Abstract
Immunity to human group A rotavirus (RV), a major cause of viral gastroenteritis in infants, involves B lymphocytes that provide RV-specific antibodies. Additionally, some arguments suggest that naive B cells could be implicated in the first steps of the immune response against RV. The aim of our study was to analyze the interaction of VP6 and VP7 RV capsid proteins with human B cells depending on the immune status of the individual, i.e., naive or RV experienced. For this purpose, a two-color virus-like particle flow cytometry assay was devised to evaluate the blood B-lymphocyte reactivity to VP6 and VP7 proteins from healthy RV-exposed adults, recently infected infants, and neonates at birth. Both VP6 and VP7 interactions with B cells were mediated by surface immunoglobulins and probably by their Fab portions. VP7-reactive B lymphocytes were mainly detected from RV-experienced patients and almost exclusively in the CD27-positive memory cell fraction. Conversely, VP6-reactive B lymphocytes were detected at similar and high frequencies in adult, infant, and neonate samples. In adult samples, VP6 reacted with about 2% of the CD27-negative (CD27neg) naive B cells. These results demonstrated that the VP6 RV protein interacted with a large fraction of naive B lymphocytes from both adults and neonates. We propose that naive B cell-VP6 interaction might influence the strength and quality of the acquired immune response and should be considered for elaborating RV vaccine strategies.
Human group A rotavirus (RV) is recognized as a leading cause of severe dehydrating diarrhea in young children. The worldwide impact of the disease has led to extensive research to develop RV vaccines (15, 16, 29). However, RV vaccines only partially achieve protective immunity in humans, as do natural primary exposures. The previously released Rotashield vaccine, which has been withdrawn because of adverse effects, conferred only a 60% level of protection against RV-induced diarrhea (1, 35). The bases underlying the variable efficacy of RV vaccines are unknown. Efforts remain to be made to better understand the protective mechanisms against RV for improving vaccine strategies.
RV possesses a triple-layered icosahedral protein capsid, and three of the RV structural proteins (VP4, VP6, and VP7) have important antigenic properties. The intermediate-layer capsid protein VP6 mediates group and subgroup specificity, while the outer-layer proteins VP4 and VP7 mediate serotype P and serotype G specificities, respectively (20). VP6 is the most immunogenic RV protein (20, 34). VP6 does not induce neutralizing antibodies (Abs), although some VP6-specific polymeric immunoglobulins A (IgA) are protective in vivo, probably via transcytosis through epithelial cells (6, 32). VP7 is known as the major antigen inducing neutralizing Abs (20). These Abs can passively protect experimental animals from RV-induced diarrhea (26, 30, 31). In humans, RV-induced Abs probably play an important role in the resolution of viral infection and against reinfections, as suggested by studies with adult volunteers, naturally RV-infected children, and infants from candidate vaccine clinical trials (19). The B-lymphocyte population, which provides the specific anti-RV Abs, appears to be involved in other aspects of the host response, especially in the early phase of infection. Actually, intestinal infection with RV induces a rapid and massive T-lymphocyte-independent expansion of B cells that results in early anti-RV IgM production (5). Furthermore, naive B lymphocytes were shown to be the antigen-presenting cells responsible for intestinal IgA production after subcutaneous RV injection in mice (12). Because RV does not infect B cells, naive B lymphocytes probably take up RV via pinocytosis or receptor-mediated endocytosis. Among the hypotheses, a high frequency of naive B-cell-expressing surface Igs reactive to RV antigens could explain both the extent of RV antigen presentation by B cells and the early and massive expansion of the naive B-cell population. Whether such an innate recognition of RV proteins by naive B cells does exist in humans and, if so, the nature of the RV protein and B-cell receptor involved in this interaction remain to be established.
The goal of our study was to determine whether naive B cells spontaneously interacted via surface Ig with RV proteins by comparison with immune RV-experienced B cells. This study was conducted with humans to address relevant clinical implications. The two candidate RV proteins that we focused on were the VP6 major capsid protein and the VP7 outer-capsid protein. We developed a flow cytometry assay based on two-color fluorescent virus-like particles (VLP). This assay was designed for the simultaneous detection and discrimination of B cells interacting with VP6 and VP7. By using this approach, we found that VP6 interacted equally via surface Ig with a large number of B lymphocytes in blood from healthy RV-exposed adults, RV-infected infants, and RV-naive neonates, whereas VP7 mostly interacted with B cells from RV-experienced patients. The VP6-B cell interaction in adult samples was predominantly found associated to the CD27-negative (CD27neg) B cells that represent naive B lymphocytes (22). The high frequency of VP6 interaction with naive B lymphocytes could explain the early involvement of naive B cells during RV infection and might have a significant impact in shaping adaptive immune responses after primary infection or vaccination.
MATERIALS AND METHODS
Sample collection.
Stool and blood samples were obtained during the acute phase of RV disease from five infants (median age, 7 months; range, 4 to 10 months) who were hospitalized in the pediatric gastroenterology unit of the Hôpital Armand Trousseau, Paris, France, in January and February 2001. A child was considered infected if RV antigens were detected by enzyme-linked immunosorbent assay (ELISA) in a stool sample. Blood samples were collected at a median time of 7 days (range, 1 to 18 days) after the onset of diarrhea. During the same RV season, blood samples were obtained from 13 healthy and asymptomatic pediatricians of the Hôpital Armand Trousseau emergency room. This medical staff is regularly exposed to infants with RV infection. The detection of anti-RV Abs in the pediatricians' blood samples by ELISA confirmed their previous infection. Lastly, cord blood samples were collected from 13 healthy neonates at birth. Neonates of a mother with any positive medical history were excluded. This study was conducted according to the guidelines of the local ethical committee, and informed consent for participation in this study was obtained from the parents of the infants, the pediatricians, and the mothers of the neonates.
Peripheral blood mononuclear cells (PBMC) were isolated from blood samples by density gradient centrifugation with Ficoll-Hypaque (Eurobio, Les Ullis, France). PBMC were either tested freshly or kept frozen in liquid nitrogen in fetal calf serum (FCS) containing 10% dimethyl sulfoxide (Braun, Boulogne, France) prior to flow cytometry testing.
B-cell-enriched PBMC were obtained from fresh whole blood with a depletion cocktail of monoclonal Abs (MAbs) (RosetteSep B cell enrichment cocktail; Stemcell, Meylan, France) according to the manufacturer's recommendations and analyzed by flow cytometry as soon as they were prepared.
Detection of RV antigens in feces.
Detection of group A RV antigens in fecal samples was performed with a commercial ELISA (IDEIA RV; Dako, Cambridgeshire, United Kingdom) according to the manufacturer's recommendations.
Production of fluorescent VLP.
The production of fluorescent group A RV VLP in the baculovirus expression system has been previously described in detail (8).
To obtain fluorescent VP2 proteins, the first 92 amino acids of the VP2 protein from the group A bovine RV strain RF were substituted with either the green fluorescent protein (GFP) or the red fluorescent protein (DsRed), and the resulting fusion proteins were cloned into a baculovirus vector (pFASTBAC1; Gibco, Paisley, United Kingdom). Sf9 cells were then coinfected (approximately 5 PFU/cell) with the recombinant baculovirus containing either the GFP-VP2 or the DsRed-VP2 fusion protein and with a baculovirus containing the RV VP6 protein (strain RF) to obtain green fluorescent VLP (GFP-VLP2/6, consisting of VP2 and VP6) or red fluorescent VLP (DsRed-VLP2/6). Alternatively, the recombinant baculovirus containing the GFP-VP2 fusion protein was used together with a baculovirus containing the VP6 protein and a baculovirus containing the VP7 protein derived from the human RV strain Wa serotype G1 to infect Sf9 cells and to obtain GFP-VLP2/6/7. Nonfluorescent VLP2/6 was obtained by coinfecting Sf9 cells with two baculoviruses expressing, respectively, wild-type VP2 and VP6 proteins from the bovine RV strain RF. VLP were purified from infected Sf9 cultures by density gradient centrifugation in cesium chloride, and the protein concentration of the VLP preparations was determined by a micro-BCA protein assay kit (Pierce, Rockford, Ill.).
Flow cytometry assays.
For the detection of RV-specific B cells, we used a two-color flow cytometry assay modified from a previously described technique validated in the mouse model (24, 38) and recently used with samples from humans (17). PBMC (fresh or frozen and thawed) were washed once with RPMI 1640 (Gibco, Paisley, United Kingdom) plus 4% FCS. Pellets containing at least 106 cells were resuspended and incubated with a mixture containing 1 μg of PerCP-conjugated mouse anti-human CD19 MAbs (anti-CD19 PerCP; Becton Dickinson, San Jose, Calif.) per ml, 5 μg of DsRed-VLP2/6 per ml, and 5 μg of GFP-VLP2/6/7 per ml. The fluorescent VLP concentration of 5 μg/ml corresponds to 4,000 and 6,150 VLP per cell for GFP-VLP2/6/7 and DsRed-VLP2/6, respectively, and this concentration was found optimal to stain RV-reactive B lymphocytes. After 30 min of incubation in the dark at 4°C, the cells were washed in RPMI 1640 plus 4% FCS, resuspended in 10% CellFix (Becton Dickinson) and analyzed with a flow cytometer (FACSCalibur, Becton-Dickinson). At least 105 cells were acquired per sample. As a guide for setting markers to delineate specific and nonspecific staining, control cells were stained only with anti-CD19 PerCP MAbs. The analysis of data files was performed with CellQuest Software (Becton-Dickinson).
To test the possibility of maternal Abs binding to neonate B lymphocytes, cells were incubated for 1 h at 37°C, washed once with RPMI 1640 alone, and reincubated 30 min at 4°C prior to being pelleted for staining as described above.
In the competitive experiments, freshly B-cell-enriched PBMC were stained with fluorescent RV VLP and anti-CD19 PerCP MAbs as described above in the presence of increasing concentrations of nonfluorescent RV VLP2/6, RV double-layered particles (DLPs) obtained from the bovine RF strain or nonfluorescent birnavirus VP2-based VLP used as the control (11).
In the inhibition experiments with anti-surface Ig Abs, B-cell-enriched PBMC were first incubated with increasing concentrations (from 0,01 to 100 μg/ml) of affinity-purified goat Fab′2 Abs directed to human Fab′2 Ig (anti-human Fab′2 Ab; Jackson, West Grove, Pa.). The affinity-purified goat Fab′2 Abs directed to the mouse μ chain (Jackson) were used as controls. After 30 min of incubation in the dark on ice, the cells were washed and stained with fluorescent RV VLP as described above.
For the analysis of CD27 marker on B cells, B-cell-enriched PBMC were incubated with allophycocin-conjugated mouse anti-CD19 Abs (anti-CD19 APC; Becton Dickinson), phycoerythrin-conjugated mouse anti-CD27 Abs (anti-CD27 PE; Becton Dickinson) and GFP-VLP2/6 or GFP-VLP2/6/7.
Statistical analysis.
Statistical analysis was performed with StatView software, version 4.2 (SAS Institute), with nonparametric tests. VP6- or VP7-specific B-lymphocyte global data were evaluated with the Kruskal-Wallis test and the Mann-Whitney test for pairwise comparisons between the VP6- or VP7-specific cell values in adult, infant, and neonate samples. The correlation between VP6- and VP7-specific cell numbers in each group was evaluated with the Spearman test. Significance was established if P was <0.05.
RESULTS
Two-color fluorescent RV VLP assay allows the simultaneous and specific detection of VP6- and VP7-reactive B lymphocytes.
In order to analyze VP6 and VP7 RV protein interaction with human B lymphocytes, we established a two-color-based VLP flow cytometry assay for the analysis of blood B-cell samples from healthy RV-exposed adults, recently infected infants, and neonates at birth. DsRed-VLP2/6 and GFP-VLP2/6/7 were used simultaneously in fluorescence-activated cell sorter (FACS) analysis. The B cells reacting with VLP2/6 were expected to appear in red and the B cells reacting with VLP2/6/7 were expected to appear in green. The VP7 protein in VLP2/6/7 was of the G1 serotype, which is the most frequently encountered in patient populations in Western European countries, so that VLP2/6/7 were expected to react with B cells from most RV-immune individuals. B cells were labeled with a PerCP conjugated anti-CD19 MAb (anti-CD19 PerCP) and gated for the analysis of VLP2/6 and VLP2/6/7 binding. As exemplified in Fig. 1, clear labeling of CD19-positive (CD19pos) B cells with VLP2/6 or VLP2/6/7 could be simultaneously detected in samples from RV-exposed adults and from recently infected infants. It is noteworthy that no VLP binding could be detected on CD19neg cells (data not shown). B cells double labeled with DsRed-VLP2/6 and GFP-VLP2/6/7 were not detected in these experiments. Conversely, when DsRed-VLP2/6 and GFP-VLP2/6 were used together, double-labeled B cells could be detected (data not shown). This indicates that the antigenic determinants of VP6 in VLP2/6/7 were probably masked by the VLP's outer protein, VP7. The lack of a double-labeled B-cell population allowed us to consider that the binding of VLP2/6 and VLP2/6/7 on CD19 B cells unambiguously involved VP6 and VP7, respectively. Interestingly, FACS analysis performed on samples from neonates revealed that VP6 and not VP7 reacted broadly with cord blood B cells (Fig. 1). Over 95% of the cord blood B cells were found to be negative for CD27 expression, a marker of memory B lymphocytes (22, 27), confirming that cord blood B cells were naive cells (data not shown). The possibility of VP6 binding via maternal Abs present in cord blood and bound to neonatal B cells seemed unlikely because B lymphocytes are not known to express the high affinity FcγR1, which is the major receptor of cytophilic Abs (3). Furthermore, when cells were first incubated at 37°C to allow for potential Ig endocytosis and subsequently reacted at 4°C with fluorescent VLP, no difference in VP6 binding could be detected (data not shown).
FIG. 1.
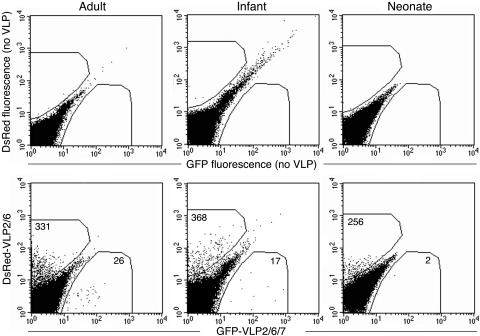
FACS detection of RV-reactive B lymphocytes. Examples of adult, infant, and neonate blood samples are presented. The cells were first gated on CD19pos cells, and dot plots of GFP-VLP- versus DsRed-VLP-reactive B cells were then created. In each panel, results of analyzing approximately 5 × 105 CD19pos B cells are shown. Gates of fluorescence positivity were determined by comparison with identical B cells incubated without VLP (top panels).
To determine the specificity of fluorescent VLP binding to B lymphocytes from adults and neonates, nonfluorescent VLP2/6 were used in competition experiments. In adult as well as in neonate samples, an increasing concentration of nonfluorescent VLP2/6 progressively inhibited DsRed-VLP2/6 binding (Fig. 2). Similarly, an increasing concentration of RV-DLP inhibited fluorescent VLP binding to adult cells (Fig. 2A). Irrelevant VLP made of birnavirus VP2 proteins did not compete with DsRed-VLP2/6 binding to adult B cells, further confirming the specificity of VLP2/6 interaction with a membranous receptor on B cells (Fig. 2A).
FIG. 2.
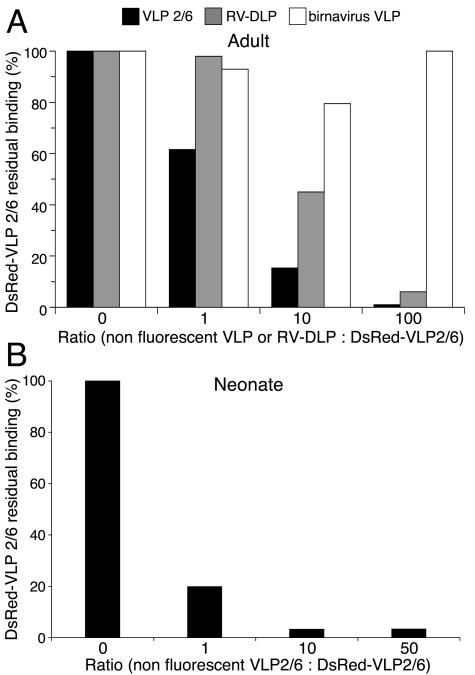
Binding competition of DsRed-VLP2/6 with nonfluorescent VLP2/6 and RV-DLP. (A) Enriched B-cell PBMC from a sample taken from an adult were incubated with fluorescent DsRed-VLP2/6 and with increasing concentrations of nonfluorescent VLP2/6, RV-DLP, or nonfluorescent birnavirus VP2-VLP. (B) PBMC from a neonate were incubated with fluorescent DsRed-VLP2/6 and increasing concentrations of nonfluorescent VLP2/6. The results are expressed as the percentages of residual binding of fluorescent DsRed-VLP2/6 to CD19pos cells.
Taken together, these results indicated that VLP2/6 and VLP2/6/7 specifically and differentially interacted with human B cells. At first sight, VLP2/6 binding appeared much more frequent on B cells than VLP2/6/7 binding. Furthermore, conversely from VLP2/6/7, VLP2/6 could largely decorate neonate B cells, indicating that VLP2/6 interacted with naive B cells.
Both VLP2/6 and VLP2/6/7 are specifically bound to B cells by surface Ig.
We next set up our goal to determine whether VLP2/6 and VLP2/6/7 interactions with B cells were mediated by surface Ig. We found that increasing concentrations of goat polyclonal Abs directed to human Fab′2 progressively inhibited DsRed-VLP2/6 binding to the B-cell surface in adult (Fig. 3A) as well as in neonate (Fig. 3B) samples. When the same Abs were used, GFP-VLP2/6/7 binding to the B cell surface was similarly inhibited in adult samples (Fig. 3C). As the control, goat polyclonal Abs to the murine μ chain showed no significant competitive effect (Fig. 3). These results indicate that the RV-fluorescent VLP binding to the B lymphocytes was mediated by surface Ig and most probably by their Fab portion.
FIG. 3.
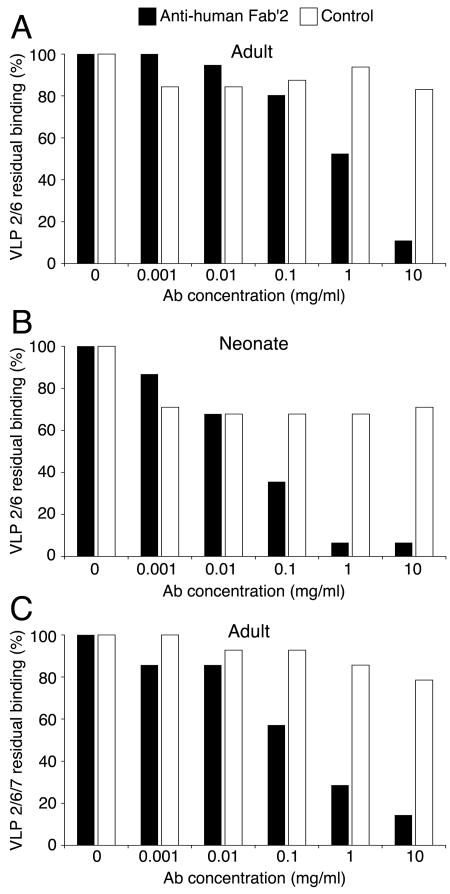
Inhibition of DsRed-VLP2/6 and GFP-VLP2/6/7 binding by anti-human Fab′2 Abs. Enriched B-cell PBMC were incubated with increasing concentrations of polyclonal Abs directed to human Fab′2 and with DsRed-VLP2/6 in samples from adults (A) and neonates (B) or with GFP-VLP2/6/7 in samples from adults (C). Goat polyclonal Abs to murine μ chain Abs were used as a control. The results are expressed as the percentages of residual binding of the corresponding fluorescent VLP to CD19pos cells.
VP6-reactive B cells are detected at high and similar frequencies in samples from neonates, infants, and adults, whereas VP7-reactive B cells are mainly detected in samples from adults.
Our preliminary results suggest possible differences in the reactivity of VLP2/6 and VLP2/6/7 with B lymphocytes between adults, infants and neonates. To further document this primary observation, we tested the reactivity of the two-colored VLP on samples from 13 healthy RV-exposed adults, five infants recently infected with RV, and 13 neonates at birth. The global distribution of the VP7-specific B-cell values was not random among the 32 samples of the three groups (Kruskal-Wallis; P < 0.0001). CD19pos B lymphocytes reactive to VLP2/6/7 were mostly detected in adult samples with a median value of 73 (range, 35 to 450) out of 105 B lymphocytes (Fig. 4A). CD19pos B lymphocytes reactive to VLP2/6/7 were detected in infant and in cord blood samples with median values of 43 (range, 14 to 49) and 12 (range, 0 to 30) out of 105 B lymphocytes, respectively (Fig. 4A). VP7-specific B lymphocyte values in infants were directly related to the number of days between symptoms and sample collection (data not shown). As a consequence, the number of VP7-specific B cells observed in infants might have been underestimated because of a too-short time period between symptoms and sampling. A pairwise comparison indicated that the number of VP7-specific B cells was significantly higher in adults than in infants (Mann-Whitney; P = 0.02) and higher in infants than in neonates (Mann-Whitney; P = 0.03). Thus, VP7 reactivity was associated with prior exposure to RV, and the higher values observed in adult samples might be related to multiple RV exposure. Conversely to VP7, global distribution of the VP6-reactive B cell values was random among the 32 patients of the three groups (Fig. 4B). CD19pos B lymphocytes reactive to VLP2/6 were detected in the adult, infant, and cord blood samples with median values of 822 (range, 190 to 1285), 879 (range, 215 to 1263), and 706 (range, 10 to 1965), respectively, out of 105 B lymphocytes, respectively. A pairwise comparison indicates that the number of VP6-specific B cells did not significantly differ between neonates, infants, and adults. Lastly, no Spearman correlation was evidenced between VP6- and VP7-specific B-cell values, neither for the overall 32 patients nor within each group, indicating that the levels of VP6- and VP7-binding B cells varied independently.
FIG. 4.
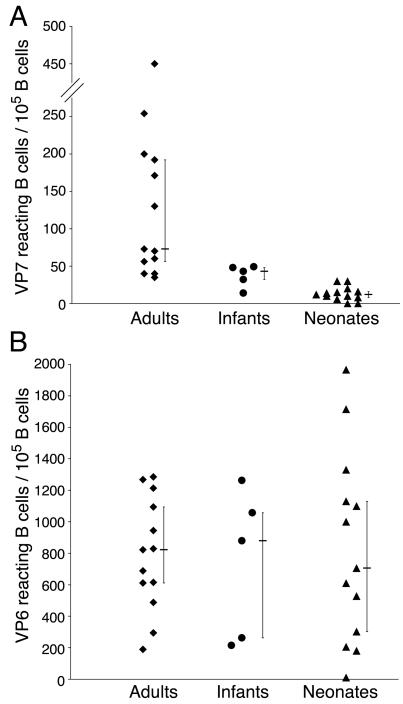
The number of VP7- and VP6-reactive B cells in naive and RV-experienced individuals. The number of VP7-reactive (A) and VP6-reactive (B) B cells in healthy adults, recently infected infants, and neonates at birth are expressed as the numbers of GFP-VLP2/6/7 and DsRed-VLP2/6-reactive B lymphocytes for 105 CD19pos cells. The bars indicate median values with upper and lower quartiles.
Overall, these results showed that VP7-specific B cells were mostly detected in blood samples from RV-immune adults, accounting for approximately 0.07% of the B cells. By contrast, VP6-specific B cells were found in blood samples from both naive neonates and RV-experienced patients at a similar and high frequency of 0.8%. These results indicate that VP7-B cell reactivity was associated with acquired recognition and that VP6-B cell reactivity could relate to innate recognition.
VP6 interacts at a high frequency with a large fraction of naive B lymphocytes in adults.
The high frequency of VP6-specific B lymphocytes in cord blood suggests that a large fraction of adult B cells reactive to VP6 could be naive cells. On the other hand, a largely represented subset of VP6-reactive B cells in neonates might be related to a superantigen type of interaction that could result in the deletion of this subset after RV infection (4). To determine the extent of VP6 interaction with naive B lymphocytes in adults, three blood samples were analyzed for their B-cell reactivity to RV VLP in association with the expression of CD27, a marker of memory B lymphocytes (22, 27). The overall results are given in Table 1 and examples of flow cytometry results are shown in Fig. 5. CD27neg and CD27pos cells accounted for 34 and 66% of the CD19pos B cells, respectively. Interestingly, a large part (mean, 61%; range, 57 to 65%) of the VP6-specific lymphocytes were of the CD 27neg naive type, and 2.1% of the CD27neg lymphocytes were reactive to VP6. By contrast, a minority (mean, 10%; range, 4 to 14%) of the VP7-specific B lymphocytes were of the CD27neg naive type and only 0.021% of the CD27neg lymphocytes were reactive to VP7. Thus, VP6 interacted with a large fraction of naive B cells and bound these cells at a high frequency. Conversely, VP7-specific B lymphocytes were mostly (mean, 90%; range, 86 to 96%) of the CD27pos memory type, in accordance with the association between VP7 reactivity and RV-immune experience. As expected, a significant part (mean, 39%; range, 35 to 43%) of the VP6-reactive lymphocytes were of the CD27pos memory type. Besides, 0.76% of the CD27pos B cells were reactive to VP6, whereas only 0.08% of the CD27pos B cells were involved in VP7 binding.
TABLE 1.
VP6- and VP7-specific naive (CD27neg) and memory (CD27pos) B cells in three adults
| Adult | No. (%) of CD19pos B cellsa
|
No. (%) of VP6-specific B cellsb
|
No. (%) of VP7-specific B cellsb
|
No. of CD27neg B cellsc reacting with:
|
No. of CD27pos B cellsc reacting with:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD27neg | CD27pos | CD27neg | CD27pos | CD27neg | CD27pos | VP6 | VP7 | VP6 | VP7 | |
| 1 | 27,237 (45) | 33,107 (55) | 741 (65) | 397 (35) | 2 (4) | 45 (96) | 2.2 | 0.03 | 1.2 | 0.079 |
| 2 | 23,614 (29) | 57,100 (71) | 430 (57) | 323 (43) | 8 (12) | 57 (88) | 1.8 | 0.026 | 0.5 | 0.117 |
| 3 | 15,800 (28) | 40,700 (72) | 358 (59) | 246 (41) | 4 (14) | 25 (86) | 2.3 | 0.007 | 0.6 | 0.045 |
| Mean | 66,651 (34) | 130,907 (66) | 510 (61) | 322 (39) | 4 (10) | 42 (90) | 2.1 | 0.021 | 0.76 | 0.08 |
Number of CD27neg and CD27pos cells in 2 × 105 enriched B-cell PBMCs.
Number (percentage) of VP6- or VP7-reactive B lymphocytes belonging to the CD27neg naive or CD27pos memory type cells.
Frequency of CD27neg or CD27pos B cells reacting with VP6 or VP7 out of 102 cells.
FIG. 5.
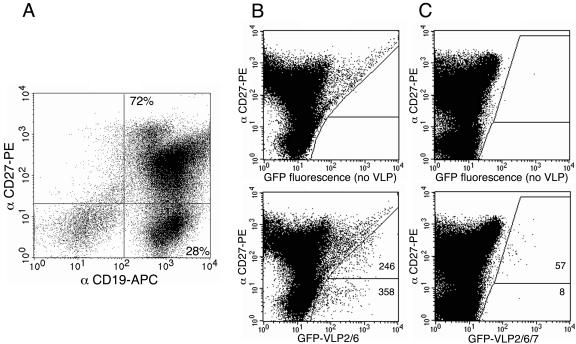
FACS detection of CD27neg naive and CD27pos memory B cells reacting with VLP2/6 and VLP2/6/7. An example of dot plotting with CD27-PE- versus CD19-APC-labeled cells is presented (A). Dot plots of GFP-VLP2/6 (B) and GFP-VLP2/6/7 (C) versus CD27-PE-labeled cells gated on CD19pos cells were created. The number of CD19pos B cells analyzed were 5 × 105 (B) and 106 (C), respectively. Gates of fluorescence positivity were determined by comparison with identical B cells incubated without VLP (B and C, top panels).
Taken together, these results indicated that memory B lymphocytes in adults bound VP6 at a frequency approximately 10 times higher than VP7. Most interestingly, VP6, as opposed to VP7, interacted at a high frequency with naive B cells, suggesting that despite exposure to RV, the corresponding Ig repertoire was probably not deleted in adults.
DISCUSSION
In this study, we used a two-color VLP-based flow cytometry assay to determine VP6 and VP7 B-cell reactivity in blood samples from healthy adults, infected infants, and neonates. The determination of the absolute VP6- and VP7-specific cell numbers may be affected by the VLP concentration, the type of fluorophore, and the gating strategies that are inherent to this type of assay. However, the values obtained for VP6 and VP7 reactivity were clearly distinct and allowed us to reliably conclude that VP6-specific B cells were around 10 times more frequent than VP7-specific B cells.
In a previous study that used a similar fluorescent VLP cytofluorimetry-based technique, Gonzalez et al. focused on RV-specific interaction with adult and infant IgDneg memory B cells (17). The authors found that approximately 0.2% of the memory-type B lymphocytes in healthy adults interacted with GFP-VLP2/6, which is on the same order of magnitude as what we observed with CD27pos B cells, that is, 0.76%. In addition, we observed that VLP2/6 B-cell-binding frequencies in recently infected children and healthy adults were similar (0.8%). However, VLP2/6 B-cell-binding frequencies may vary with the delay after onset of infection. Actually, Gonzalez et al. also showed that samples from acutely infected adults displayed much higher levels of VLP2/6-binding memory B lymphocytes (approximately 3%) than samples from noninfected adults (17).
The major finding of our work is that a large fraction of naive CD27neg B lymphocytes in adult and cord blood samples interacted with RV VP6 via surface Ig. Conversely, VP7-reactive B lymphocytes were mostly of the CD27pos memory type and were predominantly found in RV-experienced individuals. Overall, our results suggest that the VP6 RV capsid protein interacts with a natural Ig repertoire that is broadly, but variably, represented in every individual. This might be of importance in modulating RV immune response, by linking innate to adaptive immunity.
Interestingly, we already observed with species other than humans that VP6-reactive cells interact with presumably naive B lymphocytes. For instance, it has been reported that VP6 reacts with 0.1% of splenic B cells in mice (32). Furthermore, it has been found that B cells reacted at a high frequency with VLP2/6 (1% ± 0.2%) but not with VLP2/6/7 (<0.01%) in neonate goats (X. Roux, unpublished data). These findings suggest that VP6 binding with nonimmune B cells is a common phenomenon among mammals, although its extent seems to vary between species.
We show that the molecular interaction of VLP2/6 with B cells was mediated by surface Ig and possibly by their Fab portions. The binding site could involve the conventional paratope of natural Abs. These natural Abs are involved in the spontaneous recognition of several viruses, such as influenza (2), vesicular stomatitis virus, lymphocytic choriomeningitis virus, and vaccinia virus (28). Natural Abs represent a high percentage of the Ig primary repertoire, being expressed by 10 to 30% of the circulating human B cells (9, 10). They are usually polyreactive Ig of the IgM isotype (36) that display a low affinity (18) and react with some self and non-self antigens at different frequencies, depending on the antigen (7, 36). Such natural polyreactive Abs could be involved in a VP6 interaction with B cells. In agreement with this, IgM directed to VP6 was detected in the serum of nonimmune mice at a 1/200 dilution (C. Fourgeux, unpublished data). B-1 cells, which can be characterized by surface marker expression such as CD5 and CD11b, are considered to be the major producers of natural Abs in mice and humans (23). Colabeling experiments are under way to evaluate the implication of the B-1 subset in the interaction of VP6 with B lymphocytes.
Alternatively to binding to a paratope of natural Abs, VP6 may display a nonconventional interaction with Ig motifs outside the traditional antigen-binding site, similarly to that of B-cell superantigens (13, 14). Indeed, some viral proteins have been reported to behave like B-cell superantigens. For instance, a conserved region of the HIV-1 gp120 glycoprotein specifically binds the VH3 Ig family of healthy individuals (21). In HIV-infected hosts, the VH3 repertoire is initially expanded and then deleted, as reported for bacterial superantigens on T and B cells (4). In the case of RV-exposed adults, the naive B-cell population reacting with VP6 is globally maintained, suggesting that VP6 does not behave as a classical superantigen. Actually, Weitkamp et al. found that the VH gene segment usage in VP6-specific B cells was similar in adults and infants and mainly involved the VH4 family (37). However, whether the VH4 family bias resides in the memory and/or in the naive VP6-specific B-cell compartment remains to be determined. Interestingly, a nonconventional virus-surface Ig interaction has also been documented for the core antigen of hepatitis B virus (HBcAg), which involves a linear motif of the VH1 germ line family and results in HBcAg binding to 4 to 8% of the splenic B cells in both humans and mice (25). Although RV VP6 interacts with fewer B cells, such a mechanism of nonconventional association with a surface Ig should be considered.
We propose as a hypothesis that an innate Ig-mediated recognition of VP6 by naive B cells may possibly influence disease course and resolution of infection by rapidly providing antiviral Abs via natural anti-VP6 Abs as well as via early T-cell-independent B-cell responses. Indeed, the basal presence of circulating anti-VP6 natural Abs may have a direct effect on viral replication, as these natural IgMs may neutralize the virus in an intracellular compartment (6, 32). Besides, early T-cell-independent B-cell responses have been related not only to the repetitive paracrystalline structure of antigens but also to immune parameters such as B-lymphocyte frequency (18). Thus, the high frequency of VP6-binding B cells, together with the large amounts of VP6 assembled in DLPs that are probably released from infected cells, may contribute to the early T-cell-independent massive response of B cells observed in RV infection (5). This early B-cell response probably contributes to a rapid enhanced anti-VP6 IgM production, which in turn may promote the establishment of CD8-T cell responses, as shown in the case of natural Abs to leishmania (33).
Moreover, the high frequency of VP6-reactive B lymphocytes may contribute to the involvement of B cells in RV antigen presentation. It has been shown that antigen-presenting B cells are responsible for short-lived RV-specific IgA production in the gut (12). However in the same study, dendritic cells and monocytes were shown to be involved in long-lived intestinal IgG production (12). It can be hypothesized that a large fraction of B lymphocytes reacting with VP6 compete with dendritic cells for antigen presentation and thus favor short-term over long-term Ig production. Furthermore, as B lymphocytes are much less efficient than dendritic cells in T-cell priming (36), a large targeting of VP6 to B lymphocytes could be, overall, advantageous to the virus. Thus, VP6-mediated antigen presentation by B cells may be related to recurring RV infections in children.
It is noteworthy that, compared to VP7, VP6 interacts with 10 times more memory-type CD27pos B lymphocytes. This observation could be related to VP6 immunodominance, which is observed in RV infections (34). Innate and acquired B-cell responses have been shown to be supported by distinct B cells in murine viral infection (2). Thus, the VP6-specific CD27pos B cells are unlikely to derive from the VP6-specific naive CD27neg B cells described herein. The mechanisms underlying the difference in relative frequency between VP6- and VP7-specific CD27pos B lymphocytes are unknown. They may involve the larger amounts of VP6 than of VP7 protein produced in RV infection, a higher affinity of B cell receptors for VP6 epitopes, or a higher number of VP6-specific B-cell precursors of effectors and/or memory cells.
A large fraction of naive B lymphocytes reacting with VP6 provides a possible mechanistic explanation to the early B-cell response to RV and antigen presentation by naive B cells. The targeting of naive B cells via surface Ig has been documented for several viruses, either by external viral proteins or by inner-capsid proteins as in the present case with RV. The question remains whether naive B-cell targeting is fortuitous or selected for optimal viral adaptation to the host. It could be the result of coevolution between a putative ancestral RV made of double-layered capsids, including VP2 and VP6 proteins, and their hosts that may have adapted with an innate repertoire to bind directly to virus capsids. The external proteins (VP7 and VP4) may thus be considered viral escapes to the immune system, helping the virus to avoid natural Ig and surface Ig-mediated endocytosis by naive B lymphocytes. Since innate immunity to VP6 may modulate the host immune response to RV, it should be studied in depth, to evaluate whether it can be detrimental to the host or not. As a working hypothesis, we propose that variations in the extent of naive B cells interacting with VP6 may affect the individual response to RV and RV vaccines, either positively or negatively. A contribution of B cells to the immune response should be taken into account for the design of optimal RV vaccines, especially when VP6 is concerned.
Acknowledgments
We are grateful to J. P. Girardet, J. L. Bénifla, and E. Grimprel for providing help with the sample collection. We thank Xavier Roux for performing VLP binding to neonatal goat B lymphocytes, Bernard Delmas for providing birnavirus VPL, Sabine Riffault and Mathieu Epardaud for critical reading of the manuscript, Wendy Brand-Williams for editing assistance, and Chantal Kang for technical assistance.
This work was supported in part by the MESRT grant Programme de Recherches Fondamentales en Microbiologie, Maladies Infectieuses et Parasitologie “Réseau de Recherche sur les Gastro-Entérites à Rotavirus.”
REFERENCES
- 1.1998. American Academy of Pediatrics. Prevention of rotavirus disease: guidelines for use of rotavirus vaccine. Pediatrics 102:1483-1491. [DOI] [PubMed] [Google Scholar]
- 2.Baumgarth, N., O. C. Herman, G. C. Jager, L. Brown, L. A. Herzenberg, and L. A. Herzenberg. 1999. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc. Natl. Acad. Sci. USA 96:2250-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell, J., and D. Gray. 2003. Antigen-capturing cells can masquerade as memory B cells. J. Exp. Med. 19:1233-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berberian, L., L. Goodglick, T. J. Kipps, and J. Braun. 1993. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science 261:1588-1591. [DOI] [PubMed] [Google Scholar]
- 5.Blutt, S. E., K. L. Warfield, D. E. Lewis, and M. E. Conner. 2002. Early response to rotavirus infection involves massive B cell activation. J. Immunol. 168:5716-5721. [DOI] [PubMed] [Google Scholar]
- 6.Burns, J. W., M. Siadat-Pajouh, A. A. Krishnaney, and H. B. Greenberg. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272:104-107. [DOI] [PubMed] [Google Scholar]
- 7.Casali, P., and A. L. Notkins. 1989. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu. Rev. Immunol. 7:513-535. [DOI] [PubMed] [Google Scholar]
- 8.Charpilienne, A., M. Nejmeddine, M. Berois, N. Parez, E. Neumann, E. Hewat, G. Trugnan, and J. Cohen. 2001. Individual rotavirus-like particles containing 120 molecules of fluorescent protein are visible in living cells. J. Biol. Chem. 276:29361-29367. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Z. J., F. Shimizu, J. Wheeler, and A. L. Notkins. 1996. Polyreactive antigen-binding B cells in the peripheral circulation are IgD+ and B7−. Eur. J. Immunol. 26:2916-2923. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Z. J., J. Wheeler, and A. L. Notkins. 1995. Antigen-binding B cells and polyreactive antibodies. Eur. J. Immunol. 25:579-586. [DOI] [PubMed] [Google Scholar]
- 11.Chevalier, C., J. Lepault, I. Erk, B. Da Costa, and B. Delmas. 2002. The maturation process of pVP2 requires assembly of infectious bursal disease virus capsids. J. Virol. 76:2384-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffin, S. E., S. L. Clark, N. A. Bos, J. O. Brubaker, and P. A. Offit. 1999. Migration of antigen-presenting B cells from peripheral to mucosal lymphoid tissues may induce intestinal antigen-specific IgA following parenteral immunization. J. Immunol. 163:3064-3070. [PubMed] [Google Scholar]
- 13.Domiati-Saad, R., J. F. Attrep, H. P. Brezinschek, A. H. Cherrie, D. R. Karp, and P. E. Lipsky. 1996. Staphylococcal enterotoxin D functions as a human B cell superantigen by rescuing VH4-expressing B cells from apoptosis. J. Immunol. 156:3608-3620. [PubMed] [Google Scholar]
- 14.Domiati-Saad, R., and P. E. Lipsky. 1998. Staphylococcal enterotoxin A induces survival of VH3-expressing human B cells by binding to the VH region with low affinity. J. Immunol. 161:1257-1266. [PubMed] [Google Scholar]
- 15.Estes, M. K. 1996. Advances in molecular biology: impact on rotavirus vaccine development. J. Infect. Dis. 174(Suppl. 1):S37-S46. [DOI] [PubMed] [Google Scholar]
- 16.Glass, R. I., D. R. Lang, B. N. Ivanoff, and R. W. Compans. 1996. Introduction: rotavirus—from basic research to a vaccine. J. Infect. Dis. 174(Suppl. 1):S1-S2. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez, A. M., M. C. Jaimes, I. Cajiao, O. L. Rojas, J. Cohen, P. Pothier, E. Kohli, E. C. Butcher, H. B. Greenberg, J. Angel, and M. A. Franco. 2003. Rotavirus-specific B cells induced by recent infection in adults and children predominantly express the intestinal homing receptor α4β7. Virology 305:93-105. [DOI] [PubMed] [Google Scholar]
- 18.Hangartner, L., B. M. Senn, B. Ledermann, U. Kalinke, P. Seiler, E. Bucher, R. M. Zellweger, K. Fink, B. Odermatt, K. Burki, R. M. Zinkernagel, and H. Hengartner. 2003. Antiviral immune responses in gene-targeted mice expressing the immunoglobulin heavy chain of virus-neutralizing antibodies. Proc. Natl. Acad. Sci. USA 100:12883-12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, B., J. R. Gentsch, and R. I. Glass. 2002. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin. Infect. Dis. 34:1351-1361. [DOI] [PubMed] [Google Scholar]
- 20.Kapikian, A., Y. Hoshino, and R. M. Chanock. 2001. Rotaviruses, p. 1787-1833. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.) Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 21.Karray, S., and M. Zouali. 1997. Identification of the B cell superantigen-binding site of HIV-1 gp120. Proc. Natl. Acad. Sci. USA 94:1356-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein, U., K. Rajewsky, and R. Kuppers. 1998. Hum. immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohler, H., J. Bayry, A. Nicoletti, and S. V. Kaveri. 2003. Natural autoantibodies as tools to predict the outcome of immune response? Scand. J. Immunol. 58:285-289. [DOI] [PubMed] [Google Scholar]
- 24.Kuklin, N. A., L. Rott, N. Feng, M. E. Conner, N. Wagner, W. Müller, and H. B. Greenberg. 2001. Protective intestinal anti-rotavirus B cell immunity is dependent on α4β7 integrin expression but does not require IgA antibody production. J. Immunol. 166:1894-1902. [DOI] [PubMed] [Google Scholar]
- 25.Lazdina, U., T. Cao, J. Steinbergs, M. Alheim, P. Pumpens, D. L. Peterson, D. R. Milich, G. Leroux-Roels, and M. Sallberg. 2001. Molecular basis for the interaction of the hepatitis B virus core antigen with the surface immunoglobulin receptor on naive B cells. J. Virol. 75:6367-6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui, S. M., P. A. Offit, P. T. Vo, E. R. Mackow, D. A. Benfield, R. D. Shaw, L. Padilla-Noriega, and H. B. Greenberg. 1989. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to the heterotypic neutralization domain of VP7 and the VP8 fragment of VP4. J. Clin. Microbiol. 27:780-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagumo, H., K. Agematsu, N. Kobayashi, K. Shinozaki, S. Hokibara, H. Nagase, M. Takamoto, K. Yasui, K. Sugane, and A. Komiyama. 2002. The different process of class switching and somatic hypermutation; a novel analysis by CD27− naive B cells. Blood 99:567-575. [DOI] [PubMed] [Google Scholar]
- 28.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156-2159. [DOI] [PubMed] [Google Scholar]
- 29.Offit, P. A. 2002. The future of rotavirus vaccines. Semin. Pediatr. Infect. Dis. 13:190-195. [DOI] [PubMed] [Google Scholar]
- 30.Offit, P. A., and H. F. Clark. 1985. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J. Virol. 54:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Offit, P. A., R. D. Shaw, and H. B. Greenberg. 1986. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J. Virol. 58:700-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz-Cornil, I., Y. Benureau, H. Greenberg, B. A. Hendrickson, and J. Cohen. 2002. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins. J. Virol. 76:8110-8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stager, S., J. Alexander, A. C. Kirby, M. Botto, N. V. Rooijen, D. F. Smith, F. Brombacher, and P. M. Kaye. 2003. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat. Med. 9:1287-1292. [DOI] [PubMed] [Google Scholar]
- 34.Svensson, L., H. Sheshberadaran, S. Vene, E. Norrby, M. Grandien, and G. Wadell. 1987. Serum antibody responses to individual viral polypeptides in human rotavirus infections. J. Gen. Virol. 68:643-651. [DOI] [PubMed] [Google Scholar]
- 35.Vazquez, J., Y. Boher, M. Perez, M. J. Guntinas, and A. M. Rojas. 1998. Immune response to three doses of quadrivalent rotavirus vaccine: 1-year follow-up. Vaccine 16:1179-1183. [DOI] [PubMed] [Google Scholar]
- 36.Wang, Z., Z. J. Chen, J. Wheeler, S. Shen, and A. L. Notkins. 2001. Characterization of murine polyreactive antigen-binding B cells: presentation of antigens to T cells. Eur. J. Immunol. 31:1106-1114. [DOI] [PubMed] [Google Scholar]
- 37.Weitkamp, J.-H., N. Kallewaard, K. Kusuhara, E. Bures, J. V. Williams, B. LaFleur, H. B. Greenberg, and J. E. Crowe, Jr. 2003. Infant and adult human B cell responses to rotavirus share common immunodominant variable gene repertoires. J. Immunol. 171:4680-4688. [DOI] [PubMed] [Google Scholar]
- 38.Youngman, K. R., M. A. Franco, N. A. Kuklin, L. S. Rott, E. C. Butcher, and H. B. Greenberg. 2002. Correlation of tissue distribution, developmental phenotype, and intestinal homing receptor expression of antigen-specific B cells during the murine anti-rotavirus immune response. J. Immunol. 168:2173-2181. [DOI] [PubMed] [Google Scholar]


