraepithelial neoplasia lesions analysed by PCR. J. Gen. Virol. 76:2589-2593. [DOI] [PubMed] [Google Scholar]
- 13.Danos, O., M. Katinka, and M. Yaniv. 1980. Molecular cloning, refined physical map and heterogeneity of methylation sites of papilloma virus type 1a DNA. Eur. J. Biochem. 109:457-461. [DOI] [PubMed] [Google Scholar]
- 14.Dodge, J. E., B. H. Ramsahoye, Z. G. Wo, M. Okano, and E. Li. 2002. De novo methylation of MMLV provirus in embryonic stem cells: CpG versus non-CpG methylation. Gene 289:41-48. [DOI] [PubMed] [Google Scholar]
- 15.Doerfler, W., Remus, R., Muller, K., Heller, H., Hohlweg, U., and R. Schubbert. 2001. The fate of foreign DNA in mammalian cells and organisms. Dev. Biol. (Basel) 106:89-97. [PubMed] [Google Scholar]
- 16.Frommer, M., L. E. McDonald, D. S. Millar, C. M. Collis, F. Watt, G. W. Grigg, P. L. Molloy,and C. L. Paul. 1992. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl. Acad. Sci. USA 89:1827-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington, M. A., P. A. Jones, M. Imagawa, and M. Karin. 1988. Cytosine methylation does not affect binding of transcription factor Sp1. Proc. Natl. Acad. Sci. USA 85:2066-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman, J. G., and S. B. Baylin. 2000. Promoter-region hypermethylation and gene silencing in human cancer, p. 35-54. In P. A. Jones and P. K. Vogt (ed.), DNA methylation and cancer. Springer, Berlin, Germany. [DOI] [PubMed]
- 19.Jaenisch, R., and A. Bird. 2003. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33:245-254. [DOI] [PubMed] [Google Scholar]
- 20.Jones, P. A. 2003. Epigenetics in carcinogenesis and cancer prevention. Ann. N. Y. Acad. Sci. 983:213-219. [DOI] [PubMed] [Google Scholar]
- 21.Jones, P. L., P. A. Wade, and A. P. Wolffe. 2001. Purification of the MeCP2/histone deacetylase complex from Xenopus laevis. Methods Mol. Biol. 181:297-307. [DOI] [PubMed] [Google Scholar]
- 22.Kim, K., P. A. Garner-Hamrick, C. Fisher, D. Lee, and P. F. Lambert. 2003. Methylation patterns of papillomavirus DNA, its influence on E2 function, and implications in viral infection. J. Virol. 77:12450-12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang, G., M. F. Chan, Y. Tomigahara, Y. C. Tsai, F. A. Gonzales, E. Li, P. W. Laird, and P. A. Jones. 2002. Cooperativity between DNA methyltransferases in the maintenance methylation of repetitive elements. Mol. Cell. Biol. 22:480-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers, G., H. U. Bernard, H. Delius, M. Favre, J. Iconogle, M. van Ranst, and C. Wheeler (ed.). 1994. Human papillomaviruses: 1994 compendium, p. 1-A-3 to 1-A-8. Los Alamos National Laboratory, Los Alamos, N. Mex.
- 25.Nguyen, C., G. Liang, T. T. Nguyen, D. Tsao-Wei, S. Groshen, M. Lubbert, J. H. Zhou, W. F. Benedict, and P. A. Jones. 2001. Susceptibility of nonpromoter CpG islands to de novo methylation in normal and neoplastic cells. J. Natl. Cancer Inst. 93:1465-1472. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor, M. J., W. Stünkel, C.-H. Koh, H. Zimmermann, and H.-U. Bernard. 2000. The differentiation-specific factor CDP/Cut represses transcription and replication of human papillomaviruses through a conserved silencing element. J. Virol. 70:401-410. [PMC free article] [PubMed] [Google Scholar]
- 27.Okano, M., D. W. Bell, D. A. Haber, and E. Li. 1999. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99:247-257. [DOI] [PubMed] [Google Scholar]
- 28.Okuwaki, M., and A. Verreault. 2004. Maintenance DNA methylation of nucleosome core particles. J. Biol. Chem. 279:2904-2912. [DOI] [PubMed] [Google Scholar]
- 29.Pater, M. M., G. A. Hughes, D. E. Hyslop, H. Nakshatri, and A. Pater. 1988. Glucocorticoid-dependent oncogenic transformation by type 16 but not type 11 human papilloma virus DNA. Nature 335:832-835. [DOI] [PubMed] [Google Scholar]
- 30.Ramsahoye, B. H., D. Biniszkiewicz, F. Lyko, V. Clark, A. P. Bird, and R. Jaenisch. 2000. Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a. Proc. Natl. Acad. Sci. USA 97:5237-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee, I., K. E. Bachman, B. H. Park, K. W. Jair, R. W. Yen, K. E. Schuebel, H. Cui, A. P. Feinberg, C. Lengauer, K. W. Kinzler, S. B. Baylin, and B. Vogelstein. 2002. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature 416:552-556. [DOI] [PubMed] [Google Scholar]
- 32.Robertson, K. D., E. Uzvolgyi, G. Liang, C. Talmadge, J. Sumegi, F. A. Gonzales, and P. A. Jones. 1999. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res. 27:2291-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson, K.D. 2000. The role of DNA methylation in modulating Epstein-Barr virus gene expression, p. 21-34. In P. A. Jones and P. K. Vogt (ed.), DNA methylation and cancer. Springer Verlag, Berlin, Germany. [DOI] [PubMed]
- 34.Rosl, F., A. Arab, B. Klevenz, and H. zur Hausen. 1993. The effect of DNA methylation on gene regulation of human papillomaviruses. J. Gen. Virol. 74: 791-801. [DOI] [PubMed] [Google Scholar]
- 35.Schiffman, M. H., and L. A. Brinton. 1995. The epidemiology of cervical carcinogenesis. Cancer 76:1888-1901. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz, E., U. K. Freese, L. Gissmann, W. Mayer, B. Roggenbuck, A. Stremlau, and H. zur Hausen. 1985. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 314:111-114. [DOI] [PubMed] [Google Scholar]
- 37.Stoler, M. H., C. R. Rhodes, A. Whitbeck, S. M. Wolinsky, L. T. Chow, and T. R. Broker. 1992. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum. Pathol. 23:117-128. [DOI] [PubMed] [Google Scholar]
- 38.Stünkel, W., Z. Huang,S. H. Tan, M. O'Connor, and H.-U. Bernard. 2000. Nuclear matrix attachment regions of human papillomavirus type 16 repress or activate the E6 promoter, depending on the physical state of the viral DNA. J. Virol. 74:2489-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stünkel, W., and H.-U. Bernard. 1999. The chromatin structure of the long control region of human papillomavirus type 16 represses viral oncoprotein expression. J. Virol. 73:1918-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugawara, K., K. Fujinaga, Y. Yamashita, and Y. Ito. 1983. Integration and methylation of shope papilloma virus DNA in the transplantable Vx2 and Vx7 rabbit carcinomas. Virology 131:88-99. [DOI] [PubMed] [Google Scholar]
- 41.Sutter, D., and W. Doerfler. 1980. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc. Natl. Acad. Sci. USA 77:253-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan, S. H., C. C. Baker, W. Stünkel, and H. U. Bernard. 2003. A transcriptional initiator overlaps with a conserved YY1 site in the long control region of human papillomavirus type 16. Virology 305:486-501. [DOI] [PubMed] [Google Scholar]
- 43.Tan, S. H., L. E. C. Leong, P. A. Walker, and H. U. Bernard. 1994. The human papillomavirus type 16 transcription factor E2 binds with low cooperativity to two flanking binding sites and represses the E6 promoter through displacement of Sp1 and TFIID. J. Virol. 68:6411-6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thain, A., O. Jenkins, A. R. Clarke, and K. Gaston. 1996. CpG methylation directly inhibits binding of the human papillomavirus type 16 E2 protein to specific DNA sequences. J. Virol. 70:7233-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Toth, M., U. Muller, and W. Doerfler. 1990. Establishment of de novo DNA methylation patterns. Transcription factor binding and deoxycytidine methylation at CpG and non-CpG sequences in an integrated adenovirus promoter. J. Mol. Biol. 214:673-683. [DOI] [PubMed] [Google Scholar]
- 45.Van Tine, B. A., J. Knops, T. R. Broker, L. T. Chow, and P. T. Moen. 2001. In situ analysis of the transcriptional activity of integrated viral DNA using tyramide-FISH. Dev. Biol. (Basel) 106:381-385. [PubMed] [Google Scholar]
- 46.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 47.Wettstein, F. O., and J. G. Stevens. 1983. Shope papilloma virus DNA is extensively methylated in non-virus-producing neoplasms. Virology 126:493-504. [DOI] [PubMed] [Google Scholar]
- 47a.Woodcock, D. M., P. J. Crowther, S. Jefferson, and W. P. Diver. 1988. Methylation at dinucleotides other than CpG: implications for human maintenance methylation. Gene 74:151-152. [DOI] [PubMed] [Google Scholar]
- 48.Yoder, J. A., C. P. Walsh, and T. H. Bestor. 1997. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 13:335-340. [DOI] [PubMed] [Google Scholar]
- 49.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2:342-350. [DOI] [PubMed] [Google Scholar]