Abstract
Background
Emerging research points to innate immune mechanisms in the neuropathological and behavioral consequences of heavy alcohol use. Alcohol use is common among people living with HIV infection (PLWH), a chronic condition that carries its own set of long-term effects on brain and behavior. Notably, neurobiological and cognitive profiles associated with heavy alcohol use and HIV infection share several prominent features. This observation raises questions about interacting biological mechanisms as well as compounded impairment when HIV infection and heavy drinking co-occur.
Objective and Method
This narrative overview discusses peer-reviewed research on specific immune mechanisms of alcohol that exhibit apparent potential to compound the neurobiological and psychiatric sequelae of HIV infection. These include microbial translocation, systemic immune activation, blood-brain barrier compromise, microglial activation, and neuroinflammation.
Results
Clinical and preclinical evidence supports overlapping mechanistic actions of HIV and alcohol use on peripheral and neural immune systems. In preclinical studies, innate immune signaling mediates many of the detrimental neurocognitive and behavioral effects of alcohol use. Neuropsychopharmacological research suggests potential for a feed-forward cycle in which heavy drinking induces innate immune signaling, which in turn stimulates subsequent alcohol use behavior.
Conclusion
Alcohol-induced immune activation and neuroinflammation are a serious health concern for PLWH. Future research to investigate specific immune effects of alcohol in the context of HIV infection has potential to identify novel targets for therapeutic intervention.
1. Introduction
Alcohol is the most commonly used drug among people living with HIV infection (PLWH) in the United States: 51% consumed alcohol and 15% reported binge drinking in the past 30 days (1). Alcohol use is linked to outcomes with serious consequences for the individual and for public health, including medication non-adherence (2), high-risk sexual behaviors (3, 4), HIV progression (5), and increased mortality (6, 7). Strikingly, even moderate drinking (30 drinks per month, or an average of one drink per day) was associated with significantly greater risk of mortality in men living with HIV (8). This level of consumption is below the current threshold for high-risk drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as >14 drinks for men and >7 drinks for women per week, or >5 drinks for men and >4 drinks for women on any single occasion (9). Therefore, it is imperative to examine interactions of HIV and alcohol use that may place PLWH at greater risk of harm.
Immune interactions of alcohol and HIV are complex and not well understood, particularly with regard to effects on brain, behavior, and cognition. Over the past 30 years, evolving treatment approaches to HIV have complicated systematic research on its neural effects and possible interactions with alcohol. A significant shift occurred with the advent of “combination” (or “highly active”) antiretroviral therapy (ART) in 1996. Although rates of HIV-associated dementia have declined in the combination ART era, milder HIV-associated neurocognitive disorders persist, affecting 44% of PLWH without severe comorbidities (10). The observation that rates and patterns of cognitive impairment have changed following the introduction of combination ART (11) suggests that these drugs modulate central nervous system (CNS) effects of HIV, yet CNS profiles of various drug regimens are not well characterized. Lack of clarity also arises from inconsistency in alcohol research methodology, including definitions of heavy drinking and assessment timeframes (12). Heterogeneity across study samples in HIV clinical status (e.g., comorbidities, viral load, history of severe immunosuppression) and alcohol use characteristics (e.g., current vs. remote heavy drinking) is another source of variability. Going forward, greater specificity of neural targets and consistency in research methodology will help to clarify HIV-alcohol interactions.
This review focuses on specific immune mechanisms of alcohol that have apparent potential to compound the neurobiological and psychiatric sequelae of HIV infection. Recently, immune effects of alcohol have emerged as a major contributor to systemic and neurobiological damage associated with chronic heavy drinking (13). Notably, several immune pathways implicated in heavy alcohol use overlap with those affected by HIV infection. Mounting evidence indicates that HIV infection and heavy drinking are likely to produce compounded damage to brain and cognition when they co-occur (14, 15). Independently, alcohol use disorder (AUD) and HIV infection are associated with abnormality in the brain’s frontal lobes, limbic system, and subcortical structures, which are substrates of higher-order cognition and reward processing (16–20). Both AUD and HIV infection are associated with deficits in executive function, learning, memory, and processing speed (11, 21, 22). Not surprisingly, chronic heavy alcohol use exacerbates information processing and psychomotor speed impairment in PLWH (23–26). Therefore, it is of utmost importance to understand how alcohol may potentiate HIV consequences at systemic and neural levels. Alcohol-HIV interactions with respect to HIV transmission, acute infection, viral replication, adaptive immunity, and disease progression have been reviewed in detail elsewhere (5, 12, 27). This conceptual overview focuses on specific immune mechanisms with potential for additive or synergistic consequences in co-occurring heavy drinking and HIV infection.
2. Systemic immune activation
Mechanisms through which alcohol and HIV may affect the brain via indirect effects on other organ systems, particularly the gastrointestinal tract, are a rapidly growing area of interest. Independently, HIV and alcohol use induce translocation of microbial products from the gastrointestinal tract into systemic circulation (28), leading to immune activation and inflammation. Figure 1 illustrates this model, and Table 1 provides a summary of key terms and biomarkers. Briefly, lipopolysaccharide (LPS) in plasma is used as a marker of microbial translocation, and immune activation is indexed by plasma levels of lipopolysaccharide binding protein (LBP), soluble cluster of differentiation 14 (sCD14), and endotoxin immunoglobulin core antibody IgM (EndoCAb). As an aid to interpretation, acute LPS challenge typically results in increased plasma levels of LBP and sCD14 and depletion of EndoCAb (29–31). HIV and alcohol first will be addressed separately, followed by evidence on co-occurring HIV and alcohol use.
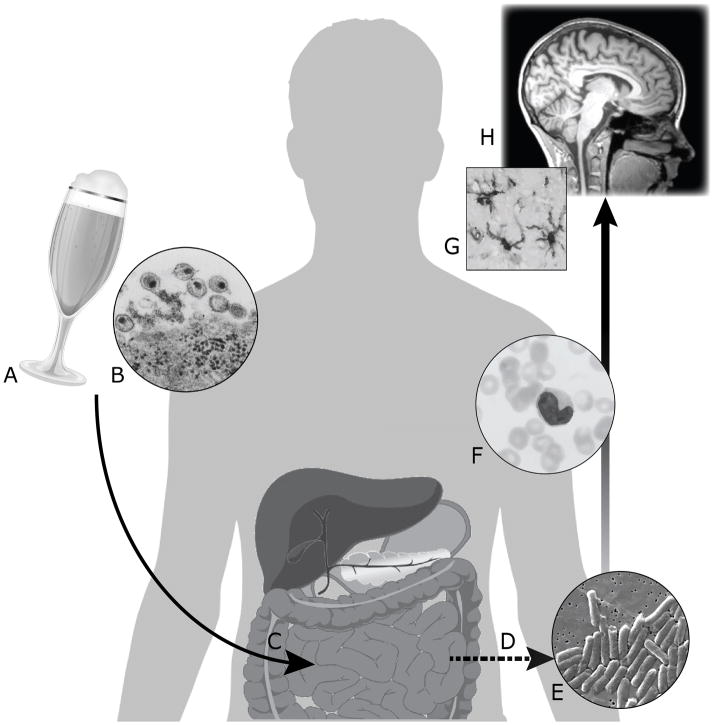
Figure 1.
Alcohol (A) and HIV (B) are associated with deleterious effects on the gastrointestinal tract (C), including intestinal hyperpermeability, dysbiosis of the gut microbiota, and epithelial cell damage (33, 42, 76, 78, 182, 191). Research suggests potential for additive effects in co-occurring HIV infection and heavy drinking. These mechanisms contribute to microbial translocation (D), the unphysiological movement of microbial components into systemic circulation. LPS, a component of cell walls of Gram-negative bacteria (E), stimulates pro-inflammatory signaling by monocytes in circulation (F). Systemic inflammation may be propagated to the central nervous system via infiltration of infected monocytes, free virus, and cytokines. Neuroinflammation mediated by microglia (G) is thought to underlie many of the changes observed on MRI (H) in alcohol use disorders or HIV infection and to contribute to cognitive and behavioral dysfunction.
Table 1.
Key terms in microbial translocation and immune activation
| Key Term | Description |
|---|---|
| Endotoxin core antibody immunoglobulin M (EndoCAb) | Antibody against the LPS core antigen that binds to and neutralizes LPS |
| Innate immune activation | Rapid, nonspecific immune response to infectious agents initiated by pathogen recognition and exercised through pro-inflammatory signaling pathways |
| Lipopolysaccharide (LPS; also referred to as endotoxin) | Component of cell walls of Gram-negative bacteria and a major ligand for TLR4; LPS in plasma is used as a measure of microbial translocation |
| Lipopolysaccharide binding protein (LBP) | Acute phase protein in plasma that binds to and presents LPS to sCD14 or the membrane-bound CD14/TLR4 receptor complex |
| Microglial activation | Response of the brain’s resident innate immune cells (i.e., microglia) to pathogens, viral proteins, and toxins that involves production and secretion of pro-inflammatory mediators (e.g., cytokines, reactive oxygen species); chronic and/or excessive microglial activation is a recognized factor in neurodegeneration |
| Microbial translocation | Movement of microbial components normally confined to the gut into systemic circulation, as a result of structural and/or functional compromise of the intestinal barrier |
| Neuroinflammation | Central nervous system condition marked by elevated pro-inflammatory cytokines and oxidative stress signals that create a neurotoxic milieu, leading to neurodegeneration when chronic |
| Proinflammatory cytokines | Broad class of proteins that are critical to immune response to contain infection but are harmful with prolonged or extreme elevations; types include interleukins (e.g., IL-6), interferons (e.g., interferon-α), and tumor necrosis factor (e.g., TNF-α) |
| Soluble cluster of differentiation 14 (sCD14) | CD14 is the co-receptor for TLR4 and is expressed on monocytes and macrophages; the soluble form, sCD14, is a plasma marker of monocyte activation and presents LPS to the TLR4 receptor complex |
| Toll-like receptor 4 (TLR4) | Innate immune receptor dedicated to recognition of pathogens such as LPS; binding of LPS to TLR4 initiates a pro-inflammatory response that involves production and secretion of cytokines |
2A. Microbial translocation and immune activation in HIV infection
Following a landmark study that identified microbial translocation as a source of chronic immune activation in HIV infection (32), numerous studies have documented elevated LPS, LBP, and sCD14 and lower EndoCAb in PLWH relative to healthy control groups (32–37). Chronic immune activation is a hallmark of HIV that persists despite virologic suppression and appears to stoke disease progression independent of viral replication [e.g., (38, 39); see (40, 41) for reviews]. Basic research has uncovered pathophysiological mechanisms of microbial translocation and evidence of its independent contribution to chronic immune activation in HIV infection. HIV exposure causes intestinal epithelial barrier compromise via disruption of tight junctions proteins, such as occludin and zona occludens-1 (ZO-1), and increased production of proinflammatory cytokine tumor necrosis factor alpha (TNF-α) (42). At all stages of infection, HIV profoundly depletes CD4+ T cells in the gut, particularly the Th17 CD4+ type critical to multiple gut immune functions (43, 44). Perturbed composition of the gut microbiome, characterized by decreased diversity and/or enrichment of pathogenic bacteria, also is a factor in microbial translocation (33, 45–47). In a simian immunodeficiency virus (SIV) model, researchers directly linked gut barrier compromise to microbial translocation and immune activation independent of viral activity (48). Furthermore, experimentally induced microbial translocation in uninfected non-human primates caused immune activation and systemic inflammation of a magnitude comparable to SIV infection (49). Finally, SIV-infected non-human primate species that do not progress to AIDS have robust viral replication yet lack microbial translocation and other key pathogenic processes (50).
Immune activation is a powerful predictor of clinical outcomes in HIV infection. sCD14, a marker of monocyte activation, consistently and uniquely predicts cognitive impairment, virologic failure (i.e., failure of ART to suppress viral load), disease progression, and mortality in PLWH (36, 51–57). One study found that PLWH in the highest quartile for sCD14 had a 6-fold increased risk of mortality compared to those in the lowest quartile (37). In a large veteran cohort, sCD14 in combination with other biomarkers accounted for a modest proportion of mortality risk in PLWH with HIV RNA ≥10,000 copies/ml (58). Microbial translocation and systemic immune activation also interact with cardiovascular risk factors in PLWH, who are at significantly greater risk of cardiovascular disease (59). Decreased or dysfunctional high density lipoprotein (HDL) in serum is an important risk factor that disproportionately affects PLWH (60–62). Under certain conditions, sCD14 and LBP transfer LPS to non-immunoreactive lipoproteins, particularly HDL (63, 64), as an alternative to monocyte activation via TLR4 binding (63, 65). One possibility is that low or dysfunctional HDL characteristic of HIV infection limits access to this inhibitory pathway, thereby impeding the natural “sink” that removes LPS from circulation and promoting chronic immune activation.
2B. Microbial translocation and immune activation in heavy drinking
Alcohol is a potent immunomodulator, with effects contingent on dose and chronicity of exposure. Acute alcohol produces an initial pro-inflammatory phase followed by an anti-inflammatory or immune-inhibitory phase [e.g., (66–68); see (69) for review]. In contrast, chronic alcohol upregulates pro-inflammatory innate immune response, e.g., by sensitizing monocyte response to LPS (70, 71). Alcohol inhibits adaptive immunity by suppressing T-cell proliferation, inducing T cell dysfunction, and disrupting homeostasis among T cell subtypes (72–74). Similar to HIV infection, alcohol disrupts gut immune function via dysbiosis, structural barrier damage, and perturbation of lymphocyte populations. Rodent models have shown that acute or chronic alcohol feeding increases intestinal permeability and alters composition of the gut microbiome (75–78). Intestinal epithelial compromise results from upregulation of oxidative stress and disruption of tight junctions via acetaldehyde-mediated redistribution of tight junction proteins such as ZO-1 (78–81). Chronic alcohol further disrupts gut immune function by diminishing colonic CD4+ T cells, specifically the Th17 subtype depleted in HIV infection (82).
Perhaps due to shared pathophysiological processes, immune biomarker perturbations seen in heavy drinking are similar to those observed in HIV infection, i.e., elevated LPS, LBP, and sCD14 and decreased EndoCAb. Observational studies have reported higher LPS, LBP, and sCD14 levels in individuals with alcoholic liver disease compared to healthy controls (83–85). Even in the absence of liver disease, individuals in early abstinence from alcohol dependence have shown higher plasma LPS, LBP, and sCD14 than healthy controls (86, 87). Moreover, intestinal permeability and plasma LPS levels decreased significantly after 19 days of abstinence, suggesting remediation of gut function with cessation of drinking (86). EndoCAb, which typically is suppressed in chronic HIV infection, also is significantly lower in community heavy drinkers compared to light drinkers (88). In animal models, chronic alcohol treatment impairs synthesis of this LPS-clearing antibody in mesenteric lymph nodes (89).
To date, two experimental studies have assessed acute changes in LPS, LBP, and/or sCD14 from moderate-to-heavy alcohol consumption by healthy individuals. A peak blood alcohol level (BAL) of approximately .085 g/dL increased markers of microbial translocation and immune activation in healthy participants (90). Specifically, serum LPS, LBP, and sCD14 increased within 30 minutes of alcohol consumption; both LBP and sCD14 remained significantly elevated at 24 hours post-consumption (90). Another study that administered a comparable alcohol dose (1 g/kg; median peak BAL of .070 g/dL) to healthy men reported an increase in a marker of intestinal epithelial cell damage (91). sCD14 also increased in the alcohol condition, but the effect was difficult to interpret as it was similar to the water control condition (91).
2C. Experimental alcohol-induced microbial translocation in HIV
Although controlled studies of alcohol-induced microbial translocation in PLWH have not been reported, an animal model using HIV-transgenic rats indicated a synergistic effect of alcohol and HIV on this specific outcome (92). HIV-transgenic rats model chronic virus effects in the absence of active viral replication, analogous to virologic suppression in PLWH (93). Binge alcohol exposure caused significantly greater microbial translocation in the HIV-transgenic animals, compared to alcohol-only or HIV-only conditions, and was linked to increased TLR4 expression, pro-inflammatory cytokines, and liver damage (92).
2D. Clinical research on co-occurring HIV infection and heavy drinking
As noted previously, a biomarker profile indicative of microbial translocation and immune activation has emerged independently in HIV infection and heavy drinking. However, empirical evidence on the combined effects of HIV infection and heavy drinking is sparse, as HIV clinical research studies typically have excluded individuals with AUD or heavy drinking. A review of the current literature identified four observational studies to date examining associations of LPS, LBP, or sCD14 with alcohol use in PLWH. One study of PLWH with an AIDS diagnosis (87% on ART) compared participants with AUD to participants without substance use disorder. The AUD group had higher LPS and LBP, but there were no group differences in sCD14 or EndoCAb (36). Carrico et al. (2015) analyzed sCD14 levels as a function of alcohol use in a sample of PLWH who were not receiving ART (90% with CD4 cell count ≥350 cells/mm3; 83% with viral load ≥1000 copies/ml). Individuals who screened positive for heavy drinking in the past three months had significantly higher sCD14 than non-drinkers in analyses adjusted for demographics, smoking status, and HIV clinical characteristics (94). Our lab recently reported a pilot study of immune biomarkers and alcohol use in men living with HIV infection who met NIAAA criteria for heavy drinking in the past month. All participants were receiving ART and had achieved virologic suppression (95). Alcohol use quantity and frequency positively predicted sCD14 even after controlling for demographic and clinical variables. In addition, participants who reduced their drinking over a three-month period showed a significant decrease in sCD14 (95). Although alcohol use was not associated with either LPS or EndoCAb, power was limited by the small sample size. In contrast to those positive findings, a cohort study of PLWH (77% on ART; 73% with virologic suppression) reported that having >5 drinks per day at least once in the past 30 days was not associated with sCD14 (96). Notably, studies differed in operational definitions of heavy drinking and in sample characteristics such as ART usage and clinical status. Taken together, however, they offer preliminary support for connections among heavy drinking, microbial translocation, and innate immune activation in PLWH.
2E. Peripheral immune activation as contributor to neuroinflammation
Although the blood brain barrier (BBB) protects the privileged immune status of the CNS, the BBB is selectively permeable to immune cells and often is altered in neuropathology (97). Several known mechanisms propagate peripheral pro-inflammatory signals across the BBB, potentially contributing to neuroinflammation. Some cytokines, including interleukin-6 (IL-6) and TNF-α, cross the BBB via cytokine-specific transporters (97). Both alcohol and HIV increase permeability of the BBB via pro-inflammatory mechanisms that resemble processes observed at the level of the intestinal epithelial barrier (98). Specifically, alcohol and HIV induce oxidative stress in endothelial cells of the BBB, leading to barrier compromise via disruption and redistribution of tight junction proteins, including ZO-1 and occludin (98–103). LPS is not thought to cross the intact BBB, but LPS in peripheral circulation increases the permeability of the BBB to cell-free virus (104). At the same time, HIV sensitizes the BBB to LPS insult, and HIV-infected monocytes exhibit an enhanced capacity to migrate into the brain (103, 104). Alcohol facilitates migration of monocytes across the BBB via oxidative stress mechanisms (99). Systemic administration of alcohol to rodents increased levels of proinflammatory cytokines TNF-α and monocyte chemoattractant protein-1 (MCP-1; also called CCL2) in the brain, and alcohol prior to LPS administration potentiated and temporally extended this response (105). In short, HIV, alcohol, and LPS exposure interact to compromise the BBB and promote influx of pro-inflammatory cytokines and HIV-infected monocytes to the CNS.
3. Neuroimmune mechanisms of alcohol use and HIV infection
3A. Brief overview of HIV-related neuroinflammation
Viral proteins, infected monocytes, and cytokines are significant contributors to neuronal damage. Neuroinflammation is mediated to a large extent by glial cells (i.e., microglia, astrocytes, oligodendrocytes), which populate the human brain in quantities similar to neurons and are a major constituent of white matter (106). Microglia, the brain’s resident immune defense cells, are activated by pathogens such as bacteria or viruses to secrete cytokines, both pro- and anti-inflammatory (107). Even with virologic suppression, the CNS serves as a viral reservoir for HIV [see (108) for review]. Microglia are uniquely susceptible to HIV infection, whereas neurons and astrocytes do not appear to harbor productive infection [see (109) for review]. Infected microglia emit neurotoxic viral proteins such as Tat and gp120, contributing to neuronal apoptosis and drawing additional cytokines and infected monocytes across the BBB (109). Although somewhat counterintuitive, the largely non-productive nature of HIV infection in astrocytes also contributes to HIV persistence in the CNS (110). Because antiretroviral drugs only are able to target cells with active viral replication, astrocytes serve as a latent viral reservoir. Thus, viral reservoirs throughout the body are a major obstacle to HIV eradication and a focus of current research. For example, a recent preclinical study reported that HIV replication in astrocytes could be turned “on” by inhibiting a specific host restriction factor (110).
Neuroinflammation marked by glial activation is evident in postmortem brain tissue from PLWH and is associated with neurocognitive impairment (111, 112). There is strong evidence that neurodegenerative processes in HIV infection persist despite effective ART. A longitudinal imaging study reported that, compared to a healthy control group, PLWH with virologic suppression showed progressive white matter atrophy over a 2-year period (113). Although virologic suppression typically is associated with better cognitive function, ART does not fully eradicate the virus from the CNS, and findings are mixed with regard to possible neuroprotective or neurotoxic effects of ART (114). Ongoing CNS insult despite appropriate ART may contribute to the high prevalence of HIV-associated neurocognitive disorders, which are estimated to affect 44% of PLWH without confounding comorbidities (10).
3B. Neuroimmune mechanisms of alcohol use
As in HIV, glial activation is central to alcohol-induced neuroinflammation and neurodegeneration (115–118). It is well known that alcohol readily crosses the BBB and affects a host of neurotransmitter systems, including dopaminergic, opioid, and serotonergic systems (119). Emerging research indicates that some neurotoxic and behavioral consequences of alcohol use are mediated by innate immune receptors, particularly the TLR4 complex. TLR4, the receptor that recognizes gut-derived LPS, is expressed not only on monocytes in systemic circulation but also on the brain’s microglia and astrocytes (120, 121). A remarkable series of in vitro and in vivo murine models demonstrated that TLR4 signaling is the primary pathway through which chronic or binge alcohol induces glial activation (116, 118). The mechanistic role of TLR4 in alcohol neurotoxicity has been established in experiments with mice that are genetically deficient in TLR4 (i.e., TLR4-knockouts). TLR4 signaling following alcohol treatment causes glia to produce and secrete cytokines (e.g., MCP-1, TNF-α, IL-6), reactive oxygen species, and other inflammatory mediators (115, 122, 123). These proinflammatory cascades promote neuronal apoptosis and inhibit neurogenesis (118, 123, 124). In addition, alcohol-induced TLR4 signaling led to white matter degeneration through downregulation of myelin proteins and death of oligodendrocytes, the cells that produce myelin (125). Increased expression of microglial TLR4 was observed after 10 minutes of alcohol exposure, suggesting a feed-forward mechanism of neuroinflammation (123). Postmortem studies corroborate the presence of increased pro-inflammatory cytokines MCP-1 and interleukin-1beta (IL-1β), microglial markers, and TLR4 expression in brain tissue of individuals with a history of heavy drinking (124, 126, 127). Intriguingly, alcohol-preferring (“P”) rats had higher levels of TLR4 in the ventral tegmental area and central amygdala predating alcohol exposure, suggesting a genetic role of TLR4 in vulnerability to excessive drinking (128).
Although the exact mechanisms through which alcohol activates TLR4 are a focus of ongoing research, it appears that alcohol mimics actions of endogenous TLR4 ligands such as LPS and IL-1β (123, 129, 130). Essentially, alcohol is an exogenous substance that replicates some effects of endogenous bacterial antigens like LPS on glial cells. This parallel is significant in light of the fact that LPS normally does not infiltrate the brain. Alcohol may not be unique among addictive drugs in this capacity, as other researchers have shown that some opioid effects are mediated by TLR4 signaling (131). Indeed, the full significance of overlapping neural mechanisms responding to bacterial ligands and addictive drugs has yet to be explored.
In summary, chronic and binge models of alcohol exposure have identified neuronal death, myelin damage, and inhibited neurogenesis as major contributors to alcohol-related neurodegeneration. Alcohol neurotoxicity is mediated to a great extent by innate immune mechanisms, specifically, glial activation, cytokine production, oxidative stress, and innate immune gene induction. Upregulation of innate immune gene transcription amplifies proinflammatory signaling and, in the context of repeated neurotoxic insults, can stoke a self-perpetuating or “feed-forward” cycle (132).
3C. Neurodegenerative interactions of HIV infection and alcohol
Complete discussion of possible interactions of HIV and alcohol in the CNS is outside the scope of this review [see (15) for further discussion]. However, recent research has highlighted several mechanisms with potential for converging neurodegenerative effects in co-occurring HIV infection and heavy drinking. These include epigenetic changes, regulation of synaptic plasticity, and generation of oxidative stress. HIV infection of cultured astrocytes resulted in downregulation of genes involved in synaptic plasticity and dendritic spine density, as well as induction of apoptosis (133). Effects of alcohol on synaptic plasticity are complex and vary according to many factors, including the specific brain region, developmental stage, neurotransmitter system, and alcohol concentration [see (134) for review]. Although acute alcohol transiently increases synaptic plasticity, chronic exposure or withdrawal inhibits plasticity through decrease in dendritic spine density (135). These effects of alcohol use or HIV infection on dendritic architecture are paralleled by epigenetic changes that influence synaptic plasticity, specifically through expression of histone deacetylase 2 (HDAC2) (135). The HIV viral protein Tat induced epigenetic changes in neurons by upregulating HDAC2, which in turn decreased expression of genes that facilitate synaptic plasticity via long-term potentiation (136). Although initial alcohol exposure reduced HDAC2 in rodent models, chronic binge exposure led to increased HDAC2 in blood and amygdala (137). Similarly, individuals presenting with acute alcohol intoxication in a healthcare setting had elevated HDAC2 levels (137). In neuronal cell culture, alcohol produced a dose-dependent increase in HDAC2 expression (138). Important to note, upregulation of HDACs appears to mediate neuronal dysfunction and cognitive impairment across a diverse array of neurodegenerative conditions (139). In preclinical trials, HDAC inhibiting agents have shown potential to ameliorate cognitive and affective symptoms in both HIV infection and AUD, e.g., (140, 141).
Closely tied to neuroinflammation and a known contributor to alcohol neurotoxicity (142), oxidative stress is thought to play a role in epigenetic alterations associated with alcohol use and HIV. In neuronal culture, alcohol-induced HDAC2 upregulation was linked to levels of oxidative stress and was blocked by antioxidant administration (138). In an animal model of co-occurring HIV infection and heavy drinking, alcohol increased plasma viremia, reduced clearance of infected macrophages from the brain, and increased microglial activation and oxidative stress markers (143). It is important to note that, although some animal models and in vitro studies [e.g., (144, 145)] have demonstrated that alcohol increases levels of HIV viral proteins and rates of cellular infection, in vivo evidence in humans is inconclusive (27). At the same time, mounting evidence suggests that alcohol and other drugs of abuse may mechanistically accelerate HIV-related neurodegeneration through oxidative stress mechanisms. For example, treating mice with the HIV viral protein Tat plus alcohol potentiated increases in oxidative stress markers and pro-inflammatory cytokines IL-1β and MCP-1 in brain tissue, compared to Tat or alcohol alone (146). Elevated MCP-1 is significant because MCP-1 enhances migration of infected monocytes across the BBB (147) and predicts HIV-related neurocognitive dysfunction and neurodegeneration (56, 148, 149). In another study, combined treatment with alcohol and HIV viral protein gp120 synergistically augmented free radical production and suppressed antioxidant activity in rat brain (150). Indeed, oxidative stress signaling may be an important common pathway by which alcohol and other drugs exacerbate HIV-related neurodegeneration. For example, exposing microglia to HIV plus morphine amplified markers of oxidative stress and cellular DNA damage more than either HIV or morphine alone (151). In summary, research provides strong evidence that HIV infection and heavy drinking share mechanisms of neuroinflammation and neurodegeneration, but preclinical findings require confirmation in human clinical research.
3D. Preliminary clinical evidence linking immune activation, inflammation, and brain abnormality
Clinical research has begun to link systemic immune activation and inflammation to brain abnormality and cognitive impairment in PLWH. First, immune biomarkers have shown considerable potential to serve as indicators of risk for HIV-related neurodegeneration and/or cognitive decline. Most studies have found positive associations of cognitive impairment with plasma LPS and/or sCD14 levels in PLWH [(36, 53, 152); but see also (153)]. In a study comparing predictive validity of several biomarkers, sCD14 and MCP-1 were the strongest predictors of cognitive impairment in PLWH (56). Second, peripheral levels of cytokines were correlated with several domains of cognitive functioning in PLWH and tended to be better predictors of cognition than HIV clinical characteristics (154, 155). Associations between plasma cytokines and cognition support a relationship between peripheral and CNS inflammation, with further studies needed to establish directionality. Third, non-invasive magnetic resonance imaging (MRI) measures of neurometabolism and white matter microstructure give evidence of CNS pathology in HIV infection [see brief review (156)]. Specifically, elevated myo-inositol, elevated choline, decreased N-acetyl-aspartate, and abnormal white matter microstructure in PLWH are consistent with neuroinflammatory processes (156). Large neuroimaging studies of PLWH have reported that immune activation markers (e.g., MCP-1, sCD14) in plasma and cerebrospinal fluid are associated with elevated choline, elevated myo-inositol, and decreased N-acetyl-aspartate (149, 157–159). Neuroimaging studies also support a link between peripheral and CNS immune activation. When comparing healthy controls to PWLH with various degrees of cognitive impairment, markers of inflammation (myo-inositol & choline) were elevated in PLWH, regardless of cognitive status; and a marker of neuronal integrity (N-acetyl-aspartate) was decreased only in HIV-positive participants with marked cognitive impairment (160). The researchers noted that results were consistent with a progressive model of primary neuroinflammation leading to neuronal injury. Another study reported that peripheral cytokines involved in monocyte activation were overexpressed in PLWH and were negatively correlated with N-acetyl-aspartate in frontal white matter and anterior cingulate cortex (159). As a caveat, abnormalities detected by currently available clinical imaging techniques are not specific to neuroinflammatory states (161).
Neuroimaging research on effects of heavy drinking in HIV infection is limited, as previous neuroimaging studies of PLWH generally have excluded individuals with AUD. A handful of MRI studies of comorbid AUD and HIV have found significantly greater ventricular expansion (typical of brain atrophy), neurometabolic abnormality, and white matter damage in comorbid AUD and HIV compared to either condition alone (16, 162–164). These findings point to neurodegeneration yet do not identify neuroinflammation as a causal factor per se, given the limitations of current clinical MRI methods. Further research is needed to determine the neural correlates of peripheral inflammation and to characterize patterns of neuroinflammation in heavy drinking and HIV, separately and concomitantly.
4. Behavioral consequences of HIV- and alcohol-related neuroinflammation
4A. Affective and behavioral dysregulation
Peripheral levels of LPS and cytokines have distinct relevance for affective and behavioral symptom presentations in both AUD and HIV infection. Pro-inflammatory cytokines used in clinical practice, e.g., interferon-α treatment for chronic hepatitis, have long been recognized for their ability to induce or exacerbate dysphoric mood and somatic symptoms (165). As previously noted, it is not necessary for LPS to cross the BBB to exert deleterious neuroinflammatory effects. In fact, peripheral injection of LPS, standard vaccine, or cytokines is used as an experimental model of low-grade inflammation with rapid onset (i.e., 2–4 hours) (166). Experimental studies confirm the ability of these agents to cause “sickness behaviors” in otherwise healthy humans and animals [see review (167)]. Sickness behaviors include fatigue, decreased motor activity, and reduced pursuit of natural reinforcers, e.g., food, novelty, and socialization. For example, LPS injection in mice caused reductions in food intake, novel object exploration, and social interaction, even at relatively low doses (168). In humans, responses to pro-inflammatory challenge can manifest as neuropsychiatric symptoms of anhedonia, social withdrawal, and depressed mood (167, 169, 170).
Recent neuroimaging studies offer insight into the neural bases of these symptoms. On functional MRI (fMRI), researchers detected suppressed activity in the striatum, a neural substrate of reward, in healthy participants who received a low-dose LPS injection (171). An fMRI study of individuals receiving interferon-α treatment for hepatitis C found a similar reduction of striatal activity in the treatment group compared to wait-list control, in conjunction with perturbations in dopaminergic activity on positron emission tomography (PET) (172). In healthy participants, induction of low-grade inflammation produced significant metabolic changes in the insula, a brain region key to interoception (i.e., internal representation of physiological states) (173). Across studies, ratings of depressed mood, decreased motivation, and fatigue closely corresponded to neural changes.
There is reason to posit that PLWH may be especially susceptible to affective and behavioral changes caused by elevated LPS. In response to acute LPS challenge, PLWH had significantly greater pro-inflammatory (TNF-α, IL-6, and IL-8) and lower anti-inflammatory (IL-10) cytokine response than HIV-negative individuals (174). An observational study found that IL-6, TNF-α, and monocyte counts were higher in PLWH who endorsed predominantly somatic depressive symptoms, particularly fatigue (175). Another study found higher levels of several pro-inflammatory cytokines and oxidative stress markers in PLWH who endorsed depressive symptoms, compared to PLWH without depressive symptoms (176). As a large proportion of PLWH receiving ART continue to experience burdensome but nonspecific symptoms such as fatigue and anxiety (177), controlled, prospective studies to identify root causes of these symptoms is essential to improving quality of life.
The centrality of innate immune signaling, especially involving TLR4, to alcohol-related neuropathology extends to cognitive and behavioral consequences. Chronic or binge alcohol treatment caused long-term memory impairment and anxiety behaviors in wild type mice, but not TLR4-knockouts (178, 179). Persistent glial activation and epigenetic alterations accompanied these behavioral changes in wild-type mice but were absent in TLR4-knockout mice (178, 179). Importantly, behavioral effects were not attributable to differences in voluntary alcohol intake for the mouse strains (178).
4B. Increased voluntary alcohol intake
Preclinical and clinical evidence suggests that heavy alcohol exposure may interact with innate immune mechanisms to increase subsequent alcohol consumption. Again, TLR4-mediated immune signaling appears to play a critical role. LPS exposure in rodents either a week or month prior to alcohol availability caused a significant and durable increase in voluntary alcohol consumption (180). Because the LPS-induced increase in drinking was abolished in mice lacking CD14, the TLR4 co-receptor, the researchers inferred that TLR4 signaling mediated the effect of LPS on drinking behavior (180). In another study, LPS or cytokine treatment prior to alcohol feeding sensitized rodents to social interaction anxiety during alcohol withdrawal (181). Immune activation also may contribute to effects of adolescent alcohol exposure on adult substance use. Adolescent wild-type mice receiving intermittent heavy alcohol treatment showed greater alcohol preference and conditioned cocaine reward later in development, but these effects were abrogated in TLR4-knockouts (179). Alcohol-preferring (“P”) rats have innately higher levels of TLR4 expression in behaviorally relevant brain regions prior to alcohol exposure, and inhibiting gene expression of either TLR4 or MCP-1 reduced their voluntary alcohol intake (128). Although translational significance for human behavior remains to be determined, observational studies are consistent with animal models. Individuals in early abstinence from alcohol showed positive correlations of intestinal permeability and plasma cytokines with depression, anxiety, and alcohol craving (86, 182).
5. Conclusion and future directions
With access to combination ART, life expectancy of PLWH approaches that of uninfected individuals (183). However, increased mortality was observed at a considerably lower level of drinking for men living with HIV (≥30 drinks per month) than for HIV-negative men (≥70 drinks per month) (8). Understanding the mechanisms by which even moderate alcohol intake may contribute to mortality and morbidity in PLWH is a pressing public health concern. The absence of evidence-based guidelines for alcohol consumption in PLWH underscores the import of this issue. Research reviewed here strongly suggests that heavy alcohol use may exacerbate specific inflammatory processes already present in HIV infection, including microbial translocation, immune activation, BBB compromise, microglial activation, and neuroinflammation.
Currently, long-term effects of alcohol use on the clinical course of HIV infection are not well understood. Research linking systemic immune activation to neuroinflammatory processes remains in early stages. Because most previous neuroimaging studies of PLWH have excluded individuals with heavy drinking, integrated and multidisciplinary research on this complex comorbidity is imperative. Animal models permit hypothesis-driven studies of additive or synergistic effects of alcohol and HIV on key inflammatory processes. As animal models have certain limitations in the study of HIV, however, clinical neuroimaging research in cohorts of PLWH with varied alcohol use patterns will be critical to understanding these relationships. Development of advanced neuroimaging methods with specificity for neuroinflammation and microglial activation will aid in diagnosis and targeted treatment of HIV- and alcohol-related neuropathology. Moreover, peripheral markers of immune activation warrant further investigation as proxy biomarkers of neuroinflammation [e.g., (56)]. In clinical settings without ready access to neuroimaging tools, such biomarkers would be highly useful for identifying individuals in need of more intensive assessment and treatment.
Microglial activation, particularly the TLR4-mediated cascade, is a novel and promising target for neuropharmacological interventions (184, 185). In addition, prebiotic and/or probiotic supplements are receiving attention as an adjunctive therapy in HIV infection and AUD. Prebiotic and/or probiotic supplements have shown initial promise to mitigate inflammation in small randomized, controlled trials with PLWH (186–188) and in rodent models of alcoholic liver disease (189, 190). An important question for such trials is whether therapeutic interventions that reduce systemic inflammation by way of the gut microbiome also have benefits for neural health and psychological functioning.
In conclusion, basic and clinical findings on immune mechanisms of alcohol have critical implications in the setting of HIV infection. Up to this point, alcohol and HIV research agendas have proceeded largely independent of each other. The possibility for heavy drinking to compound systemic, neurobiological, and cognitive complications in HIV infection presents a serous cause for concern, as well as a compelling rationale to integrate these lines of research. Studies on specific immune mechanisms shared by heavy drinking and HIV infection will help to identify critical targets for behavioral and biomedical interventions in this complex comorbidity.
Acknowledgments
Funding: This work was supported by National Institute on Alcohol Abuse and Alcoholism grants T32AA007459 (PI: Monti) and K23AA024704-01A1 (PI: Monnig).
Footnotes
Declaration of Interest: The author has no relevant financial disclosures or conflicts of interest.
Bibliography
- 1.Centers for Disease Control and Prevention. Behavioral and clinical characteristics of persons receiving medical care for HIV infection--Medical Monitoring Project, United States, 2010. 2014 [PubMed] [Google Scholar]
- 2.Kalichman SC, Grebler T, Amaral CM, McNerey M, White D, Kalichman MO, Cherry C, Eaton L. Intentional non-adherence to medications among HIV positive alcohol drinkers: prospective study of interactive toxicity beliefs. J Gen Intern Med. 2013;28(3):399–405. doi: 10.1007/s11606-012-2231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott-Sheldon LA, Walstrom P, Carey KB, Johnson BT, Carey MP Team tMR. Alcohol Use and Sexual Risk Behaviors among Individuals Infected with HIV: A Systematic Review and Meta-Analysis 2012 to Early 2013. Curr HIV/AIDS Rep. 2013 doi: 10.1007/s11904-013-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahler CW, Wray TB, Pantalone DW, Kruis RD, Mastroleo NR, Monti PM, Mayer KH. Daily Associations Between Alcohol Use and Unprotected Anal Sex Among Heavy Drinking HIV-Positive Men Who Have Sex with Men. AIDS Behav. 2014 doi: 10.1007/s10461-014-0896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandrea I, Happel KI, Amedee AM, Bagby GJ, Nelson S. Alcohol’s role in HIV transmission and disease progression. Alcohol Res Health. 2010;33(3):203–218. [PMC free article] [PubMed] [Google Scholar]
- 6.Braithwaite RS, Conigliaro J, Roberts MS, Shechter S, Schaefer A, McGinnis K, Rodriguez MC, Rabeneck L, Bryant K, Justice AC. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19(4):459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neblett RC, Hutton HE, Lau B, McCaul ME, Moore RD, Chander G. Alcohol consumption among HIV-infected women: impact on time to antiretroviral therapy and survival. J Womens Health (Larchmt) 2011;20(2):279–286. doi: 10.1089/jwh.2010.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, Edelman EJ, Fiellin LE, Freiberg MS, Gordon AJ, Kraemer KL, Marshall BD, Williams EC, Fiellin DA. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016 doi: 10.1016/j.drugalcdep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United States Department of Health and Human Services - National Institute on Alcohol Abuse and Alcoholism. The Physicians’ Guide to Helping Patients with Alcohol Problems. NIH Publication No95-3796. Bethesda, MD: National Institutes of Health; 1995. [Google Scholar]
- 10.Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CG HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CGHG HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn JA, Samet JH. Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep. 2010;7(4):226–233. doi: 10.1007/s11904-010-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N, Vetreno RP. Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol Research: Current Reviews. 2015;37(2):331–351. [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbloom MJ, Sullivan EV, Pfefferbaum A. Focus on the brain: HIV infection and alcoholism: comorbidity effects on brain structure and function. Alcohol Res Health. 2010;33(3):247–257. [PMC free article] [PubMed] [Google Scholar]
- 15.Persidsky Y, Ho W, Ramirez SH, Potula R, Abood ME, Unterwald E, Tuma R. HIV-1 infection and alcohol abuse: neurocognitive impairment, mechanisms of neurodegeneration and therapeutic interventions. Brain Behav Immun. 2011;25(Suppl 1):S61–70. doi: 10.1016/j.bbi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfefferbaum A, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, Sullivan EV. Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biol Psychiatry. 2012;72:361–370. doi: 10.1016/j.biopsych.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gongvatana A, Harezlak J, Buchthal S, Daar E, Schifitto G, Campbell T, Taylor M, Singer E, Algers J, Zhong J, Brown M, McMahon D, So YT, Mi D, Heaton R, Robertson K, Yiannoutsos C, Cohen RA, Navia B. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J Neurovirol. 2013;19:209–218. doi: 10.1007/s13365-013-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre SL, Alhassoon OM, Jacobus J, Woods SP, Jernigan TL, Ellis RJ, Frank LR, Grant I. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol. 2009;15:187–195. doi: 10.1080/13550280902769756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mon A, Durazzo TC, Abe C, Gazdzinski S, Pennington D, Schmidt T, Meyerhoff DJ. Structural brain differences in alcohol-dependent individuals with and without comorbid substance dependence. Drug Alcohol Depend. 2014;144:170–177. doi: 10.1016/j.drugalcdep.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ragin AB, Du H, Ochs R, Wu Y, Sammet CL, Shoukry A, Epstein LG. Structural brain alterations can be detected early in HIV infection. Neurology. 2012;79(24):2328–2334. doi: 10.1212/WNL.0b013e318278b5b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- 22.Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2012;18:203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- 23.Fama R, Rosenbloom MJ, Nichols BN, Pfefferbaum A, Sullivan EV. Working and episodic memory in HIV infection, alcoholism, and their comorbidity: baseline and 1-year follow-up examinations. Alcohol Clin Exp Res. 2009;33:1815–1824. doi: 10.1111/j.1530-0277.2009.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sassoon SA, Fama R, Rosenbloom MJ, O’Reilly A, Pfefferbaum A, Sullivan EV. Component cognitive and motor processes of the digit symbol test: differential deficits in alcoholism, HIV infection, and their comorbidity. Alcohol Clin Exp Res. 2007;31:1315–1324. doi: 10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 25.Durvasula RS, Myers HF, Mason K, Hinkin C. Relationship between alcohol use/abuse, HIV infection and neuropsychological performance in African American men. J Clin Exp Neuropsychol. 2006;28:383–404. doi: 10.1080/13803390590935408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, Weiner MW. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11:70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagby GJ, Amedee AM, Siggins RW, Molina PE, Nelson S, Veazey RS. Alcohol and HIV Effects on the Immune System. Alcohol Research: Current Reviews. 2015;37(2):287–297. [PMC free article] [PubMed] [Google Scholar]
- 28.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64(5):1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barclay GR. Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: a review. Prog Clin Biol Res. 1995;392:263–272. [PubMed] [Google Scholar]
- 31.Schumann RR, Zweigner J. A novel acute-phase marker: lipopolysaccharide binding protein (LBP) Clin Chem Lab Med. 1999;37(3):271–274. doi: 10.1515/CCLM.1999.047. [DOI] [PubMed] [Google Scholar]
- 32.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 33.Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, Wanke CA, Ward HD. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211(1):19–27. doi: 10.1093/infdis/jiu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, Kirk GD, Mehta SH, Cox AL, Thomas DL, Ray SC. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135(1):226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volpe GE, Ward H, Mwamburi M, Dinh D, Bhalchandra S, Wanke C, Kane AV. Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J Stud Alcohol Drugs. 2014;75(2):347–357. doi: 10.15288/jsad.2014.75.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, Singer EJ, Wolinsky SM, Gabuzda D. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC, Group ISS. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leon A, Leal L, Torres B, Lucero C, Inciarte A, Arnedo M, Plana M, Vila J, Gatell JM, Garcia F. Association of microbial translocation biomarkers with clinical outcome in controllers HIV-infected patients. Aids. 2015;29(6):675–681. doi: 10.1097/QAD.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 39.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104(4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 40.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2012;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26:2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6(4):e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan ES, Micci L, Fromentin R, Paganini S, McGary CS, Easley K, Chomont N, Paiardini M. Loss of Function of Intestinal IL-17 and IL-22 Producing Cells Contributes to Inflammation and Viral Persistence in SIV-Infected Rhesus Macaques. PLoS Pathog. 2016;12(2):e1005412. doi: 10.1371/journal.ppat.1005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, French A, Demarais P, Sun Y, Koenig L, Cox S, Engen P, Chakradeo P, Abbasi R, Gorenz A, Burns C, Landay A. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10(2):e1003829. doi: 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vazquez-Castellanos JF, Serrano-Villar S, Latorre A, Artacho A, Ferrus ML, Madrid N, Vallejo A, Sainz T, Martinez-Botas J, Ferrando-Martinez S, Vera M, Dronda F, Leal M, Del Romero J, Moreno S, Estrada V, Gosalbes MJ, Moya A. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 2015;8(4):760–772. doi: 10.1038/mi.2014.107. [DOI] [PubMed] [Google Scholar]
- 47.Zevin AS, McKinnon L, Burgener A, Klatt NR. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS. 2016;11(2):182–190. doi: 10.1097/COH.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM, Hirsch VM, Silvestri G, Douek DC, Miller CJ, Haase AT, Lifson J, Brenchley JM. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6(8):e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hao XP, Lucero CM, Turkbey B, Bernardo ML, Morcock DR, Deleage C, Trubey CM, Smedley J, Klatt NR, Giavedoni LD, Kristoff J, Xu A, Del Prete GQ, Keele BF, Rao SS, Alvord WG, Choyke PL, Lifson JD, Brenchley JM, Apetrei C, Pandrea I, Estes JD. Experimental colitis in SIV-uninfected rhesus macaques recapitulates important features of pathogenic SIV infection. Nat Commun. 2015;6:8020. doi: 10.1038/ncomms9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ploquin MJ, Silvestri G, Muller-Trutwin M. Immune activation in HIV infection: what can the natural hosts of simian immunodeficiency virus teach us? Curr Opin HIV AIDS. 2016;11(2):201–208. doi: 10.1097/COH.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 51.Krastinova E, Lecuroux C, Leroy C, Seng R, Cabie A, Rami A, Venet A, Meyer L, Goujard C. High Soluble CD14 Levels at Primary HIV-1 Infection Predict More Rapid Disease Progression. J Infect Dis. 2015 doi: 10.1093/infdis/jiv145. [DOI] [PubMed] [Google Scholar]
- 52.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, Morgello S, Gabuzda D. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57:371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, Fiellin DA, Vanasse GJ, Butt AA, Rodriguez-Barradas MC, Gibert C, Oursler KA, Deeks SG, Bryant K. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54(7):984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, Deeks SG, Meinert CL, Van Natta ML, Jabs DA, Lederman MM. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210(8):1228–1238. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marcotte TD, Deutsch R, Michael BD, Franklin D, Cookson DR, Bharti AR, Grant I, Letendre SL. A concise panel of biomarkers identifies neurocognitive functioning changes in HIV-infected individuals. J Neuroimmune Pharmacol. 2013;8(5):1123–1135. doi: 10.1007/s11481-013-9504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karris MY, Kao YT, Patel D, Dawson M, Woods SP, Vaida F, Spina C, Richman D, Little S, Smith DM. Predictors of virologic response in persons who start antiretroviral therapy during recent HIV infection. Aids. 2014;28(6):841–849. doi: 10.1097/QAD.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.So-Armah KA, Tate JP, Chang CH, Butt AA, Gerschenson M, Gibert CL, Leaf D, Rimland D, Rodriguez-Barradas MC, Budoff MJ, Samet JH, Kuller LH, Deeks SG, Crothers KA, Tracy RP, Crane HM, Sajadi MM, Tindle HA, Justice AC, Freiberg MS. Do Biomarkers Of Inflammation, Monocyte Activation And Altered Coagulation Explain Excess Mortality Between HIV Infected and Uninfected People? J Acquir Immune Defic Syndr. 2016 doi: 10.1097/QAI.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hemkens LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart J. 2014;35(21):1373–1381. doi: 10.1093/eurheartj/eht528. [DOI] [PubMed] [Google Scholar]
- 60.Gillard BK, Raya JL, Ruiz-Esponda R, Iyer D, Coraza I, Balasubramanyam A, Pownall HJ. Impaired lipoprotein processing in HIV patients on antiretroviral therapy: aberrant high-density lipoprotein lipids, stability, and function. Arterioscler Thromb Vasc Biol. 2013;33(7):1714–1721. doi: 10.1161/ATVBAHA.113.301538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siegel MO, Borkowska AG, Dubrovsky L, Roth M, Welti R, Roberts AD, Parenti DM, Simon GL, Sviridov D, Simmens S, Bukrinsky M, Fitzgerald ML. HIV infection induces structural and functional changes in high density lipoproteins. Atherosclerosis. 2015;243(1):19–29. doi: 10.1016/j.atherosclerosis.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bernal E, Masia M, Padilla S, Gutierrez F. High-density lipoprotein cholesterol in HIV-infected patients: evidence for an association with HIV-1 viral load, antiretroviral therapy status, and regimen composition. AIDS Patient Care STDS. 2008;22(7):569–575. doi: 10.1089/apc.2007.0186. [DOI] [PubMed] [Google Scholar]
- 63.Kitchens RL, Thompson PA, Viriyakosol S, O’Keefe GE, Munford RS. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J Clin Invest. 2001;108:485–493. doi: 10.1172/JCI13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wurfel MM, Hailman E, Wright SD. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med. 1995;181(5):1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11(4):225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 66.Afshar M, Richards S, Mann D, Cross A, Smith GB, Netzer G, Kovacs E, Hasday J. Acute immunomodulatory effects of binge alcohol ingestion. Alcohol. 2015;49(1):57–64. doi: 10.1016/j.alcohol.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandrekar P, Catalano D, White B, Szabo G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol Clin Exp Res. 2006;30(1):135–139. doi: 10.1111/j.1530-0277.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 68.Muralidharan S, Ambade A, Fulham MA, Deshpande J, Catalano D, Mandrekar P. Moderate alcohol induces stress proteins HSF1 and hsp70 and inhibits proinflammatory cytokines resulting in endotoxin tolerance. J Immunol. 2014;193:1975–1987. doi: 10.4049/jimmunol.1303468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barr T, Helms C, Grant K, Messaoudi I. Opposing effects of alcohol on the immune system. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:242–251. doi: 10.1016/j.pnpbp.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30(4):720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 72.Pascual M, Fernandez-Lizarbe S, Guerri C. Role of TLR4 in ethanol effects on innate and adaptive immune responses in peritoneal macrophages. Immunol Cell Biol. 2011;89(6):716–727. doi: 10.1038/icb.2010.163. [DOI] [PubMed] [Google Scholar]
- 73.Ghare S, Patil M, Hote P, Suttles J, McClain C, Barve S, Joshi-Barve S. Ethanol inhibits lipid raft-mediated TCR signaling and IL-2 expression: potential mechanism of alcohol-induced immune suppression. Alcohol Clin Exp Res. 2011;35(8):1435–1444. doi: 10.1111/j.1530-0277.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H, Meadows GG. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78(5):1070–1080. doi: 10.1189/jlb.0605317. [DOI] [PubMed] [Google Scholar]
- 75.Chaudhry KK, Shukla PK, Mir H, Manda B, Gangwar R, Yadav N, McMullen M, Nagy LE, Rao R. Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice. J Nutr Biochem. 2016;27:16–26. doi: 10.1016/j.jnutbio.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferrier L, Berard F, Debrauwer L, Chabo C, Langella P, Bueno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168(4):1148–1154. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 1998;22(8):1724–1730. [PubMed] [Google Scholar]
- 80.Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol. 2001;280(6):G1280–1288. doi: 10.1152/ajpgi.2001.280.6.G1280. [DOI] [PubMed] [Google Scholar]
- 81.Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol Clin Exp Res. 2009;33(7):1220–1230. doi: 10.1111/j.1530-0277.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Asquith M, Pasala S, Engelmann F, Haberthur K, Meyer C, Park B, Grant KA, Messaoudi I. Chronic ethanol consumption modulates growth factor release, mucosal cytokine production, and microRNA expression in nonhuman primates. Alcohol Clin Exp Res. 2014;38(4):980–993. doi: 10.1111/acer.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schafer C, Parlesak A, Schutt C, Bode JC, Bode C. Concentrations of lipopolysaccharide-binding protein, bactericidal/permeability-increasing protein, soluble CD14 and plasma lipids in relation to endotoxaemia in patients with alcoholic liver disease. Alcohol Alcohol. 2002;37:81–86. doi: 10.1093/alcalc/37.1.81. [DOI] [PubMed] [Google Scholar]
- 84.Parlesak A, Schafer C, Bode C. IgA against gut-derived endotoxins: does it contribute to suppression of hepatic inflammation in alcohol-induced liver disease? Dig Dis Sci. 2002;47:760–766. doi: 10.1023/a:1014783815433. [DOI] [PubMed] [Google Scholar]
- 85.Urbaschek R, McCuskey RS, Rudi V, Becker KP, Stickel F, Urbaschek B, Seitz HK. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res. 2001;25(2):261–268. [PubMed] [Google Scholar]
- 86.Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, Delzenne NM, de Timary P. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun. 2012;26:911–918. doi: 10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Frank J, Witte K, Schrodl W, Schutt C. Chronic alcoholism causes deleterious conditioning of innate immunity. Alcohol Alcohol. 2004;39:386–392. doi: 10.1093/alcalc/agh083. [DOI] [PubMed] [Google Scholar]
- 88.Kazbariene B, Krikstaponiene A, Monceviciute-Eringiene E. Disturbance of human immunohomeostasis by environmental pollution and alcohol consumption. Acta Microbiol Immunol Hung. 2006;53(2):209–218. doi: 10.1556/AMicr.53.2006.2.7. [DOI] [PubMed] [Google Scholar]
- 89.Tabata T, Meyer AA. Immunoglobulin M synthesis after burn injury: the effects of chronic ethanol on postinjury synthesis. J Burn Care Rehabil. 1995;16(4):400–406. doi: 10.1097/00004630-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 90.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9:e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Jong WJ, Cleveringa AM, Greijdanus B, Meyer P, Heineman E, Hulscher JB. The effect of acute alcohol intoxication on gut wall integrity in healthy male volunteers; a randomized controlled trial. Alcohol. 2015;49(1):65–70. doi: 10.1016/j.alcohol.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 92.Banerjee A, Abdelmegeed MA, Jang S, Song BJ. Increased Sensitivity to Binge Alcohol-Induced Gut Leakiness and Inflammatory Liver Disease in HIV Transgenic Rats. PLoS One. 2015;10(10):e0140498. doi: 10.1371/journal.pone.0140498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vigorito M, Connaghan KP, Chang SL. The HIV-1 transgenic rat model of neuroHIV. Brain Behav Immun. 2015;48:336–349. doi: 10.1016/j.bbi.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carrico AW, Hunt PW, Emenyonu NI, Muyindike W, Ngabirano C, Cheng DM, Winter MR, Samet JH, Hahn JA. Unhealthy Alcohol Use is Associated with Monocyte Activation Prior to Starting Antiretroviral Therapy. Alcohol Clin Exp Res. 2015 doi: 10.1111/acer.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Monnig MA, Kahler CW, Cioe PA, Tucker L, Monti PM, Mayer KH, Ramratnam B. Alcohol use predicts elevation in inflammatory marker soluble CD14 in men living with HIV. AIDS Care. 2016:1–7. doi: 10.1080/09540121.2016.1189497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cioe PA, Baker J, Kojic E, Onen N, Hammer J, Patel P, Kahler CW. Elevated soluble CD14 and lower D-dimer are associated with cigarette smoking and heavy episodic alcohol use in persons living with HIV (PLWH) J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 2005;11(8):973–984. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- 98.Shiu C, Barbier E, Di Cello F, Choi HJ, Stins M. HIV-1 gp120 as well as alcohol affect blood-brain barrier permeability and stress fiber formation: involvement of reactive oxygen species. Alcohol Clin Exp Res. 2007;31(1):130–137. doi: 10.1111/j.1530-0277.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- 99.Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- 100.Abdul Muneer PM, Alikunju S, Szlachetka AM, Haorah J. Inhibitory effects of alcohol on glucose transport across the blood-brain barrier leads to neurodegeneration: preventive role of acetyl-L: -carnitine. Psychopharmacology (Berl) 2011;214(3):707–718. doi: 10.1007/s00213-010-2076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alikunju S, Abdul Muneer PM, Zhang Y, Szlachetka AM, Haorah J. The inflammatory footprints of alcohol-induced oxidative damage in neurovascular components. Brain Behav Immun. 2011;25(Suppl 1):S129–136. doi: 10.1016/j.bbi.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol. 2005;64(6):498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- 103.Wang H, Sun J, Goldstein H. Human immunodeficiency virus type 1 infection increases the in vivo capacity of peripheral monocytes to cross the blood-brain barrier into the brain and the in vivo sensitivity of the blood-brain barrier to disruption by lipopolysaccharide. J Virol. 2008;82:7591–7600. doi: 10.1128/JVI.00768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dohgu S, Banks WA. Brain pericytes increase the lipopolysaccharide-enhanced transcytosis of HIV-1 free virus across the in vitro blood-brain barrier: evidence for cytokine-mediated pericyte-endothelial cell crosstalk. Fluids Barriers CNS. 2013;10:23. doi: 10.1186/2045-8118-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62(9):1377–1391. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- 107.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1–12. doi: 10.1016/j.bbi.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 110.Pilakka-Kanthikeel S, Raymond A, Atluri VS, Sagar V, Saxena SK, Diaz P, Chevelon S, Concepcion M, Nair M. Sterile alpha motif and histidine/aspartic acid domain-containing protein 1 (SAMHD1)-facilitated HIV restriction in astrocytes is regulated by miRNA-181a. J Neuroinflammation. 2015;12:66. doi: 10.1186/s12974-015-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T. Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Curr HIV Res. 2014;12(2):97–110. doi: 10.2174/1570162x12666140526114956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tauber SC, Staszewski O, Prinz M, Weis J, Nolte K, Bunkowski S, Bruck W, Nau R. HIV encephalopathy: glial activation and hippocampal neuronal apoptosis, but limited neural repair. HIV Med. 2016;17(2):143–151. doi: 10.1111/hiv.12288. [DOI] [PubMed] [Google Scholar]
- 113.Cardenas VA, Meyerhoff DJ, Studholme C, Kornak J, Rothlind J, Lampiris H, Neuhaus J, Grant RM, Chao LL, Truran D, Weiner MW. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol. 2009;15(4):324–333. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Underwood J, Robertson KR, Winston A. Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? Aids. 2015;29(3):253–261. doi: 10.1097/QAD.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 115.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175:6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- 117.Valles SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- 119.Gilpin NW, Koob GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health. 2008;31(3):185–195. [PMC free article] [PubMed] [Google Scholar]
- 120.Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43(3):281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- 122.Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-kappaB and proinflammatory cytokines. Alcohol Clin Exp Res. 2010;34(5):777–789. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- 123.Fernandez-Lizarbe S, Montesinos J, Guerri C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J Neurochem. 2013;126:261–273. doi: 10.1111/jnc.12276. [DOI] [PubMed] [Google Scholar]
- 124.Zou J, Crews FT. Inflammasome-IL-1beta Signaling Mediates Ethanol Inhibition of Hippocampal Neurogenesis. Front Neurosci. 2012;6:77. doi: 10.3389/fnins.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alfonso-Loeches S, Pascual M, Gomez-Pinedo U, Pascual-Lucas M, Renau-Piqueras J, Guerri C. Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia. 2012;60:948–964. doi: 10.1002/glia.22327. [DOI] [PubMed] [Google Scholar]
- 126.Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2013;73(7):602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D, Puche A, Aurelian L. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology. 2015;40(6):1549–1559. doi: 10.1038/npp.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Blanco AM, Perez-Arago A, Fernandez-Lizarbe S, Guerri C. Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J Neurochem. 2008;106:625–639. doi: 10.1111/j.1471-4159.2008.05425.x. [DOI] [PubMed] [Google Scholar]
- 130.Fernandez-Lizarbe S, Pascual M, Gascon MS, Blanco A, Guerri C. Lipid rafts regulate ethanol-induced activation of TLR4 signaling in murine macrophages. Mol Immunol. 2008;45(7):2007–2016. doi: 10.1016/j.molimm.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 131.Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci. 2012;32(33):11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Atluri VS, Kanthikeel SP, Reddy PV, Yndart A, Nair MP. Human synaptic plasticity gene expression profile and dendritic spine density changes in HIV-infected human CNS cells: role in HIV-associated neurocognitive disorders (HAND) PLoS One. 2013;8(4):e61399. doi: 10.1371/journal.pone.0061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zorumski CF, Mennerick S, Izumi Y. Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol. 2014;48(1):1–17. doi: 10.1016/j.alcohol.2013.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kyzar EJ, Pandey SC. Molecular mechanisms of synaptic remodeling in alcoholism. Neurosci Lett. 2015;601:11–19. doi: 10.1016/j.neulet.2015.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saiyed ZM, Gandhi N, Agudelo M, Napuri J, Samikkannu T, Reddy PV, Khatavkar P, Yndart A, Saxena SK, Nair MP. HIV-1 Tat upregulates expression of histone deacetylase-2 (HDAC2) in human neurons: implication for HIV-associated neurocognitive disorder (HAND) Neurochem Int. 2011;58(6):656–664. doi: 10.1016/j.neuint.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lopez-Moreno JA, Marcos M, Calleja-Conde J, Echeverry-Alzate V, Buhler KM, Costa-Alba P, Bernardo E, Laso FJ, Rodriguez de Fonseca F, Nadal R, Viveros MP, Maldonado R, Gine E. Histone Deacetylase Gene Expression Following Binge Alcohol Consumption in Rats and Humans. Alcohol Clin Exp Res. 2015;39(10):1939–1950. doi: 10.1111/acer.12850. [DOI] [PubMed] [Google Scholar]
- 138.Agudelo M, Gandhi N, Saiyed Z, Pichili V, Thangavel S, Khatavkar P, Yndart-Arias A, Nair M. Effects of alcohol on histone deacetylase 2 (HDAC2) and the neuroprotective role of trichostatin A (TSA) Alcohol Clin Exp Res. 2011;35(8):1550–1556. doi: 10.1111/j.1530-0277.2011.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Penney J, Tsai LH. Histone deacetylases in memory and cognition. Sci Signal. 2014;7(355):re12. doi: 10.1126/scisignal.aaa0069. [DOI] [PubMed] [Google Scholar]
- 140.Atluri VS, Pilakka-Kanthikeel S, Samikkannu T, Sagar V, Kurapati KR, Saxena SK, Yndart A, Raymond A, Ding H, Hernandez O, Nair MP. Vorinostat positively regulates synaptic plasticity genes expression and spine density in HIV infected neurons: role of nicotine in progression of HIV-associated neurocognitive disorder. Mol Brain. 2014;7:37. doi: 10.1186/1756-6606-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28(14):3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation. 2012;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Potula R, Haorah J, Knipe B, Leibhart J, Chrastil J, Heilman D, Dou H, Reddy R, Ghorpade A, Persidsky Y. Alcohol abuse enhances neuroinflammation and impairs immune responses in an animal model of human immunodeficiency virus-1 encephalitis. Am J Pathol. 2006;168(4):1335–1344. doi: 10.2353/ajpath.2006.051181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Agudelo M, Figueroa G, Yndart A, Casteleiro G, Munoz K, Samikkannu T, Atluri V, Nair MP. Alcohol and Cannabinoids Differentially Affect HIV Infection and Function of Human Monocyte-Derived Dendritic Cells (MDDC) Front Microbiol. 2015;6:1452. doi: 10.3389/fmicb.2015.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bagasra O, Bachman SE, Jew L, Tawadros R, Cater J, Boden G, Ryan I, Pomerantz RJ. Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: potential immunopathogenic mechanisms. J Infect Dis. 1996;173(3):550–558. doi: 10.1093/infdis/173.3.550. [DOI] [PubMed] [Google Scholar]
- 146.Flora G, Pu H, Lee YW, Ravikumar R, Nath A, Hennig B, Toborek M. Proinflammatory synergism of ethanol and HIV-1 Tat protein in brain tissue. Exp Neurol. 2005;191(1):2–12. doi: 10.1016/j.expneurol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 147.Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, Morgello S, Berman JW. Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One. 2013;8:e69270. doi: 10.1371/journal.pone.0069270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ragin AB, Wu Y, Ochs R, Scheidegger R, Cohen BA, Edelman RR, Epstein LG, McArthur J. Biomarkers of neurological status in HIV infection: a 3-year study. Proteomics Clin Appl. 2010;4(3):295–303. doi: 10.1002/prca.200900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anderson AM, Fennema-Notestine C, Umlauf A, Taylor MJ, Clifford DB, Marra CM, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Simpson DM, Morgello S, Grant I, Letendre SL. CSF biomarkers of monocyte activation and chemotaxis correlate with magnetic resonance spectroscopy metabolites during chronic HIV disease. J Neurovirol. 2015 doi: 10.1007/s13365-015-0359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Singh AK, Gupta S, Jiang Y. Oxidative stress and protein oxidation in the brain of water drinking and alcohol drinking rats administered the HIV envelope protein, gp120. J Neurochem. 2008;104(6):1478–1493. doi: 10.1111/j.1471-4159.2007.05094.x. [DOI] [PubMed] [Google Scholar]
- 151.Samikkannu T, Ranjith D, Rao KV, Atluri VS, Pimentel E, El-Hage N, Nair MP. HIV-1 gp120 and morphine induced oxidative stress: role in cell cycle regulation. Front Microbiol. 2015;6:614. doi: 10.3389/fmicb.2015.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ryan LA, Zheng J, Brester M, Bohac D, Hahn F, Anderson J, Ratanasuwan W, Gendelman HE, Swindells S. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis. 2001;184:699–706. doi: 10.1086/323036. [DOI] [PubMed] [Google Scholar]
- 153.Sun B, Abadjian L, Rempel H, Calosing C, Rothlind J, Pulliam L. Peripheral biomarkers do not correlate with cognitive impairment in highly active antiretroviral therapy-treated subjects with human immunodeficiency virus type 1 infection. J Neurovirol. 2010;16(2):115–124. doi: 10.3109/13550280903559789. [DOI] [PubMed] [Google Scholar]
- 154.Cohen RA, de la Monte S, Gongvatana A, Ombao H, Gonzalez B, Devlin KN, Navia B, Tashima KT. Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. J Neuroimmunol. 2010;233:204–210. doi: 10.1016/j.jneuroim.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Correia S, Cohen R, Gongvatana A, Ross S, Olchowski J, Devlin K, Tashima K, Navia B, Delamonte S. Relationship of plasma cytokines and clinical biomarkers to memory performance in HIV. J Neuroimmunol. 2013;265:117–123. doi: 10.1016/j.jneuroim.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ances BM, Hammoud DA. Neuroimaging of HIV-associated neurocognitive disorders (HAND) Curr Opin HIV AIDS. 2014;9:545–551. doi: 10.1097/COH.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Letendre SL, Zheng JC, Kaul M, Yiannoutsos CT, Ellis RJ, Taylor MJ, Marquie-Beck J, Navia B. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011;17(1):63–69. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Anderson AM, Harezlak J, Bharti A, Mi D, Taylor MJ, Daar ES, Schifitto G, Zhong J, Alger JR, Brown MS, Singer EJ, Campbell TB, McMahon DD, Buchthal S, Cohen R, Yiannoutsos C, Letendre SL, Navia BA. Plasma and Cerebrospinal Fluid Biomarkers Predict Cerebral Injury in HIV-Infected Individuals on Stable Combination Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2015;69(1):29–35. doi: 10.1097/QAI.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pulliam L, Rempel H, Sun B, Abadjian L, Calosing C, Meyerhoff DJ. A peripheral monocyte interferon phenotype in HIV infection correlates with a decrease in magnetic resonance spectroscopy metabolite concentrations. Aids. 2011;25(14):1721–1726. doi: 10.1097/QAD.0b013e328349f022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. Aids. 2011;25:625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Quarantelli M. MRI/MRS in neuroinflammation: methodology and applications. Clin Transl Imaging. 2015;3:475–489. doi: 10.1007/s40336-015-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- 163.Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, Sullivan EV. Contribution of alcoholism to brain dysmorphology in HIV infection: effects on the ventricles and corpus callosum. Neuroimage. 2006;33(1):239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 164.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Cortical NAA deficits in HIV infection without dementia: influence of alcoholism comorbidity. Neuropsychopharmacology. 2005;30(7):1392–1399. doi: 10.1038/sj.npp.1300723. [DOI] [PubMed] [Google Scholar]
- 165.Valentine AD, Meyers CA. Neurobehavioral effects of interferon therapy. Curr Psychiatry Rep. 2005;7(5):391–395. doi: 10.1007/s11920-005-0042-3. [DOI] [PubMed] [Google Scholar]
- 166.DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci Biobehav Rev. 2010;34(1):130–143. doi: 10.1016/j.neubiorev.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33:315–327. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Haba R, Shintani N, Onaka Y, Wang H, Takenaga R, Hayata A, Baba A, Hashimoto H. Lipopolysaccharide affects exploratory behaviors toward novel objects by impairing cognition and/or motivation in mice: Possible role of activation of the central amygdala. Behav Brain Res. 2012;228:423–431. doi: 10.1016/j.bbr.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 169.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24(4):558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex Differences in Depressive and Socioemotional Responses to an Inflammatory Challenge: Implications for Sex Differences in Depression. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harrison NA, Cooper E, Dowell NG, Keramida G, Voon V, Critchley HD, Cercignani M. Quantitative Magnetization Transfer Imaging as a Biomarker for Effects of Systemic Inflammation on the Brain. Biol Psychiatry. 2015;78(1):49–57. doi: 10.1016/j.biopsych.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.da Silva B, Singer W, Fong IW, Ottaway CA. In vivo cytokine and neuroendocrine responses to endotoxin in human immunodeficiency virus-infected subjects. J Infect Dis. 1999;180:106–115. doi: 10.1086/314819. [DOI] [PubMed] [Google Scholar]
- 175.Norcini Pala A, Steca P, Bagrodia R, Helpman L, Colangeli V, Viale P, Wainberg ML. Subtypes of depressive symptoms and inflammatory biomarkers: An exploratory study on a sample of HIV-positive patients. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rivera-Rivera Y, Garcia Y, Toro V, Cappas N, Lopez P, Yamamura Y, Rivera-Amill V. Depression Correlates with Increased Plasma Levels of Inflammatory Cytokines and a Dysregulated Oxidant/Antioxidant Balance in HIV-1-Infected Subjects Undergoing Antiretroviral Therapy. J Clin Cell Immunol. 2014;5(6) doi: 10.4172/2155-9899.1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wilson NL, Azuero A, Vance DE, Richman JS, Moneyham LD, Raper JL, Heath SL, Kempf MC. Identifying Symptom Patterns in People Living With HIV Disease. J Assoc Nurses AIDS Care. 2016;27(2):121–132. doi: 10.1016/j.jana.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pascual M, Balino P, Alfonso-Loeches S, Aragon CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun. 2011;25(Suppl 1):S80–91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 179.Montesinos J, Pascual M, Rodriguez-Arias M, Minarro J, Guerri C. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 180.Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25(Suppl 1):S92–s105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Breese GR, Knapp DJ, Overstreet DH, Navarro M, Wills TA, Angel RA. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology. 2007;33:867–876. doi: 10.1038/sj.npp.1301468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, Windey K, Tremaroli V, Backhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111(42):E4485–4493. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis. 2013;26(1):17–25. doi: 10.1097/QCO.0b013e32835ba6b1. [DOI] [PubMed] [Google Scholar]
- 184.Bachtell R, Hutchinson MR, Wang X, Rice KC, Maier SF, Watkins LR. Targeting the Toll of Drug Abuse: The Translational Potential of Toll-Like Receptor 4. CNS Neurol Disord Drug Targets. 2015;14(6):692–699. doi: 10.2174/1871527314666150529132503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ray LA, Roche DJ, Heinzerling K, Shoptaw S. Opportunities for the development of neuroimmune therapies in addiction. Int Rev Neurobiol. 2014;118:381–401. doi: 10.1016/B978-0-12-801284-0.00012-9. [DOI] [PubMed] [Google Scholar]
- 186.Villar-Garcia J, Hernandez JJ, Guerri-Fernandez R, Gonzalez A, Lerma E, Guelar A, Saenz D, Sorli L, Montero M, Horcajada JP, Knobel Freud H. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: a double-blind, randomized, placebo-controlled trial. J Acquir Immune Defic Syndr. 2015;68(3):256–263. doi: 10.1097/QAI.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 187.Gonzalez-Hernandez LA, Jave-Suarez LF, Fafutis-Morris M, Montes-Salcedo KE, Valle-Gutierrez LG, Campos-Loza AE, Enciso-Gomez LF, Andrade-Villanueva JF. Synbiotic therapy decreases microbial translocation and inflammation and improves immunological status in HIV-infected patients: a double-blind randomized controlled pilot trial. Nutr J. 2012;11:90. doi: 10.1186/1475-2891-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gori A, Rizzardini G, Van’t Land B, Amor KB, van Schaik J, Torti C, Quirino T, Tincati C, Bandera A, Knol J, Benlhassan-Chahour K, Trabattoni D, Bray D, Vriesema A, Welling G, Garssen J, Clerici M. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the “COPA” pilot randomized trial. Mucosal Immunol. 2011;4:554–563. doi: 10.1038/mi.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163–172. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chiu YH, Tsai JJ, Lin SL, Lin MY. Lactobacillus casei MYL01 modulates the proinflammatory state induced by ethanol in an in vitro model. J Dairy Sci. 2014;97(4):2009–2016. doi: 10.3168/jds.2013-7514. [DOI] [PubMed] [Google Scholar]
- 191.Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, Hernandez RD, Lederman MM, Huang Y, Somsouk M, Deeks SG, Hunt PW, Lynch SV, McCune JM. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5:193ra191. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]