Abstract
White matter (WM) occupies a large volume of the human cerebrum and is mainly composed of myelinated axons and myelin-producing glial cells. The myelinated axons within WM are the structural foundation for efficient neurotransmission between cortical and subcortical areas. Similar to neuron-enriched gray matter areas, WM undergoes a series of changes during the process of aging. WM malfunction can induce serious neurobehavioral and cognitive impairments. Thus, age-related changes in WM may contribute to the functional decline observed in the elderly. In addition, aged WM becomes more susceptible to neurological disorders, such as stroke, traumatic brain injury (TBI), and neurodegeneration. In this review, we summarize the structural and functional alterations of WM in natural aging and speculate on the underlying mechanisms. We also discuss how age-related WM changes influence the progression of various brain disorders, including ischemic and hemorrhagic stroke, TBI, Alzheimer’s disease, and Parkinson’s disease. Although the physiology of WM is still poorly understood relative to gray matter, WM is a rational therapeutic target for a number of neurological and psychiatric conditions.
Keywords: Aging, white matter, stroke, traumatic brain injury, neurodegeneration, myelin, axon
1. Introduction
The central nervous system (CNS) has long been divided into gray and white matter based on the appearance of human brain tissue at autopsy. Gray matter is mainly composed of neuronal cell bodies, dendrites, axons, and glial cells and serves to process electrical and chemical signals for the purpose of interneuronal communication. White matter (WM) is mostly composed of bundled myelinated or unmyelinated axons and myelin-producing glial cells, among other glial cell types. WM volume has expanded during primate evolution (Hofman, 2014) such that WM occupies almost 50% of total cerebral volume in humans (Zhang and Sejnowski, 2000). In turn, most of the space within WM (up to 87%) is occupied by myelinated axons (Wang et al., 2008). WM is essential for the transmission of electrical signals across different brain regions, and WM malfunction can therefore lead to serious neurobehavioral and cognitive impairments (Bennett and Madden, 2014).
As with other organs, the brain undergoes a series of structural and functional changes during the aging process. Longitudinal and cross-sectional imaging studies have reported smaller global brain volumes (Driscoll et al., 2009; Seidler et al., 2010), reduced cortical thickness (Salat et al., 2004), and expansion of the ventricular system (Scahill et al., 2003) in the brains of older adults. Cerebral pathologies such as WM lesions, infarction, and cerebral microbleeds are also more common in older brains (Salat et al., 2004). As anatomical structure reflects physiological function, it is not surprising that many brain functions are also affected by aging. For example, declines in motor abilities (Seidler et al., 2010), sensory function (Brodoehl et al., 2013), and cognitive skills have all been observed with natural aging. Along with these functional declines, the cerebral levels of neurotransmitters such as dopamine (Wenk et al., 1989), acetylcholine (Gottfries, 1990), serotonin (Gottfries, 1990), and norepinephrine (Mei et al., 2015), and neurotrophic factors such as brain-derived neurotrophic factor (BDNF) (Terry et al., 2011) and nerve growth factor (NGF) (Zeng et al., 2011) are dramatically reduced in aging brains.
Although a number of age-related cerebral changes have been identified, the identity of those changes directly responsible for age-related declines in function remains controversial. It was previously held that the functional decline in aging brains was associated with significant and progressive neuronal loss in gray matter (Colon, 1972). However, this view has been challenged by Pakkenberg and Gundersen, who used an unbiased stereological approach to estimate the precise number of cortical neurons in three dimensions (Pakkenberg and Gundersen, 1997). They reported only 10% loss in the total number of neurons in the cortex of both sexes from 20 to 90 years of age. Subsequent studies further confirmed that the age-related decline in cognitive function is not accompanied by robust neuronal loss (Morrison and Hof, 1997). Instead, normal aging led to a reduction in WM volume by as much as 28% (Pakkenberg and Gundersen, 1997). With the advent of advanced imaging methods (to be discussed in section 2), particularly diffusion tender imaging (DTI), higher resolution visualization of WM pathways has become possible. Thus, many studies have explored WM alterations in aging brains and have identified WM atrophy (Lemaitre et al., 2005), WM tract disruption (Shenkin et al., 2005), vessel impairments (Pantoni, 2002), increased inflammation (Sloane et al., 1999), and loss of myelination (Marner et al., 2003). More importantly, these age-related changes have been associated with functional deficits, such as sensorimotor (Fleischman et al., 2015) and cognitive impairments (Kohama et al., 2012) and psychiatric disorders (Fields, 2008). Indeed, age-related changes in WM have been shown to influence the pathogenesis and progression of many brain diseases.
In this review, we summarize current knowledge of age-related structural and functional alterations of WM and speculate on the underlying mechanisms. We also discuss the influence of age-related changes in WM on the progression of various neurological conditions, including stroke, traumatic brain injury (TBI), Alzheimer’s disease (AD), and Parkinson’s disease (PD). Despite progress in our understanding of WM structure and function, the physiology and pathology of WM changes with aging are still poorly understood compared to gray matter because of the greater historical emphasis on neurons. Nevertheless, it has become evident that WM is a valid therapeutic target for a number of brain injuries and disease states.
Search Criteria
In the present review, PubMed was used to systematically identify studies investigating WM alterations in normal aging and various pathological contexts, including stroke, TBI, neurodegenerative diseases, MS, and schizophrenia. The search strategy was restricted to original studies and reviews published in English up to August 30, 2016. Search terms included (i) aging, older adults, or elderly; (ii) cerebral; brain; (iii) magnetic resonance imaging (MRI), fluid-attenuated inversion recovery (FLAIR), diffusion tensor imaging (DTI), positron emission tomography (PET), functional MRI (fMRI); (iv) Stroke; ischemia; hemorrhage; intracerebral hemorrhage (ICH) intraventricular hemorrhage (IVH); subarachnoid hemorrhage (SAH); Alzheimer disease (AD); Parkinson’s disease (PD); Huntington’s disease (HD); multiple sclerosis (MS); schizophrenia. The selected studies were screened for content to assure compliance with the aforementioned inclusion/exclusion criteria. A total of 199 publications were examined in this manner.
2. Emerging neuroimaging tools to detect age-related white matter alterations
In the middle of the 20th century, magnetic resonance imaging (MRI) measurements were developed as neuroimaging tools to identify and study WM alterations. The field of MRI has exploded since its introduction and MRI is now used to aid in clinical diagnoses.
Traditional MRI methods include T1-weighted sequences and T2-weighted sequences. T1-weighted sequences are often used to acquire high-resolution structural images of brain anatomy that typically maximize the contrast between gray matter, WM, and cerebrospinal fluid (CSF), and can thereby reveal the sizes of cortical and subcortical structures. In contrast, T2-weighted sequences are useful in detecting pathological lesions, such as damaged WM. For example, edema often accompanies pathological lesions and presents as a hyperintensity in T2 sequences. FLAIR (fluid-attenuated inversion recovery) MR images are T2-weighted images in which the CSF signal is suppressed in order to distinguish lesioned tissue from CSF. Due to this advantage, FLAIR is widely used to detect abnormalities of cerebral WM, including WM hyperintensities (Caligiuri et al., 2015) and WM lesions (WMLs) (Barkhof and Scheltens, 2002). Functional MRI (fMRI) is another type of T2-weighted imaging that is sensitive to the ratio of oxygenated to deoxygenated hemoglobin in cerebral blood. This ratio is strongly associated with neuronal activity. Thus, fMRI has been extensively used to report neural activity in clinical studies, including studies of structural and functional connectivity between brain regions.
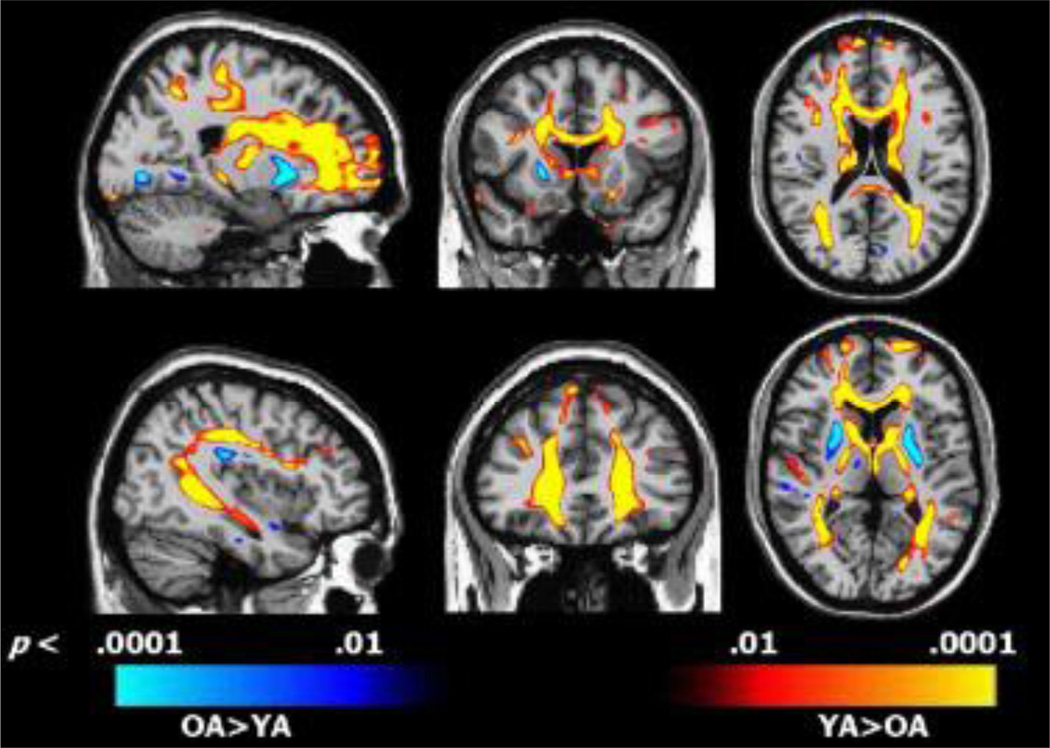
Another versatile MRI method that is widely used to study WM alterations is DTI. Taking advantage of the observation that water diffuses differently within distinct brain regions, DTI measures microscopic diffusion of water molecules in various brain tissues to infer microstructural integrity (Pierpaoli et al., 1996). In fluid-filled areas such as the cerebral ventricles, diffusion is rarely bounded, and therefore non-directional. Similarly, in gray matter, water diffusion is also relatively non-directional because the non-uniform microstructures of neuronal cell bodies and dendrites restrict the movement of water molecules. In WM, however, water diffusion is faster in the direction parallel to WM fibers than in the perpendicular direction, due to anatomical restriction by the parallel bundles of axonal membranes and myelin sheaths. In other words, compared with gray matter, diffusion in WM is far more directional (Le Bihan, 2003). As a result, the rate and directionality of water diffusion within WM can be used to measure the integrity of WM. Fractional anisotropy (FA) is a parameter indexing the restricted proportion of total diffusion. Higher FA values indicate increased diffusion directionality, suggesting higher WM integrity (Figure 1A). Mean diffusivity (MD) is a scalar measure that indexes the average rate of diffusion. Higher MD values within WM often suggest impaired WM integrity. Furthermore, the rate of diffusion along the primary (axial diffusivity, AD) and secondary (radial diffusivity, RD) axes can also be used to measure WM integrity. As reviewed by Bennett and Madden (Bennett and Madden, 2014), AD elevation is more sensitive to axonal disruptions while RD alterations are more sensitive to myelin breakdown (Figure 1). In addition to inferring WM integrity, DTI can also be applied to reconstruct cerebral WM tracts. By using DTI-based tractography, Catani and Ffytche published a map of the major WM tracts within the brain (Catani and ffytche, 2005). DTI-based tractography has been widely applied to investigate the correlation between structural and functional connectivity, i.e. to identify specific tracts involved in multiple cerebral functions. However, DTI-based tractography also suffers from a few drawbacks. For example, DTI is based on measurements of water diffusion within tracts and is therefore not a direct measure of structural connectivity (Jones et al., 2013). Furthermore, structural and functional connectivity are not identical because tight functional connections may exist between areas with limited structural connections (Honey et al., 2009).
Figure 1. Differences between young adult (YA) and old adult (OA) in fractional anisotropy (FA) overlaid on representative sagittal (left), coronal (middle), and axial (right) DTI images.
Regions depicted in red–yellow indicate areas where FA was lower in OA compared to YA; regions depicted in blue indicate areas where FA was higher in OA compared to YA. Reprinted from Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Ziegler DA1, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Neurobiol Aging. 2010 Nov;31(11):1912–26 (Ziegler et al., 2010). Copyright (2010), with permission from Elsevier.
3. White matter changes in the normal aging brains
According to the National Institute on Aging, normal aging is defined as all the changes that occur over the course of the life of an otherwise healthy person and can vary dramatically across individuals. Normal aging can start quite early and leads to changes in every major organ, such that threats to health generally take a bigger toll on older bodies. Normal aging may not be caused by active gene programming but by limitations in homeostatic maintenance of organ systems that culminate in an accumulation of damage (Kirkwood, 2005). The most common theories to explain normal aging have invoked slow increases in DNA damage over time, progressive loss of protein quality control, and/or loss of the regenerative potential of stem cells. In terms of brain fitness, normal aging is usually accompanied by some decline in cognitive functions, such as quantitative reasoning and perceptual speed, which may be associated with loss of WM volume and integrity in areas such as the prefrontal cortex (Caserta et al., 2009). However, verbal fluency and semantic memory do not decline with normal aging, perhaps because these skills are highly dependent on past experience. The impact of age-related structural changes in WM on cognitive functions is described in further detail below.
3.1 Age-related macrostructural WM changes
A variety of age-related macrostructural WM changes, including reduced WM volume, WM lesions, disrupted WM integrity, and subsequent cortical disconnection have all been observed in normal aging brains (Caligiuri et al., 2015). Remarkably, WM volume gradually increases in the first 40 years of life, peaks at around 50 years of age, and then decreases rapidly from 60 years of age onwards (Liu et al., 2016). Even with healthy aging, WM lesions (also known as leukoaraiosis) are evident as WM hyperintensities on T2-weighted MRI. WM lesions in various anatomical locations may be responsible for distinct types of functional decline. For example, WM lesions in the frontal lobe are responsible for a number of cognitive impairments in the speed of information processing, visual-motor function, verbal fluency, classification, and mental sequences (Bartres-Faz et al., 2001). Subcortical WM lesions are mainly correlated with depression in the elderly whereas periventricular WM lesions are mainly related to cognitive decline. Similarly, loss of WM integrity and subsequent cortical disconnection were reported in normal aged subjects and are correlated with reduced cognitive function (Bennett and Madden, 2014).
The influence of gender on WM changes in normal aging brains has been explored with an emphasis on loss of WM volume and integrity. Almost all studies have shown an absence of an interaction between age and gender in WM volume and therefore suggest a lack of gender differences in age-related WM volume loss (Inano et al., 2013; Liu et al., 2016; Ryan et al., 2014). However, the effect of gender on aging-induced loss of WM integrity remains controversial. Some studies claim an absence of gender differences in the WM aging process (Inano et al., 2011; Kodiweera et al., 2016), while other studies report gender differences in WM aging rates (Abe et al., 2010; Kumar et al., 2013). It is further noteworthy that gender differences in age-related loss of WM integrity are topographically organized. For example, in the right inferior fronto-temporal lobe, a faster decline in WM integrity is observed in men compared to women (Abe et al., 2010). However, females show faster WM integrity loss in the cerebellar, temporal, and frontal cortices (Kumar et al., 2013). Moreover, elderly women with the estrogen receptor 1 allele exhibit significantly smaller WM lesions, suggesting a protective role of estrogen against age-related WM impairments (Ryan et al., 2014). Nevertheless, gender differences in WM aging and the underlying mechanisms remain poorly understood and further investigations are warranted.
3.2 Age-related vascular changes
A set of age-related vascular alterations probably contributes to the increased vulnerability of aged WM to hypoperfusion. First, WM is intrinsically more vulnerable to hypoperfusion because the major blood supply for WM is via long arterioles that arise from the border zone between the middle cerebral artery and the anterior cerebral artery (Moody et al., 1990). Second, tortuous arterioles are known to form in aged WM (Brown et al., 2002) and assume a coiled shape in the cavity where brain parenchyma is lost (Moody et al., 1991). This also contributes to a decline in blood flow to WM because there is an increase in vessel length and a loss of kinetic energy in the tortuous or coiled arterioles. Third, excess collagen is deposited in the walls of veins and venules in the deep WM of aged brains (Brown et al., 2002; Moody et al., 1995), which may lead to decreased WM blood flow by constriction of the vessel lumen. Thickened venous walls resulting from collagen deposition also delay the removal of toxins via the blood stream and thereby impair perivascular drainage (Rennels et al., 1990). As a result of these changes, toxin levels may rise in aged WM, leading to inexorable WM damage. Lastly, a decline in vascular density (Klein and Michel, 1977) and impaired autoregulation in the cerebral vascular system (van Beek et al., 2008) may also exacerbate hypoperfusion in aged WM. Such hypoperfusion may result in WM ischemia, which in turn would lead to further WM damage.
3.3 Age-related changes in myelin and myelinated axons
Cerebral WM is characterized by the presence of myelin. Myelin, the outgrowth of the mature oligodendrocyte, is composed of ~70% lipid and ~30% protein. Due to its insulating properties, myelin dramatically accelerates information transfer in the brain (Baumann and Pham-Dinh, 2001). The myelin surrounding axons is anchored to the axolemma by junctional complexes located in paranodes (Sugiyama et al., 2002) and is divided into segments by small unmyelinated regions called the nodes of Ranvier. The nodes of Ranvier permit the action potentials in myelinated axons to spread in a saltatory manner, thereby increasing conduction speed by as much as 100 fold (Filley and Kleinschmidt-DeMasters, 2001). Discrete clustering of ion channels at the nodes of Ranvier is also essential for efficient action potential conduction (Hinman et al., 2006). The remarkable increase in conduction velocity with myelination is essential for efficient communication between topographically separated brain regions. As expected, higher myelination levels have consistently been shown to be associated with higher processing speeds (Kochunov et al., 2010).
The intact structure of myelin and of the axons it serves to ensheathe are the basis for efficient axon potential conduction. In aged rats, there is increased splitting of the myelin sheath, myelin balloon formation, and separation from the axon (Sugiyama et al., 2002). These pathological changes may underlie age-related cognitive decline from loss of efficient interneuronal communication. As with myelin, axons in WM also undergo a series of age-related changes. First, the integrity of paranode, which anchors the myelin to the axolemma and forms a diffusion barrier in the paranodal region, may be disrupted with aging (Sugiyama et al., 2002). This disruption may be attributed to the absence of the 21.5-kDa isoform of myelin basic protein (MBP) (Sugiyama et al., 2002) or age-related dysregulation of cyclic nucleotide phosphodiesterase (CNP), two proteins that are vital for the formation and maintenance of paranodes. Second, aged monkeys exhibit myelin alterations that result in reorganization of the cluster of ion channels at the nodes of Ranvier (Hinman et al., 2006), which would be detrimental to axonal conduction. Third, the internodal length decreases with aging (Mukoyama, 1973), which may also result in less efficient axon potential conduction. Finally, the threshold for axonal conduction increases and the slope of the stimulus-response curve decreases with aging, reflecting less overall neuronal excitability (Jankelowitz et al., 2007). Taken together, these pathological changes in myelin and axons are detrimental for nerve pulse conduction in aging brains.
3.4 Age-related changes in myelin-producing oligodendrocytes
Oligodendrocytes are myelin-producing cells in the CNS. Loss or dysfunction of oligodendrocytes is associated with increased myelin breakdown. Oligodendrocyte progenitor cells (OPCs) are present in normal adult brains and can differentiate into mature oligodendrocytes. Impairment of OPC recruitment or differentiation is also strongly correlated with reduced myelination. Degeneration of oligodendrocytes and OPCs increases with aging (Kohama et al., 2012), leading to increased myelin breakdown, decreased remyelination, fluctuations in the constituents of myelin and ultimate disruptions in WM integrity during normal aging (Hinman et al., 2006).
Lactate is important for the normal function of oligodendrocytes. During myelination, the myelin-producing oligodendrocytes require an abundant supply of energy to maintain homeostasis. Oligodendrocytes may use lactate from the blood and/or astrocytes as a source of energy and material to produce fatty acids for myelin synthesis (Rinholm and Bergersen, 2014). After myelination is complete, mature oligodendrocytes release lactate through the myelin sheath. In addition, myelin maintenance requires breakdown of fatty acids, during which lactate might be generated (Rinholm and Bergersen, 2014). Therefore, oligodendrocytes may support axons metabolically by providing lactate as a nutrient to neighboring neurons (Rinholm and Bergersen, 2014). Blocking lactate transport from oligodendrocytes to neurons through knockdown of monocarboxylate transporter 1 (MCT1) results in abnormal axon morphology and neuronal death, indicating a crucial role of oligodendrocyte-derived lactate for maintenance of axonal viability (Lee et al., 2012). Age-related decreases in lactic acid in the hippocampus have been reported in the senescence-accelerated mouse, which might be linked with cognitive impairments (Jankelowitz et al., 2007). Further studies are needed to determine if disruptions in the lactate supply with aging result in WM alterations.
3.5 Age-related changes in other glial cells
WM glial cells, including microglia and astrocytes, are also critical for the normal function of WM. Microglia serve as the resident immune cells within WM. They are essential for the maintenance of WM homeostasis under physiological conditions (Rawji and Yong, 2013). Astrocytes within WM are also believed to play a vital role in myelination. Astrocytes promote myelination and myelin maintenance by clearing extracellular ions and neurotransmitters and by releasing pro-myelination factors (Lundgaard et al., 2014). The functions of activated microglia and astrocytes under pathological conditions are complex, as they can be both detrimental and beneficial, depending partly upon their activation phenotypes. The detrimental functions include toxicity to oligodendrocyte lineage cells, release of inflammatory cytokines and free radicals, and recruitment and activation of peripheral immune cells into the CNS. In contrast, the beneficial effects include clearance of inhibitory myelin debris and the release of neurotrophic factors. Microglia and astrocytes can profoundly influence WM integrity in the following ways: 1) Mitogen and various growth factors derived from both microglia and astrocytes are essential for the survival, proliferation, or maturation of OPCs. These growth factors include nerve growth factor (NGF), neurotrophic-3 (NT-3) (Rawji and Yong, 2013), insulin-like growth factor (IGF-1), fibroblast growth factor (bFGF) from microglia, and bFGF and plate-derived growth factor (PDGF) from astrocytes (Clemente et al., 2013); 2) Iron is essential for the proliferation and maturation of OPCs. Microglia are one of the main sources of iron for OPCs. Astrocytes play a crucial role in the distribution of iron via the iron exporter ferroportin (Clemente et al., 2013). It is noteworthy that abnormally high iron levels may induce microglial activation, thereby resulting in elevated reactive oxygen species (ROS) and subsequent oligodendrocyte death. However, proinflammatory cytokines from activated microglia may also enhance astrocyte-derived growth factors, which promote OPC proliferation and maturation (Clemente et al., 2013). 3) The inflammatory factors and ROS from microglia and astrocytes may play dual roles in OPC differentiation and maturation. On one hand, several inflammatory factors, including TNF-α, IFN-γ, and ROS, are known to be toxic to oligodendrocyte lineage cells. On the other hand, inflammatory cytokines from microglia, such as IL-1β and TNF- α, may also induce astrocytic production of growth factors, which promote the survival and proliferation of OPCs (Clemente et al., 2013). Furthermore, bone morphogenetic proteins (BMPs) secreted by astrocytes inhibit the maturation of OPCs, whereas a series of chemokines derived from astrocytes, including CXCL1, promote the proliferation of OPCs (Clemente et al., 2013); 4) Microglia can promote OPC differentiation by the clearance of myelin debris and extracellular toxins such as Aβ (Clemente et al., 2013). 5) Astrocytes can facilitate myelination by providing lactate and cholesterol (Kiray et al., 2016).
Age-related changes in astrocytes have been studied for many years. Early studies revealed changes in morphology (Diniz et al., 2010), protein expression (Salminen et al., 2011) and density of transporters (Barreto et al., 2011) in astrocytes from aging brains. Ultrastructural studies of astrocytes in the aged brain demonstrate abnormal accumulations of proteins, such as lipofuscin and intermediate filament bundle (Salminen et al., 2011). In addition, astrocytes isolated from aged brains display a proinflammatory phenotype with increased production of inflammatory factors and free radicals (Garcia-Matas et al., 2008). In contrast, the release of glutathione (Rice and Russo-Menna, 1998) and trophic factors (e.g. bFGF, GDNF, etc.), which are protective for WM oligodendrocytes and axons, is reduced in aged astrocytes (Deierborg et al., 2008; Lin et al., 1993; Saavedra et al., 2006). Aged brains exhibit a decrease in the ability of astrocytes to remove toxic molecules from the extracellular medium, including α-synuclein (Braak et al., 2007; Lee et al., 2010; Song et al., 2009) and glutamate (Morales et al., 2013; Rodriguez et al., 2015). Notably, one study documented an increase in astrocyte activation in the aged brain, and this was accompanied by a significant decline in the expression of myelin proteins (Bates et al., 2013). This study suggests an important relationship between astrocyte activation and WM alterations in the aging brain.
Normal aging also affects microglial behavior. Microglial density greatly increases in WM of aged brains, while remaining stable in gray matter. Furthermore, age-related decreases in myelin proteins are linked to increased activation of microglia (Bates et al., 2013). It is widely held that microglia are primed toward a pro-inflammatory state and contribute to deficits in aged brains (Norden and Godbout, 2013). However, this concept was recently challenged by the demonstration of downregulation of neurotoxic pathways and upregulation of neuroprotective pathways in aged microglia (Hickman et al., 2013). The functional impact of age-related and region-dependent microglial changes on WM structure or function remain to be established.
4. Age-related white matter changes in stroke
Stroke is a common cerebral disease affecting mostly the elderly. In the past few decades, many neuroprotective drugs have shown promise in preclinical animal models but failed in the clinic (Gladstone et al., 2002). One potential explanation of these failures is that preclinical studies focus almost exclusively on protection of gray matter, whereas WM viability is not addressed by most therapeutic strategies, even though WM accounts for much of the lesion volume. Indeed, WM injury is an integral part of most human stroke events, accounting for about half of the lesion volume on average (Ho et al., 2005). As WM plays an essential role in transmitting signals and coordinating communication between brain regions, WM injury in stroke is expected to profoundly disrupt sensorimotor and cognitive function and elicit neurobehavioral syndromes (Schmahmann et al., 2008). It is therefore not surprising that the severity of WM injury dictates long-term motor deficits and cognitive decline in stroke-afflicted patients (Correa et al., 2011; Fu et al., 2005; Oksala et al., 2009). Thus, a better understanding of the mechanisms of ischemic and hemorrhagic WM injury may facilitate the development of strategies that protect both gray matter and WM damage after stroke.
Aging can influence the pathogenesis of stroke from many directions. First, age-related WM changes have been reported to increase the risk of stroke, particularly subcortical stroke and recurrent strokes, and elevate post-stroke mortality (Oksala et al., 2009). The rate of symptomatic intracerebral hemorrhage after thrombolysis is increased in older patients with severe WM changes and multiple lacunes (Xiong and Mok, 2011). Second, aged WM is more susceptible to ischemic injury after stroke. In the acute stages after WM stroke, aged brains exhibit enlarged infarct volumes, severe WM damage, and greater oligodendrocyte death compared to younger brains (Rosenzweig and Carmichael, 2013). Finally, aged animals have lower intrinsic capacity for WM repair. Thus, remyelination efficiency declines with aging due to impairments in oligodendrocyte progenitor recruitment and differentiation (Sim et al., 2002). Furthermore, axonal regrowth is also retarded in aged brains (Lamoureux et al., 2010). As a result, the aged brain suffers more severe WM atrophy and axonal degeneration, and worse long-term sensorimotor and cognitive deficits after stroke (Rosenzweig and Carmichael, 2013; Suenaga et al., 2015).
4.1 Age-related white matter changes in ischemic stroke
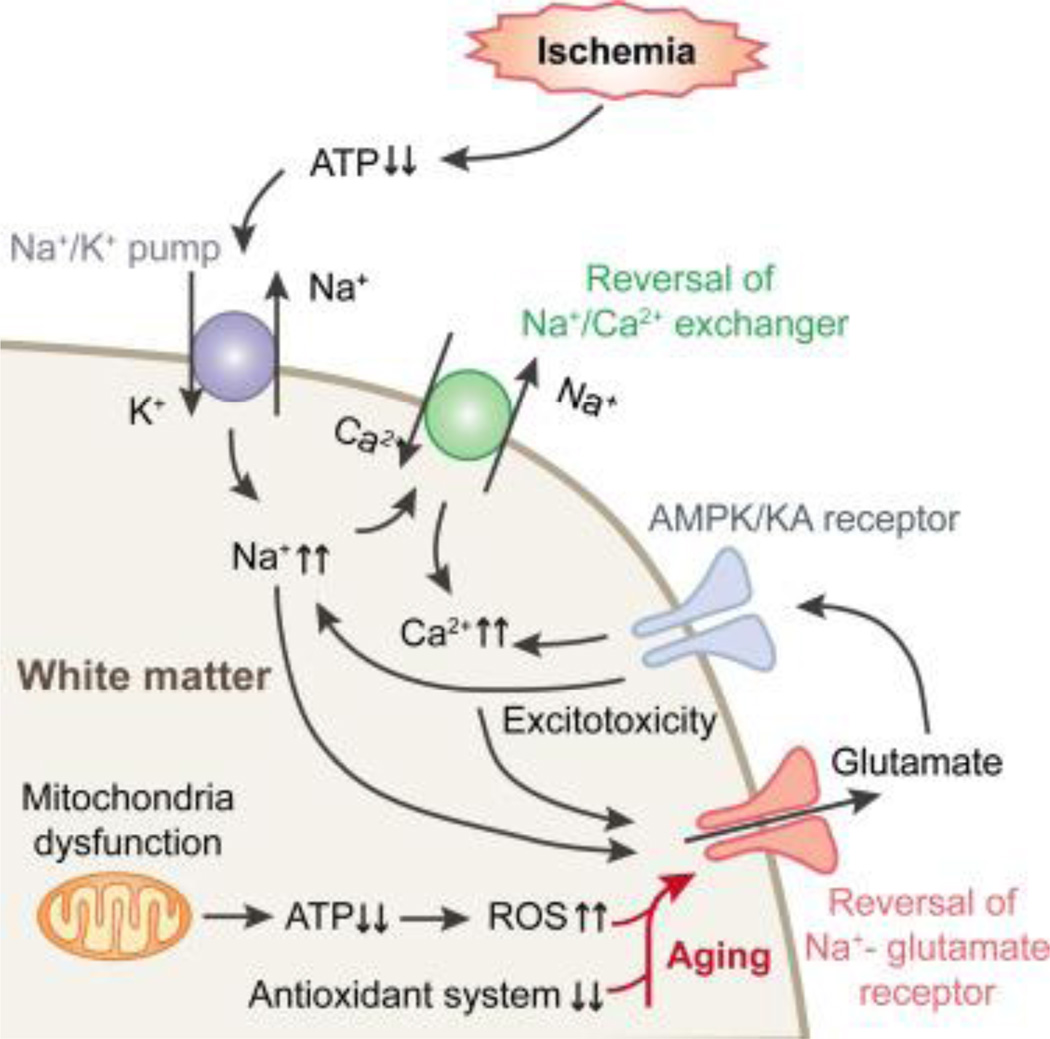
Studies investigating the molecular mechanisms underlying loss of WM integrity after ischemic stroke have shown that WM deterioration in older mice is mainly attributed to Ca2+-independent excitotoxicity after ischemia (Baltan, 2009). Ischemic injury is thought to initially stem from a lack of ATP, the energy currency of the cell. This may result in reversal of the Na+/Ca2+ exchanger, followed by increased intracellular Na+ and subsequent reversal of the Na+ dependent glutamate transporter (GLT1). The resulting glutamate overload might overactivate AMPA/kainate receptors. Furthermore, excess glutamate competes with cysteine at the glutamate-cysteine pump, resulting in eventual glutathione depletion and the production of toxic free radicals. All these changes converge to elicit the death of oligodendrocytes and disruption of the axon in WM.
GLT-1 is the principal transporter that removes glutamate and inhibits glutamate neurotoxicity. The expression of GLT-1 greatly increases in multiple cellular components in aged WM, which would be expected to counteract the elevated levels of glutamate and glutamate synthetase in aged WM. However, ischemic injury is known to induce reversal of the Na-glutamate transporter. Thus, higher expression of GLT-1 would then result in more rapid and robust glutamate release during ischemia and severe excitotoxicity in aged WM (Baltan, 2009; Baltan et al., 2008).
Excessive oxidative stress is also likely to aggravate WM injury in the aged brain (Li et al., 2011). Mitochondria are the main source of ROS after ischemia. Mitochondria are highly abundant within axons, making axons one of the main targets of oxidative stress follow ischemia (Lipton, 1999). Age-related mitochondrial dysfunction results in reduced ATP production and excessive oxidative stress. In addition, the function of endogenous antioxidant systems such as glutathione peroxidase declines with aging (Espinoza et al., 2008). All these changes increase axonal and oligodendrocyte vulnerability in aged brains and exacerbate WM injury upon ischemia (Figure 2).
Figure 2. Molecular mechanisms underlying age-related deterioration of WM after stroke.
Ischemic injury leads to ATP depletion and reversal of the Na+/Ca2+ exchanger. This is followed by increased intracellular Na+ and subsequent reversal of the Na+ dependent glutamate transporter (GLT1). The resulting glutamate overload overactivates AMPA/kainate receptors. 1) In aged WM, the expression of GLT-1 greatly increases in multiple cellular components, resulting in more rapid and robust glutamate release and severe excitotoxicity during ischemia. Excessive oxidative stress is also likely to aggravate WM injury in the aged brain. Mitochondria are the main source of reactive oxygen species (ROS) after ischemia. 2) Age-related mitochondrial dysfunction results in reduced ATP production and excessive oxidative stress. 3) The functions of endogenous antioxidant systems decline with aging in parallel with the increase in mitochondrial impairments. All these changes converge to increase axonal and oligodendrocyte vulnerability in aged brains and exacerbate WM injury upon ischemia.
In addition to the myelin-producing oligodendrocytes, other glial cells—especially microglia—also contribute to age-related aggravation of ischemic WM damage. Aged brains express higher levels of chemokines and cytokines, including MCP-1 and TNF-α, after WM injury, and these chemical mediators efficiently recruit microglia and macrophages (Rosenzweig and Carmichael, 2013). Microglia of the aged brain are primed to remain in an activated state and become resistant to regulation (Norden and Godbout, 2013). Furthermore, aging also affects microglia/macrophage phenotypic profiles. It is widely accepted that microglia/macrophages are not a uniform cell population but polarize into a spectrum of phenotypes at different stages after brain injuries. These phenotypes have been reported to exert distinct roles in WM injury and repair. Specifically, M1 (classically activated) microglia/macrophages exacerbate ischemic WM injury while M2 (alternatively activated) microglia/macrophages reduce ischemic WM injury (Hu et al., 2015). In addition, the M2 microglial phenotype promotes the differentiation of oligodendrocyte precursor cells into mature myelinating cells and is therefore essential in the process of remyelination (Miron et al., 2013). Notably, impaired microglial M2 polarization has been reported in aged brains, which might partially account for the age-related decline in WM integrity (Suenaga et al., 2015).
4.2 Age-related white matter changes in hemorrhagic stroke
Cellular and molecular mechanisms underlying WM injury in hemorrhagic stroke have been intensively investigated. Mechanical mass effects, inflammation, and edema around the hematoma are the main contributors to WM injuries in this condition (Hatakeyama et al., 2013; Zhao et al., 2014). Cellular toxicity of blood components and the degradation products of heme also promote acute and delayed hemorrhagic WM injuries. WM disruption after hemorrhagic stroke might be be exacerbated in the aging brain The aged brain is known to suffer from greater iron overload after hemorrhagic stroke and consequently higher production of ROS and inflammatory mediators, all of which may explain greater WM injury in the elderly (Hatakeyama et al., 2013; Williams et al., 2012). Furthermore, erythrocytes become much more fragile with aging, which may result in easier hemolysis after hemorrhage and subsequent release of heme and its toxic byproducts (Detraglia et al., 1974). Heme degradation by heme oxygenase (HO) may also release free iron and this may be increased in the elderly because HO levels are higher in aged brains (Gong et al., 2004). Similarly, complement activation is also higher in aged brains (Gong et al., 2008), which may further promote erythrocyte lysis (Hua et al., 2000). In addition to iron, thrombin is another major player in brain injury after ICH (Hua et al., 2007), as it converts soluble fibrinogen into insoluble fibrin, among other processes in the clotting cascade. Indeed, blood coagulation rates are accelerated with aging (Ibbotson et al., 1992), resulting in increased production of thrombin, which might exacerbate WM injuries in older brains. However, the precise roles of iron and thrombin in hemorrhagic stroke-induced WM injury in aging populations need to be further validated.
5. Age-related white matter changes in TBI
WM injury is commonly observed after TBI (Armstrong et al., 2016) and, as after ischemic stroke (see above), may account for more than half of the lesion volume (Hulkower et al., 2013). Pathological changes of WM after TBI mainly include traumatic axonal injury and demyelination (Armstrong et al., 2016), which might disrupt signal transmission and lead to poor neurological outcomes (Kinnunen et al., 2011; Spitz et al., 2013). Recent studies have shown that aging influences TBI prognosis. For example, elderly subjects exhibit worse outcomes after TBI than young adults, with regard to hospitalization, mortality, and functional disability rates (Mosenthal et al., 2004; Rutland-Brown et al., 2006; Thompson et al., 2006). Recently, studies from a number of laboratories have unveiled underlying mechanisms that might account for age-related increases in vulnerability to TBI. These studies mainly focused on increased oxidative damage (Shao et al., 2006), impaired neuroprotective responses (Shimamura et al., 2004), worse blood brain barrier damage (Onyszchuk et al., 2008), and excessive pro-inflammatory responses in the thalamus (Sandhir et al., 2004), hippocampus (Sandhir et al., 2008), and cortex (Sandhir and Berman, 2010). However, the relationship between WM injury in TBI and aging has not been widely investigated. One study demonstrated that advanced age is associated with greater WM lesion volume following TBI (Schonberger et al., 2009). Some studies indicate that WM in aged subjects is more vulnerable to TBI-associated impairments and exhibits slower recovery from TBI. For example, a decline in WM volume, along with dramatic loss of myelinated fibers, is often observed in normal aging (Marner et al., 2003; Tang et al., 1997). Thus, the aging process leaves unmyelinated fibers especially vulnerable after TBI (Reeves et al., 2005).
A decline in the rate of myelin debris clearance has been reported in the WM of aged patients; this decline can decelerate the rate of WM restoration after an insult (Linehan et al., 2014). Myelin debris generated during demyelination inhibits axon regeneration through myelin-associated molecules (Geoffroy and Zheng, 2014) and activates microglia/macrophages to promote inflammatory responses (Clarner et al., 2012). In addition, myelin debris interferes with the differentiation of oligodendrocyte progenitor cells into oligodendrocytes and therefore retards the process of remyelination after TBI (Baer et al., 2009). Further investigations of the interaction between aged WM and susceptibility to TBI as well as the underlying mechanisms are warranted.
6. Aging of white matter in neurodegenerative diseases
The phrase neurodegenerative diseases (NDs) is an umbrella term for a range of conditions with progressive loss of neuronal structure and function. Common NDs include AD, frontotemporal dementia, amyotrophic lateral sclerosis, PD, and HD. NDs are believed to stem from pathological protein aggregations, which lead to neuronal death, gross dysfunction of entire brain regions, and eventual clinical disability. It has long been known that age is the greatest risk factor for NDs. It is therefore not surprising that there is a strong correlation between the aging of WM and the risk for acquiring NDs. Studies of age-related WM alterations in NDs, with special emphasis on AD and PD, are reviewed below.
6.1 Aging of white matter in Parkinson’s disease
PD is the second most common ND worldwide, and is featured by bradykinesia and at least one of the following symptoms—tremor, rigidity, and postural instability. PD is believed to result in part from pathological aggregations of the synaptic protein α-synuclein in many brain regions, ranging from the olfactory bulb to the brainstem (Braak et al., 2003). These protein aggregations form the hallmark Lewy bodies and Lewy neurites that characterize PD. The motor deficits of PD are thought to emerge following loss of dopaminergic neurons in the substantia nigra, pars compacta (SNpc) (Damier et al., 1999). There is also neuronal loss in the neighboring ventral tegmental area (VTA) of the midbrain. Studies of PD therefore mainly focus on neural degeneration within the SNpc and VTA. The SNpc and VTA send largely unmyelinated efferents to the dorsal and ventral striatum. However, accumulating imaging-based evidence also demonstrates the presence of WM changes in PD and a correlation between WM changes and PD-associated functional decline, as reviewed below. The presence of WM changes in PD may reflect the observation that Lewy pathology in PD is much more widespread than was previously believed (Braak et al., 2003; Jellinger, 2012).
Age-related WM changes in PD include decreased WM volume, increased WM lesions, impaired WM integrity, and functional network alterations. Significant differences in WM volume between PD patients and healthy control subjects have been reported (Lee et al., 2011). In particular, Watanabe and colleagues found that PD patients with visual hallucinations exhibit significant atrophy in several areas of cerebral WM, compared to patients without such hallucinations (Watanabe et al., 2013). This finding suggests that WM atrophy may be attributed to disease processes. Furthermore, Hamasaki and colleagues discovered that PD patients with higher WM volume benefit more from subthalamic nucleus (STN) stimulation, indicating a role for WM atrophy in PD prognosis and treatment guidelines (Hamasaki et al., 2010).
WM lesions in PD may be related to the dementia that is observed in some patients at end stages (Burton et al., 2006). Consistent with this view, Ham and colleagues reported that deep WM lesions are closely associated with decreased cortical thickness, perhaps leading to the declines in executive function that are evident in PD (Ham et al., 2015). Similar to dementia patients, WM lesions are also associated with depression in PD patients (Petrovic et al., 2012). Furthermore, age-associated WM lesions may exacerbate the motor deficits associated with PD (Bohnen and Albin, 2011). Loss of WM integrity has been reported in several WM tracts in PD patients (Kim et al., 2013). For example, Luo and colleagues used resting-state functional MRI (rs-fMRI) to show that functional connectivity between homotopic brain regions was compromised in PD patients (Luo et al., 2015).
The myelinated axons within WM are organized as bundles. These bundled axons within WM are known as fiber tracts. In place of assessing global WM integrity, several studies have attempted to identify specific WM tract deficiencies that might account for particular types of functional decline in PD. As expected, impairments in specific fiber tracts are strongly associated with cognitive decline in PD patients (Agosta et al., 2014; Auning et al., 2014; Kamagata et al., 2013). For example, Agosta and colleagues reported that PD patients with mild cognitive impairment exhibit disruptions in WM integrities in the anterior and superior corona radiata, genu, body of the corpus callosum, anterior inferior fronto-occipital, uncinate, and superior longitudinal fasciculi (Agosta et al., 2014). The authors also concluded that subtle cognitive decline in PD is associated with abnormalities in frontal and interhemispheric WM connections. Similarly, Rae and colleagues reported that loss of WM integrity in the frontal lobe is associated with executive dysfunction in PD (Rae et al., 2012). In addition, Tanner and colleagues reported that disconnection between the temporal lobe and frontal-subcortical area might be associated with verbal memory impairments in PD (Tanner et al., 2015).
Microstructural disruption of specific WM tracts is also associated with other types of functional declines in PD patients. For example, loss of integrity of the bilateral pedunculopontine tracts, the corpus callosum, the corticospinal tract, the cingulum, and the superior longitudinal fasciculus is related to freezing of gait (Canu et al., 2015). Significant loss of integrity in the bilateral superior longitudinal fasciculus, the bilateral anterior corona radiata, and the left genu of the corpus callosum may account for postural instability in patients with PD (Gu et al., 2014). Finally, loss of integrity of the left uncinate fasciculus, the superior longitudinal fasciculus, the anterior thalamic radiation, the forceps minor, and the inferior longitudinal fasciculus is associated with depression in PD patients (Huang et al., 2014).
Studies on the mechanisms that lead to WM changes in PD are still relatively limited. A few studies have reported that increased demyelination (Gu et al., 2014) and transverse sinus and extracranial venous abnormalities (Liu et al., 2015) may account for WM changes in PD. As multiple pathophysiological changes are thought to underlie PD, such as enhanced glial activation and subsequent inflammatory responses, axonal disruption, and pathological protein accumulation, further investigations of the effects of these factors on age-related WM changes in PD are warranted.
6.2 Aging of white matter in Alzheimer’s disease
AD is the most common chronic ND and is characterized by progressive cognitive impairments. AD is thought to originate from abnormal accumulations of intraneuronal neurofibrillary tangles and extracellular senile plaques composed of amyloid-beta (Aβ). The abnormal accumulation of these pathological proteins develops in an age-dependent manner and eventually culminates in neuronal dysfunction and death. Thus, AD has traditionally been viewed as a neurodegenerative disorder of gray matter. However, emerging evidence demonstrates a connection between WM changes and the pathophysiology of AD. For example, a significant decrease in regional WM volume has been reported in the early phases of AD (Salat et al., 2009). A correlation between CSF biomarkers of AD, such as Aβ42, total tau, phospho-tau, with subcortical axonal impairments has also been reported (Skillback et al., 2013). Impaired WM integrity—often indicated by decreased FA or increased MD in DTI—has also been detected in pre-clinical AD (i.e. mild cognitive impairment) and clinically confirmed AD. Not surprisingly, AD patients exhibit greater impairments in WM integrity compared to patients with mild cognitive impairment, indicating a role for loss of WM integrity in the inexorable progression of AD (Kantarci, 2014; Teipel et al., 2014; Wisse et al., 2015). Specific fiber tracts within WM are often affected in AD and their correlations with functional decline have been investigated. For example, the cingulum bundle has been shown to play a critical role in memory (Wu et al., 2010) and is particularly susceptible to abnormalities in AD and mild cognitive impairment (Amlien and Fjell, 2014). Volume decreases (Copenhaver et al., 2006) and abnormal DTI measurements (Amlien et al., 2013) of the fornix have also been demonstrated in mild cognitive impairment and AD. Other tracts reported to be involved in AD include parahippocampal WM (Gold et al., 2012), the inferior fronto-occipital fascicles, the genu of the corpus callosum (Zhang et al., 2011), intracortical projecting fiber tracts (Teipel et al., 2014), and limbic WM tracts (Zhuang et al., 2013).
Several studies have investigated the mechanisms underlying the deterioration in WM in AD patients. Demyelination of the superficial WM (Fornari et al., 2012) and disrupted axons within WM (Skillback et al., 2013) may lay the foundation for WM deficits observed in AD. Age-related WM hypoperfusion may lead to WM ischemia and subsequent WM damage, which may also underlie WM disruptions in AD. Apart from the common age-related cerebral vascular alterations that contribute to WM hypoperfusion in normal aging (Section 3.2), several other mechanisms also underlie WM hypoperfusion in AD. First, compromised perivascular drainage throughout the brain resulting from venous collagenosis, tortuosity lesions, and thickened arteriolar walls in aged WM may lead to reduced clearance of Aβ (Brown and Thore, 2011). The reduction of Aβ clearance in turn leads to elevated Aβ deposition in and around vascular walls, narrowing or even closing the lumen (Roher et al., 1993), and leading to further microvascular pathology and subsequent hypoperfusion. Decreased Aβ clearance also aggravates age-related reductions in angiogenesis, perhaps by the binding of Aβ to vascular endothelial growth factor (VEGF) (Yang et al., 2004), a growth factor vital for angiogenesis. Second, the loss of cholinergic innervation of cerebral blood vessels in AD may also contribute to brain hypoperfusion. Indeed, disruptions in cholinergic neurotransmission are known to exacerbate cognitive impairments in pre-clinical AD (Lim et al., 2015). Compared to age-matched control subjects, AD patients exhibited greater blood brain barrier disruption (Erickson and Banks, 2013) and elevated expression of receptor for advanced glycation endproducts (RAGE) (Janota et al., 2016), which is essential for the influx of peripheral Aβ into the brain. Decreased expression of the efflux receptor for Aβ, lipoprotein receptor-related protein (LRP)-1 and increased influx receptor RAGE (Silverberg et al., 2010a; Silverberg et al., 2010b) has been reported in AD brains, which would be expected to enhance intracellular accumulation Aβ. These characteristics may all partially account for WM impairments in AD.
Age-related changes in microglia and astrocytes may also underlie WM alterations in AD patients. AD subjects exhibit higher microglial density compared to age-matched healthy controls (Flanary et al., 2007). Higher microglial density in the aged AD brain may exacerbate WM impairments by increased production of pro-inflammatory cytokines and chemokines. Furthermore, microglia with elevated RAGE expression in AD brains may also exacerbate WM injury (Fang et al., 2010). Similarly, higher astrocytic activation in AD brains compared to age-a matched control has been reported (Furman et al., 2012), which may lead to greater WM impairments. Eevated production of apolipoprotein E from astrocytes is associated with Aβ accumulation (Bien-Ly et al., 2012). As Aβ accumulation plays a major role in WM disruptions, these alterations leading to Aβ accumulation are likely to contribute to WM disruptions in AD.
6.3 Aging of white matter in other neurodegenerative diseases
Age-related WM changes are also observed in other NDs, including HD, frontotemporal dementia (FTD), and amyotrophic lateral sclerosis (ALS). Reduced WM volume has been reported in early HD and is significantly correlated with deteriorations in cognitive performance (Beglinger et al., 2005). Reduced WM connectivities of the sensorimotor cortex have also been observed in HD and are related to motor, oculomotor, and cognitive symptoms (Dumas et al., 2012). Novak and colleagues reported impaired WM integrity in early HD and demonstrated that the alterations were related to caudate loss and disease progression (Novak et al., 2014). Significant myelin and axonal damage have also been detected in HD (Di Paola et al., 2012) and may underlie the WM changes observed in this condition. Similarly, WM changes have also been reported in ALS (Crespi et al., 2014; Kasper et al., 2014) and FTD (Kuceyeski et al., 2012; Zhang et al., 2009); in both diseases, they are associated with functional decline as expected.
7. Aging of white matter in other brain diseases
WM changes are also present in a number of other brain diseases, which can be grouped into genetic, demyelinative, infectious, inflammatory, toxic, metabolic, vascular, traumatic, neoplastic, hydrophilic, and neurodegenerative, as reviewed by Schmahmann et al (Schmahmann et al., 2008). Because WM undergoes progressive structural and functional decline with increasing age, aging likely exacerbates the neurological effects of these conditions. Below we have summarized the evidence supporting the existence of ―WM aging║ and the crucial role that it plays in these disorders, with particular emphasis on multiple sclerosis (MS) and schizophrenia.
7.1 Multiple sclerosis
MS is one of the most common demyelinating diseases. The pathology of MS is characterized by multiple demyelinative plaques within WM, which are accompanied by robust gliosis and axonal damage. MS plaques can be divided into active, inactive, and smoldering plaques that undergo a dynamic transition depending on specific endogenous factors such as aging. An equilibrium between active and inactive plaques and a peak in smoldering plaques (characterized by microglial activation and slow expansion of pre-existing plaques) have been observed in patients by the age of 47 years (Frischer et al., 2015). Even greater impairments in myelin integrity have been detected in WM in older MS patients (Newbould et al., 2014). Hampton and colleagues reported that aged animals are more vulnerable to axonal injury and exhibit less efficient remyelination than younger animals (Hampton et al., 2012). Furthermore, remyelination in aged animals is predominantly performed by Schwann cells, whereas remyelination is predominantly performed by central oligodendrocytes in younger animals (Hampton et al., 2012). This lies in agreement with the finding that age-related remyelination failure reflects the aging of the oligodendrocyte population (Adamo, 2014). All of these studies confirm an important role for aging in WM changes in MS patients.
A series of WM changes observed in MS patient brains may play a pivotal role in functional decline. WM atrophy, as indicated by decreased WM fraction of total intracranial volumes (Chard et al., 2002) or reduced WM volume (Sacco et al., 2015), has been reported in MS. Compared to cognitively intact MS patients, cognitively impaired MS patients exhibit higher WM atrophy (Sacco et al., 2015). Impaired WM integrity and WM lesions within several tracts are strongly associated with cognitive decline in MS patients (Preziosa et al., 2016). Furthermore, Bisecco and colleagues reported more extensive microstructural WM damage in fatigued MS patients, especially in the associative tracts connected to the frontal lobes, suggesting that WM damage may be related to mental and physical exhaustion (Bisecco et al., 2016). Elevated gliosis, severe demyelination, impaired remyelination, and disruptions in axonal structure in MS may be the microstructural substrate for these WM changes (Hampton et al., 2012). Overall, the mechanism underlying WM changes in MS remains poorly studied, and focused investigations of the role of aging in WM alterations in MS are needed.
7.2 Schizophrenia
Finally, WM changes have been intensively investigated in schizophrenia. Schizophrenia is a relatively common psychological disorder characterized by abnormal social behavior, delusions, occasional hallucinations, and severe cognitive dysfunction. Schizophrenia is thought to arise from a combination of genetic and environmental factors. Both psychological and neurological mechanisms underlying schizophrenia have been widely studied. For example, cognitive biases (Broome et al., 2005), brain volume reductions (Andreone et al., 2007), and neurotransmitter system alterations (Konradi and Heckers, 2003) have been identified in patients with schizophrenia.
Accumulating evidence suggests a role for age-related WM changes in the progression of schizophrenia. Accelerated WM aging, as indicated by increased rate of FA decline in DTI imaging, has been reported in schizophrenic patients compared to healthy controls (Wright et al., 2014). This is consistent with the loss of functional brain network efficiency in schizophrenic patients (Sheffield et al., 2016). The acceleration of WM changes with aging is thought to differ across fiber tract groups. For example, WM tracts that mature late in life show higher sensitivity and susceptibility to age-related acceleration in the decline of FA values in schizophrenic patients (Kochunov et al., 2013).
Further investigations to elucidate the interaction between aging, WM changes, and schizophrenia symptoms are urgently needed. Precedence for such studies is provided by findings of age-related WM disruption (Andreone et al., 2007), reduced frontal and temporal WM volume (Okugawa et al., 2007) and corpus callosal atrophy (Rotarska-Jagiela et al., 2008) in patients with schizophrenia. These WM abnormalities appear to be strongly related to loss of cognitive function (Dwork et al., 2007). The cellular and molecular mechanisms underlying WM changes in schizophrenia have also been studied. Brain metabolite abnormalities, including decreased N-acetyl, elevated glutamate, and reduced myoinositol in WM have been reported in schizophrenia, which may reflect reduced neuronal or glial content, as well as glial dysfunction (Chang et al., 2007). Schizophrenic patients also exhibit alterations in the distribution of oligodendrocytes and oligodendrocyte cell loss in the superior frontal gyrus, which is in agreement with evidence that multiple genes encoding myelin-related proteins consistently exhibit reduced expression in schizophrenia (Hof et al., 2003).
WM changes are also present in other diseases, such as leukodystrophies, vanishing WM disease, Balo’s concentric sclerosis, Lyme encephalopathy, Wegener’s granulomatosis, Cobalamin deficiency, and Binswanger’s disease (Schmahmann et al., 2008). The pathophysiology of WM changes in these conditions is poorly understood.
8. Summary
WM occupies a significant portion of the anthropoid primate brain, a structural characteristic that probably reflects its importance in cognitive function in these higher species. WM changes with aging have garnered increasing attention in recent years as neuroscientists have shifted away from a purely neurocentric view of the brain and redoubled their efforts to study all the components of the neurovascular unit. A variety of macrostructural WM changes have been identified in normal aging and these changes are thought to contribute to the age-related decline in brain function. Potential cellular and molecular mechanisms underlying age-related WM changes have also been identified. Furthermore, age-related WM changes play a pivotal role in a number of brain diseases, including stroke, TBI, and NDs. However, the underlying mechanisms remain poorly investigated, and many controversies remain. For example, there is no consensus on whether WM deficits in AD are secondary to gray matter atrophy or result directly from abnormal aggregations of Aβ (Amlien and Fjell, 2014). Furthermore, it is still not clear if there is a correlation between loss of WM integrity and bradykinesia in PD (Vesely et al., 2016). Numerous DTI-based studies have demonstrated a potential correlation between functional decline and disruption of specific WM fiber tracts. However, DTI-based tractography is based on water diffusion, not direct measurements of tract microstructure. Furthermore, structural changes may not always elicit robust functional changes and anatomical measurements should therefore be complemented by functional measurements wherever feasible. In short, the drawbacks of DTI-based tractography make our current interpretations of WM changes relatively speculative. New methods with even higher resolution are needed to measure microstructural changes and functional connections. Nevertheless, current imaging methods to detect WM changes serve as a useful tool to accelerate clinical diagnoses compared to symptoms-based diagnoses of brain disorders and to guide treatment recommendations. Finally, if preclinical studies focused as heavily on WM as they currently do on gray matter, their findings might translate better to the clinic. This bench-to-bedside translation would be facilitated by greater numbers of studies in animals with high WM content, such as monkeys.
Highlights.
White matter changes in normal aging and correlation with age-related functional decline.
Advances in neuroimaging enable detection of age-related macrostructural changes in WM. Example: diffusion tensor imaging (DTI) measures WM integrity.
Aged WM is more susceptible to neurological disorders (stroke, traumatic brain injury, and neurodegeneration).
Further investigation of age-related changes in WM may provide novel therapeutic strategies.
Acknowledgments
Sources of support:
This work was supported by the NIH grants from NIH/National Institute of neurological disorders and stroke (NINDS) (NS094573 and NS092618 to X.H), and the American Heart Association (13SDCG14570025 to X. H).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
References
- Abe O, Yamasue H, Yamada H, Masutani Y, Kabasawa H, Sasaki H, Takei K, Suga M, Kasai K, Aoki S, Ohtomo K. Sex dimorphism in gray/white matter volume and diffusion tensor during normal aging. NMR in biomedicine. 2010;23:446–458. doi: 10.1002/nbm.1479. [DOI] [PubMed] [Google Scholar]
- Adamo AM. Nutritional factors and aging in demyelinating diseases. Genes & nutrition. 2014;9:360. doi: 10.1007/s12263-013-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Canu E, Stefanova E, Sarro L, Tomic A, Spica V, Comi G, Kostic VS, Filippi M. Mild cognitive impairment in Parkinson’s disease is associated with a distributed pattern of brain white matter damage. Human brain mapping. 2014;35:1921–1929. doi: 10.1002/hbm.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlien IK, Fjell AM. Diffusion tensor imaging of white matter degeneration in Alzheimer’s disease and mild cognitive impairment. Neuroscience. 2014;276:206–215. doi: 10.1016/j.neuroscience.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Amlien IK, Fjell AM, Walhovd KB, Selnes P, Stenset V, Grambaite R, Bjornerud A, Due-Tonnessen P, Skinningsrud A, Gjerstad L, Reinvang I, Fladby T. Mild cognitive impairment: cerebrospinal fluid tau biomarker pathologic levels and longitudinal changes in white matter integrity. Radiology. 2013;266:295–303. doi: 10.1148/radiol.12120319. [DOI] [PubMed] [Google Scholar]
- Andreone N, Tansella M, Cerini R, Rambaldelli G, Versace A, Marrella G, Perlini C, Dusi N, Pelizza L, Balestrieri M, Barbui C, Nose M, Gasparini A, Brambilla P. Cerebral atrophy and white matter disruption in chronic schizophrenia. European archives of psychiatry and clinical neuroscience. 2007;257:3–11. doi: 10.1007/s00406-006-0675-1. [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM. White matter involvement after TBI: Clues to axon and myelin repair capacity. Experimental neurology 275 Pt. 2016;3:328–333. doi: 10.1016/j.expneurol.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Auning E, Kjaervik VK, Selnes P, Aarsland D, Haram A, Bjornerud A, Hessen E, Esnaashari A, Fladby T. White matter integrity and cognition in Parkinson’s disease: a cross-sectional study. BMJ open. 2014;4:e003976. doi: 10.1136/bmjopen-2013-003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer AS, Syed YA, Kang SU, Mitteregger D, Vig R, Ffrench-Constant C, Franklin RJ, Altmann F, Lubec G, Kotter MR. Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain : a journal of neurology. 2009;132:465–481. doi: 10.1093/brain/awn334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltan S. Ischemic injury to white matter: an age-dependent process. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2009;15:126–133. doi: 10.1177/1073858408324788. [DOI] [PubMed] [Google Scholar]
- Baltan S, Besancon EF, Mbow B, Ye Z, Hamner MA, Ransom BR. White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:1479–1489. doi: 10.1523/JNEUROSCI.5137-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhof F, Scheltens P. Imaging of white matter lesions. Cerebrovascular diseases (Basel, Switzerland) 2002;(13 Suppl 2):21–30. doi: 10.1159/000049146. [DOI] [PubMed] [Google Scholar]
- Barreto GE, Capani F, Gonzalez J, Morales L. Role of astrocytes in neurodegenerative diseases. INTECH Open Access Publisher; 2011. [Google Scholar]
- Bartres-Faz D, Clemente IC, Junque C. [White matter changes and cognitive performance in aging] Revista de neurologia. 2001;33:347–353. [PubMed] [Google Scholar]
- Bates TJ, Vonica A, Heasman J, Brivanlou AH, Bell E. Coco regulates dorsoventral specification of germ layers via inhibition of TGFbeta signalling. Development (Cambridge, England) 2013;140:4177–4181. doi: 10.1242/dev.095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological reviews. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Beglinger LJ, Nopoulos PC, Jorge RE, Langbehn DR, Mikos AE, Moser DJ, Duff K, Robinson RG, Paulsen JS. White matter volume and cognitive dysfunction in early Huntington’s disease. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2005;18:102–107. doi: 10.1097/01.wnn.0000152205.79033.73. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014;276:187–205. doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien-Ly N, Gillespie AK, Walker D, Yoon SY, Huang Y. Reducing human apolipoprotein E levels attenuates age-dependent Abeta accumulation in mutant human amyloid precursor protein transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:4803–4811. doi: 10.1523/JNEUROSCI.0033-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisecco A, Caiazzo G, d’Ambrosio A, Sacco R, Bonavita S, Docimo R, Cirillo M, Pagani E, Filippi M, Esposito F, Tedeschi G, Gallo A. Multiple sclerosis. Houndmills, Basingstoke, England: 2016. Fatigue in multiple sclerosis: The contribution of occult white matter damage. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nature reviews. Neurology. 2011;7:229–236. doi: 10.1038/nrneurol.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta neuropathologica. 2007;114:231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- Brodoehl S, Klingner C, Stieglitz K, Witte OW. Age-related changes in the somatosensory processing of tactile stimulation--an fMRI study. Behavioural brain research. 2013;238:259–264. doi: 10.1016/j.bbr.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Broome MR, Woolley JB, Tabraham P, Johns LC, Bramon E, Murray GK, Pariante C, McGuire PK, Murray RM. What causes the onset of psychosis? Schizophrenia research. 2005;79:23–34. doi: 10.1016/j.schres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. Journal of the neurological sciences. 2002;203–204:159–163. doi: 10.1016/s0022-510x(02)00283-6. [DOI] [PubMed] [Google Scholar]
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathology and applied neurobiology. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Firbank MJ, O’Brien JT. Progression of white matter hyperintensities in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia: a comparison with normal aging. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2006;14:842–849. doi: 10.1097/01.JGP.0000236596.56982.1c. [DOI] [PubMed] [Google Scholar]
- Caligiuri ME, Perrotta P, Augimeri A, Rocca F, Quattrone A, Cherubini A. Automatic Detection of White Matter Hyperintensities in Healthy Aging and Pathology Using Magnetic Resonance Imaging: A Review. Neuroinformatics. 2015;13:261–276. doi: 10.1007/s12021-015-9260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canu E, Agosta F, Sarasso E, Volonte MA, Basaia S, Stojkovic T, Stefanova E, Comi G, Falini A, Kostic VS, Gatti R, Filippi M. Brain structural and functional connectivity in Parkinson’s disease with freezing of gait. Human brain mapping. 2015;36:5064–5078. doi: 10.1002/hbm.22994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, Bannon Y, Fernandez F, Giunta B, Schoenberg MR, Tan J. Normal brain aging clinical, immunological, neuropsychological, and neuroimaging features. International review of neurobiology. 2009;84:1–19. doi: 10.1016/S0074-7742(09)00401-2. [DOI] [PubMed] [Google Scholar]
- Catani M, ffytche DH. The rises and falls of disconnection syndromes. Brain : a journal of neurology. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biological psychiatry. 2007;62:1396–1404. doi: 10.1016/j.biopsych.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard DT, Griffin CM, Parker GJ, Kapoor R, Thompson AJ, Miller DH. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain : a journal of neurology. 2002;125:327–337. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- Clarner T, Diederichs F, Berger K, Denecke B, Gan L, van der Valk P, Beyer C, Amor S, Kipp M. Myelin debris regulates inflammatory responses in an experimental demyelination animal model and multiple sclerosis lesions. Glia. 2012;60:1468–1480. doi: 10.1002/glia.22367. [DOI] [PubMed] [Google Scholar]
- Clemente D, Ortega MC, Melero-Jerez C, de Castro F. The effect of glia-glia interactions on oligodendrocyte precursor cell biology during development and in demyelinating diseases. Frontiers in cellular neuroscience. 2013;7:268. doi: 10.3389/fncel.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon EJ. The elderly brain. A quantitative analysis in the cerebral cortex of two cases. Psychiatria, neurologia, neurochirurgia. 1972;75:261–270. [PubMed] [Google Scholar]
- Copenhaver BR, Rabin LA, Saykin AJ, Roth RM, Wishart HA, Flashman LA, Santulli RB, McHugh TL, Mamourian AC. The fornix and mammillary bodies in older adults with Alzheimer’s disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry research. 2006;147:93–103. doi: 10.1016/j.pscychresns.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Correa F, Gauberti M, Parcq J, Macrez R, Hommet Y, Obiang P, Hernangomez M, Montagne A, Liot G, Guaza C, Maubert E, Ali C, Vivien D, Docagne F. Tissue plasminogen activator prevents white matter damage following stroke. J Exp Med. 2011;208:1229–1242. doi: 10.1084/jem.20101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi C, Cerami C, Dodich A, Canessa N, Arpone M, Iannaccone S, Corbo M, Lunetta C, Scola E, Falini A, Cappa SF. Microstructural white matter correlates of emotion recognition impairment in Amyotrophic Lateral Sclerosis. Cortex; a journal devoted to the study of the nervous system and behavior. 2014;53:1–8. doi: 10.1016/j.cortex.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain : a journal of neurology. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Deierborg T, Soulet D, Roybon L, Hall V, Brundin P. Emerging restorative treatments for Parkinson’s disease. Progress in neurobiology. 2008;85:407–432. doi: 10.1016/j.pneurobio.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Detraglia M, Cook FB, Stasiw DM, Cerny LC. Erythrocyte fragility in aging. Biochimica et biophysica acta. 1974;345:213–219. doi: 10.1016/0005-2736(74)90259-4. [DOI] [PubMed] [Google Scholar]
- Di Paola M, Luders E, Cherubini A, Sanchez-Castaneda C, Thompson PM, Toga AW, Caltagirone C, Orobello S, Elifani F, Squitieri F, Sabatini U. Cerebral cortex. Vol. 22. New York, N.Y. : 1991: 2012. Multimodal MRI analysis of the corpus callosum reveals white matter differences in presymptomatic and early Huntington’s disease; pp. 2858–2866. [DOI] [PubMed] [Google Scholar]
- Diniz DG, Foro CA, Rego CM, Gloria DA, de Oliveira FR, Paes JM, de Sousa AA, Tokuhashi TP, Trindade LS, Turiel MC, Vasconcelos EG, Torres JB, Cunnigham C, Perry VH, Vasconcelos PF, Diniz CW. Environmental impoverishment and aging alter object recognition, spatial learning, and dentate gyrus astrocytes. The European journal of neuroscience. 2010;32:509–519. doi: 10.1111/j.1460-9568.2010.07296.x. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas EM, van den Bogaard SJ, Ruber ME, Reilman RR, Stout JC, Craufurd D, Hicks SL, Kennard C, Tabrizi SJ, van Buchem MA, van der Grond J, Roos RA. Early changes in white matter pathways of the sensorimotor cortex in premanifest Huntington’s disease. Human brain mapping. 2012;33:203–212. doi: 10.1002/hbm.21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwork AJ, Mancevski B, Rosoklija G. White matter and cognitive function in schizophrenia. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2007;10:513–536. doi: 10.1017/S1461145707007638. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33:1500–1513. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza SE, Guo H, Fedarko N, DeZern A, Fried LP, Xue QL, Leng S, Beamer B, Walston JD. Glutathione peroxidase enzyme activity in aging. The journals of gerontology. Series A, Biological sciences and medical sciences. 2008;63:505–509. doi: 10.1093/gerona/63.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Yan S, Schmidt AM, Chen JX, Yan SS. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:1043–1055. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in neurosciences. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. The New England journal of medicine. 2001;345:425–432. doi: 10.1056/NEJM200108093450606. [DOI] [PubMed] [Google Scholar]
- Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 2007;10:61–74. doi: 10.1089/rej.2006.9096. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Yang J, Arfanakis K, Arvanitakis Z, Leurgans SE, Turner AD, Barnes LL, Bennett DA, Buchman AS. Physical activity, motor function, and white matter hyperintensity burden in healthy older adults. Neurology. 2015;84:1294–1300. doi: 10.1212/WNL.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari E, Maeder P, Meuli R, Ghika J, Knyazeva MG. Demyelination of superficial white matter in early Alzheimer’s disease: a magnetization transfer imaging study. Neurobiology of aging. 2012;33 doi: 10.1016/j.neurobiolaging.2010.11.014. 428.e427-419. [DOI] [PubMed] [Google Scholar]
- Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I, Mandrekar J, Bramow S, Metz I, Bruck W, Lassmann H, Lucchinetti CF. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Annals of neurology. 2015;78:710–721. doi: 10.1002/ana.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu JH, Lu CZ, Hong Z, Dong Q, Luo Y, Wong KS. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. Journal of neurology, neurosurgery, and psychiatry. 2005;76:793–796. doi: 10.1136/jnnp.2003.032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Van Eldik LJ, Norris CM. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32:16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Matas S, Gutierrez-Cuesta J, Coto-Montes A, Rubio-Acero R, Diez-Vives C, Camins A, Pallas M, Sanfeliu C, Cristofol R. Dysfunction of astrocytes in senescence-accelerated mice SAMP8 reduces their neuroprotective capacity. Aging cell. 2008;7:630–640. doi: 10.1111/j.1474-9726.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Geoffroy CG, Zheng B. Myelin-associated inhibitors in axonal growth after CNS injury. Current opinion in neurobiology. 2014;27:31–38. doi: 10.1016/j.conb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Gold BT, Johnson NF, Powell DK, Smith CD. White matter integrity and vulnerability to Alzheimer’s disease: preliminary findings and future directions. Biochimica et biophysica acta. 2012;1822:416–422. doi: 10.1016/j.bbadis.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke; a journal of cerebral circulation. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- Gong Y, Xi G, Wan S, Gu Y, Keep RF, Hua Y. Effects of aging on complement activation and neutrophil infiltration after intracerebral hemorrhage. Acta neurochirurgica. 2008;105(Supplement):67–70. doi: 10.1007/978-3-211-09469-3_14. [DOI] [PubMed] [Google Scholar]
- Gottfries CG. Neurochemical aspects on aging and diseases with cognitive impairment. Journal of neuroscience research. 1990;27:541–547. doi: 10.1002/jnr.490270415. [DOI] [PubMed] [Google Scholar]
- Gu Q, Huang P, Xuan M, Xu X, Li D, Sun J, Yu H, Wang C, Luo W, Zhang M. Greater loss of white matter integrity in postural instability and gait difficulty subtype of Parkinson’s disease. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2014;41:763–768. doi: 10.1017/cjn.2014.34. [DOI] [PubMed] [Google Scholar]
- Ham JH, Yun HJ, Sunwoo MK, Hong JY, Lee JM, Sohn YH, Lee PH. Topography of cortical thinning associated with white matter hyperintensities in Parkinson’s disease. Parkinsonism & related disorders. 2015;21:372–377. doi: 10.1016/j.parkreldis.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Yamada K, Hirai T, Kuratsu J. A positive correlation between fractional white matter volume and the response of Parkinson disease patients to subthalamic stimulation. Acta neurochirurgica. 2010;152:997–1006. doi: 10.1007/s00701-010-0609-6. discussion 1006. [DOI] [PubMed] [Google Scholar]
- Hampton DW, Innes N, Merkler D, Zhao C, Franklin RJ, Chandran S. Focal immune-mediated white matter demyelination reveals an age-associated increase in axonal vulnerability and decreased remyelination efficiency. The American journal of pathology. 2012;180:1897–1905. doi: 10.1016/j.ajpath.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Hatakeyama T, Okauchi M, Hua Y, Keep RF, Xi G. Deferoxamine reduces neuronal death and hematoma lysis after intracerebral hemorrhage in aged rats. Translational stroke research. 2013;4:546–553. doi: 10.1007/s12975-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nature neuroscience. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman JD, Peters A, Cabral H, Rosene DL, Hollander W, Rasband MN, Abraham CR. Age-related molecular reorganization at the node of Ranvier. The Journal of comparative neurology. 2006;495:351–362. doi: 10.1002/cne.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PW, Reutens DC, Phan TG, Wright PM, Markus R, Indra I, Young D, Donnan GA. Is white matter involved in patients entered into typical trials of neuroprotection? Stroke. 2005;36:2742–2744. doi: 10.1161/01.STR.0000189748.52500.a7. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Jr, Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biological psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Hofman MA. Evolution of the human brain: when bigger is better. Frontiers in neuroanatomy. 2014;8:15. doi: 10.3389/fnana.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J. Microglial and macrophage polarization-new prospects for brain repair. Nature reviews. Neurology. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke; a journal of cerebral circulation. 2007;38:759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- Hua Y, Xi G, Keep RF, Hoff JT. Complement activation in the brain after experimental intracerebral hemorrhage. Journal of neurosurgery. 2000;92:1016–1022. doi: 10.3171/jns.2000.92.6.1016. [DOI] [PubMed] [Google Scholar]
- Huang P, Xu X, Gu Q, Xuan M, Yu X, Luo W, Zhang M. Disrupted white matter integrity in depressed versus non-depressed Parkinson’s disease patients: a tract-based spatial statistics study. Journal of the neurological sciences. 2014;346:145–148. doi: 10.1016/j.jns.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR. American journal of neuroradiology. 2013;34:2064–2074. doi: 10.3174/ajnr.A3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibbotson SH, Tate GM, Davies JA. Thrombin activity by intrinsic activation of plasma in-vitro accelerates with increasing age of the donor. Thrombosis and haemostasis. 1992;67:377–380. [PubMed] [Google Scholar]
- Inano S, Takao H, Hayashi N, Abe O, Ohtomo K. Effects of age and gender on white matter integrity. AJNR. American journal of neuroradiology. 2011;32:2103–2109. doi: 10.3174/ajnr.A2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inano S, Takao H, Hayashi N, Yoshioka N, Mori H, Kunimatsu A, Abe O, Ohtomo K. Effects of age and gender on neuroanatomical volumes. Journal of magnetic resonance imaging : JMRI. 2013;37:1072–1076. doi: 10.1002/jmri.23910. [DOI] [PubMed] [Google Scholar]
- Jankelowitz SK, McNulty PA, Burke D. Changes in measures of motor axon excitability with age. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2007;118:1397–1404. doi: 10.1016/j.clinph.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Janota C, Lemere CA, Brito MA. Dissecting the Contribution of Vascular Alterations and Aging to Alzheimer’s Disease. Molecular neurobiology. 2016;53:3793–3811. doi: 10.1007/s12035-015-9319-7. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Neuropathology of sporadic Parkinson’s disease: evaluation and changes of concepts. Movement disorders : official journal of the Movement Disorder Society. 2012;27:8–30. doi: 10.1002/mds.23795. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kamagata K, Motoi Y, Tomiyama H, Abe O, Ito K, Shimoji K, Suzuki M, Hori M, Nakanishi A, Sano T, Kuwatsuru R, Sasai K, Aoki S, Hattori N. Relationship between cognitive impairment and white-matter alteration in Parkinson’s disease with dementia: tract-based spatial statistics and tract-specific analysis. European radiology. 2013;23:1946–1955. doi: 10.1007/s00330-013-2775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K. Fractional anisotropy of the fornix and hippocampal atrophy in Alzheimer’s disease. Frontiers in aging neuroscience. 2014;6:316. doi: 10.3389/fnagi.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper E, Schuster C, Machts J, Kaufmann J, Bittner D, Vielhaber S, Benecke R, Teipel S, Prudlo J. Microstructural white matter changes underlying cognitive and behavioural impairment in ALS--an in vivo study using DTI. PloS one. 2014;9:e114543. doi: 10.1371/journal.pone.0114543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim SJ, Kim HS, Choi CG, Kim N, Han S, Jang EH, Chung SJ, Lee CS. Alterations of mean diffusivity in brain white matter and deep gray matter in Parkinson’s disease. Neuroscience letters. 2013;550:64–68. doi: 10.1016/j.neulet.2013.06.050. [DOI] [PubMed] [Google Scholar]
- Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, Patel MC, Counsell SJ, Sharp DJ. White matter damage and cognitive impairment after traumatic brain injury. Brain : a journal of neurology. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiray H, Lindsay SL, Hosseinzadeh S, Barnett SC. The multifaceted role of astrocytes in regulating myelination. Experimental neurology. 2016 doi: 10.1016/j.expneurol.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Klein AW, Michel ME. A morphometric study of the neocortex of young adult and old maze-differentiated rats. Mechanisms of ageing and development. 1977;6:441–452. doi: 10.1016/0047-6374(77)90045-8. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Coyle T, Lancaster J, Robin DA, Hardies J, Kochunov V, Bartzokis G, Stanley J, Royall D, Schlosser AE, Null M, Fox PT. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. NeuroImage. 2010;49:1190–1199. doi: 10.1016/j.neuroimage.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Rowland LM, Olvera RL, Winkler A, Yang YH, Sampath H, Carpenter WT, Duggirala R, Curran J, Blangero J, Hong LE. Testing the hypothesis of accelerated cerebral white matter aging in schizophrenia and major depression. Biological psychiatry. 2013;73:482–491. doi: 10.1016/j.biopsych.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodiweera C, Alexander AL, Harezlak J, McAllister TW, Wu YC. Age effects and sex differences in human brain white matter of young to middle-aged adults: A DTI, NODDI, and q-space study. NeuroImage. 2016;128:180–192. doi: 10.1016/j.neuroimage.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama SG, Rosene DL, Sherman LS. Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. Age (Dordrecht, Netherlands) 2012;34:1093–1110. doi: 10.1007/s11357-011-9357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacology & therapeutics. 2003;97:153–179. doi: 10.1016/s0163-7258(02)00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuceyeski A, Zhang Y, Raj A. Linking white matter integrity loss to associated cortical regions using structural connectivity information in Alzheimer’s disease and fronto-temporal dementia: the Loss in Connectivity (LoCo) score. NeuroImage. 2012;61:1311–1323. doi: 10.1016/j.neuroimage.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Chavez AS, Macey PM, Woo MA, Harper RM. Brain axial and radial diffusivity changes with age and gender in healthy adults. Brain research. 2013;1512:22–36. doi: 10.1016/j.brainres.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoureux PL, O’Toole MR, Heidemann SR, Miller KE. Slowing of axonal regeneration is correlated with increased axonal viscosity during aging. BMC neuroscience. 2010;11:140. doi: 10.1186/1471-2202-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nature reviews. Neuroscience. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim C, Lee SJ. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxidative medicine and cellular longevity. 2010;3:283–287. doi: 10.4161/oxim.3.4.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim SS, Tae WS, Lee SY, Choi JW, Koh SB, Kwon DY. Regional volume analysis of the Parkinson disease brain in early disease stage: gray matter, white matter, striatum, and thalamus. AJNR. American journal of neuroradiology. 2011;32:682–687. doi: 10.3174/ajnr.A2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Grassiot B, Alperovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. NeuroImage. 2005;26:900–911. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Li N, Kong X, Ye R, Yang Q, Han J, Xiong L. Age-related differences in experimental stroke: possible involvement of mitochondrial dysfunction and oxidative damage. Rejuvenation Res. 2011;14:261–273. doi: 10.1089/rej.2010.1115. [DOI] [PubMed] [Google Scholar]
- Lim YY, Maruff P, Schindler R, Ott BR, Salloway S, Yoo DC, Noto RB, Santos CY, Snyder PJ. Disruption of cholinergic neurotransmission exacerbates Abeta-related cognitive impairment in preclinical Alzheimer’s disease. Neurobiology of aging. 2015;36:2709–2715. doi: 10.1016/j.neurobiolaging.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science (New York, N.Y.) 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging cell. 2014;13:699–708. doi: 10.1111/acel.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiological reviews. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang L, Geng Z, Zhu Q, Song Z, Chang R, Lv H. A voxel-based morphometric study of age- and sex-related changes in white matter volume in the normal aging brain. Neuropsychiatric disease and treatment. 2016;12:453–465. doi: 10.2147/NDT.S90674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Xu H, Wang Y, Zhong Y, Xia S, Utriainen D, Wang T, Haacke EM. Patterns of chronic venous insufficiency in the dural sinuses and extracranial draining veins and their relationship with white matter hyperintensities for patients with Parkinson’s disease. Journal of vascular surgery. 2015;61 doi: 10.1016/j.jvs.2014.02.021. 1511-1520.e1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Osorio MJ, Kress BT, Sanggaard S, Nedergaard M. White matter astrocytes in health and disease. Neuroscience. 2014;276:161–173. doi: 10.1016/j.neuroscience.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Guo X, Song W, Zhao B, Cao B, Yang J, Gong Q, Shang HF. Decreased Resting-State Interhemispheric Functional Connectivity in Parkinson’s Disease. BioMed research international. 2015;2015:692684. doi: 10.1155/2015/692684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. The Journal of comparative neurology. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Mei Y, Jiang C, Wan Y, Lv J, Jia J, Wang X, Yang X, Tong Z. Aging-associated formaldehyde-induced norepinephrine deficiency contributes to age-related memory decline. Aging cell. 2015;14:659–668. doi: 10.1111/acel.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nature neuroscience. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR. American journal of neuroradiology. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis: association with leukoaraiosis. Radiology. 1995;194:469–476. doi: 10.1148/radiology.194.2.7824728. [DOI] [PubMed] [Google Scholar]
- Moody DM, Santamore WP, Bell MA. Does tortuosity in cerebral arterioles impair down-autoregulation in hypertensives and elderly normotensives? A hypothesis and computer model. Clinical neurosurgery. 1991;37:372–387. [PubMed] [Google Scholar]
- Morales I, Yanos C, Rodriguez-Sabate C, Sanchez A, Rodriguez M. Striatal glutamate degenerates thalamic neurons. Journal of neuropathology and experimental neurology. 2013;72:286–297. doi: 10.1097/NEN.0b013e31828a80ee. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science (New York, N.Y.) 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Mosenthal AC, Livingston DH, Lavery RF, Knudson MM, Lee S, Morabito D, Manley GT, Nathens A, Jurkovich G, Hoyt DB, Coimbra R. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. The Journal of trauma. 2004;56:1042–1048. doi: 10.1097/01.ta.0000127767.83267.33. [DOI] [PubMed] [Google Scholar]
- Mukoyama M. Age changes in internodal length in the human spinal roots--nerve teasing study. Nagoya journal of medical science. 1973;36:17–27. [PubMed] [Google Scholar]
- Newbould RD, Nicholas R, Thomas CL, Quest R, Lee JS, Honeyfield L, Colasanti A, Malik O, Mattoscio M, Matthews PM, Sormani MP, Waldman AD, Muraro PA. Age independently affects myelin integrity as detected by magnetization transfer magnetic resonance imaging in multiple sclerosis. NeuroImage. Clinical. 2014;4:641–648. doi: 10.1016/j.nicl.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and applied neurobiology. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak MJ, Seunarine KK, Gibbard CR, Hobbs NZ, Scahill RI, Clark CA, Tabrizi SJ. White matter integrity in premanifest and early Huntington’s disease is related to caudate loss and disease progression. Cortex; a journal devoted to the study of the nervous system and behavior. 2014;52:98–112. doi: 10.1016/j.cortex.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Oksala NK, Oksala A, Pohjasvaara T, Vataja R, Kaste M, Karhunen PJ, Erkinjuntti T. Age related white matter changes predict stroke death in long term follow-up. Journal of neurology, neurosurgery, and psychiatry. 2009;80:762–766. doi: 10.1136/jnnp.2008.154104. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Tamagaki C, Agartz I. Frontal and temporal volume size of grey and white matter in patients with schizophrenia: an MRI parcellation study. European archives of psychiatry and clinical neuroscience. 2007;257:304–307. doi: 10.1007/s00406-007-0721-7. [DOI] [PubMed] [Google Scholar]
- Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. Journal of neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Pantoni L. Cerebrovascular diseases. 13 Suppl 2. Basel, Switzerland: 2002. Pathophysiology of age-related cerebral white matter changes; pp. 7–10. [DOI] [PubMed] [Google Scholar]
- Petrovic IN, Stefanova E, Kozic D, Semnic R, Markovic V, Daragasevic NT, Kostic VS. White matter lesions and depression in patients with Parkinson’s disease. Journal of the neurological sciences. 2012;322:132–136. doi: 10.1016/j.jns.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Preziosa P, Rocca MA, Pagani E, Stromillo ML, Enzinger C, Gallo A, Hulst HE, Atzori M, Pareto D, Riccitelli GC, Copetti M, De Stefano N, Fazekas F, Bisecco A, Barkhof F, Yousry TA, Arevalo MJ, Filippi M. Structural MRI correlates of cognitive impairment in patients with multiple sclerosis: A Multicenter Study. Human brain mapping. 2016;37:1627–1644. doi: 10.1002/hbm.23125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Correia MM, Altena E, Hughes LE, Barker RA, Rowe JB. White matter pathology in Parkinson’s disease: the effect of imaging protocol differences and relevance to executive function. NeuroImage. 2012;62:1675–1684. doi: 10.1016/j.neuroimage.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawji KS, Yong VW. The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clinical & developmental immunology. 2013;2013:948976. doi: 10.1155/2013/948976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves TM, Phillips LL, Povlishock JT. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Experimental neurology. 2005;196:126–137. doi: 10.1016/j.expneurol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Rennels ML, Blaumanis OR, Grady PA. Rapid solute transport throughout the brain via paravascular fluid pathways. Advances in neurology. 1990;52:431–439. [PubMed] [Google Scholar]
- Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- Rinholm JE, Bergersen LH. White matter lactate--does it matter? Neuroscience. 2014;276:109–116. doi: 10.1016/j.neuroscience.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Rodriguez-Sabate C, Morales I, Sanchez A, Sabate M. Parkinson’s disease as a result of aging. Aging cell. 2015;14:293–308. doi: 10.1111/acel.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig S, Carmichael ST. Age-dependent exacerbation of white matter stroke outcomes: a role for oxidative damage and inflammatory mediators. Stroke; a journal of cerebral circulation. 2013;44:2579–2586. doi: 10.1161/STROKEAHA.113.001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, Schonmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DE. The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. NeuroImage. 2008;39:1522–1532. doi: 10.1016/j.neuroimage.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. The Journal of head trauma rehabilitation. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- Ryan J, Artero S, Carriere I, Scali J, Maller JJ, Meslin C, Ritchie K, Scarabin PY, Ancelin ML. Brain volumes in late life: gender, hormone treatment, and estrogen receptor variants. Neurobiology of aging. 2014;35:645–654. doi: 10.1016/j.neurobiolaging.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Saavedra A, Baltazar G, Santos P, Carvalho CM, Duarte EP. Selective injury to dopaminergic neurons up-regulates GDNF in substantia nigra postnatal cell cultures: role of neuron-glia crosstalk. Neurobiology of disease. 2006;23:533–542. doi: 10.1016/j.nbd.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Sacco R, Bisecco A, Corbo D, Della Corte M, d’Ambrosio A, Docimo R, Gallo A, Esposito F, Esposito S, Cirillo M, Lavorgna L, Tedeschi G, Bonavita S. Cognitive impairment and memory disorders in relapsing-remitting multiple sclerosis: the role of white matter, gray matter and hippocampus. Journal of neurology. 2015;262:1691–1697. doi: 10.1007/s00415-015-7763-y. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Cerebral cortex. Vol. 14. New York, N.Y. : 1991: 2004. Thinning of the cerebral cortex in aging; pp. 721–730. [DOI] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. NeuroImage. 2009;44:1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. The European journal of neuroscience. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Berman NE. Age-dependent response of CCAAT/enhancer binding proteins following traumatic brain injury in mice. Neurochemistry international. 2010;56:188–193. doi: 10.1016/j.neuint.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Experimental neurology. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Puri V, Klein RM, Berman NE. Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neuroscience letters. 2004;369:28–32. doi: 10.1016/j.neulet.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Archives of neurology. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Annals of the New York Academy of Sciences. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberger M, Ponsford J, Reutens D, Beare R, O’Sullivan R. The Relationship between age, injury severity, and MRI findings after traumatic brain injury. Journal of neurotrauma. 2009;26:2157–2167. doi: 10.1089/neu.2009.0939. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neuroscience and biobehavioral reviews. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Roberts KN, Markesbery WR, Scheff SW, Lovell MA. Oxidative stress in head trauma in aging. Free radical biology & medicine. 2006;41:77–85. doi: 10.1016/j.freeradbiomed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald AW, 3rd, Ragland JD, Silverstein SM, Godwin D, Barch DM. Evidence for Accelerated Decline of Functional Brain Network Efficiency in Schizophrenia. Schizophrenia bulletin. 2016;42:753–761. doi: 10.1093/schbul/sbv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkin SD, Bastin ME, Macgillivray TJ, Deary IJ, Starr JM, Rivers CS, Wardlaw JM. Cerebrovascular diseases. Vol. 20. Basel, Switzerland: 2005. Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people; pp. 310–318. [DOI] [PubMed] [Google Scholar]
- Shimamura M, Garcia JM, Prough DS, Hellmich HL. Laser capture microdissection and analysis of amplified antisense RNA from distinct cell populations of the young and aged rat brain: effect of traumatic brain injury on hippocampal gene expression. Brain research. Molecular brain research. 2004;122:47–61. doi: 10.1016/j.molbrainres.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Silverberg GD, Messier AA, Miller MC, Machan JT, Majmudar SS, Stopa EG, Donahue JE, Johanson CE. Amyloid efflux transporter expression at the blood-brain barrier declines in normal aging. Journal of neuropathology and experimental neurology. 2010a;69:1034–1043. doi: 10.1097/NEN.0b013e3181f46e25. [DOI] [PubMed] [Google Scholar]
- Silverberg GD, Miller MC, Messier AA, Majmudar S, Machan JT, Donahue JE, Stopa EG, Johanson CE. Amyloid deposition and influx transporter expression at the blood-brain barrier increase in normal aging. Journal of neuropathology and experimental neurology. 2010b;69:98–108. doi: 10.1097/NEN.0b013e3181c8ad2f. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skillback T, Zetterberg H, Blennow K, Mattsson N. Cerebrospinal fluid biomarkers for Alzheimer disease and subcortical axonal damage in 5,542 clinical samples. Alzheimer’s research & therapy. 2013;5:47. doi: 10.1186/alzrt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane JA, Hollander W, Moss MB, Rosene DL, Abraham CR. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiology of aging. 1999;20:395–405. doi: 10.1016/s0197-4580(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Song YJ, Halliday GM, Holton JL, Lashley T, O’Sullivan SS, McCann H, Lees AJ, Ozawa T, Williams DR, Lockhart PJ, Revesz TR. Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. Journal of neuropathology and experimental neurology. 2009;68:1073–1083. doi: 10.1097/NEN.0b013e3181b66f1b. [DOI] [PubMed] [Google Scholar]
- Spitz G, Maller JJ, O’Sullivan R, Ponsford JL. White matter integrity following traumatic brain injury: the association with severity of injury and cognitive functioning. Brain topography. 2013;26:648–660. doi: 10.1007/s10548-013-0283-0. [DOI] [PubMed] [Google Scholar]
- Suenaga J, Hu X, Pu H, Shi Y, Hassan SH, Xu M, Leak RK, Stetler RA, Gao Y, Chen J. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol. 2015;272:109–119. doi: 10.1016/j.expneurol.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama I, Tanaka K, Akita M, Yoshida K, Kawase T, Asou H. Ultrastructural analysis of the paranodal junction of myelinated fibers in 31-month-old-rats. Journal of neuroscience research. 2002;70:309–317. doi: 10.1002/jnr.10386. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiology of aging. 1997;18:609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Tanner JJ, Mareci TH, Okun MS, Bowers D, Libon DJ, Price CC. Temporal Lobe and Frontal-Subcortical Dissociations in Non-Demented Parkinson’s Disease with Verbal Memory Impairment. PloS one. 2015;10:e0133792. doi: 10.1371/journal.pone.0133792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Grothe MJ, Filippi M, Fellgiebel A, Dyrba M, Frisoni GB, Meindl T, Bokde AL, Hampel H, Kloppel S, Hauenstein K. Fractional anisotropy changes in Alzheimer’s disease depend on the underlying fiber tract architecture: a multiparametric DTI study using joint independent component analysis. Journal of Alzheimer’s disease : JAD. 2014;41:69–83. doi: 10.3233/JAD-131829. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Kutiyanawalla A, Pillai A. Age-dependent alterations in nerve growth factor (NGF)-related proteins, sortilin, and learning and memory in rats. Physiology & behavior. 2011;102:149–157. doi: 10.1016/j.physbeh.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HJ, McCormick WC, Kagan SH. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. Journal of the American Geriatrics Society. 2006;54:1590–1595. doi: 10.1111/j.1532-5415.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- Vesely B, Antonini A, Rektor I. The contribution of white matter lesions to Parkinson’s disease motor and gait symptoms: a critical review of the literature. Journal of neural transmission (Vienna, Austria : 1996) 2016;123:241–250. doi: 10.1007/s00702-015-1470-9. [DOI] [PubMed] [Google Scholar]
- Wang SS, Shultz JR, Burish MJ, Harrison KH, Hof PR, Towns LC, Wagers MW, Wyatt KD. Functional trade-offs in white matter axonal scaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:4047–4056. doi: 10.1523/JNEUROSCI.5559-05.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Senda J, Kato S, Ito M, Atsuta N, Hara K, Tsuboi T, Katsuno M, Nakamura T, Hirayama M, Adachi H, Naganawa S, Sobue G. Cortical and subcortical brain atrophy in Parkinson’s disease with visual hallucination. Movement disorders : official journal of the Movement Disorder Society. 2013;28:1732–1736. doi: 10.1002/mds.25641. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiology of aging. 1989;10:11–19. doi: 10.1016/s0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]
- Williams R, Buchheit CL, Berman NE, LeVine SM. Pathogenic implications of iron accumulation in multiple sclerosis. Journal of neurochemistry. 2012;120:7–25. doi: 10.1111/j.1471-4159.2011.07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse LE, Reijmer YD, ter Telgte A, Kuijf HJ, Leemans A, Luijten PR, Koek HL, Geerlings MI, Biessels GJ. Hippocampal disconnection in early Alzheimer’s disease: a 7 tesla MRI study. Journal of Alzheimer’s disease : JAD. 2015;45:1247–1256. doi: 10.3233/JAD-142994. [DOI] [PubMed] [Google Scholar]
- Wright SN, Kochunov P, Chiappelli J, McMahon RP, Muellerklein F, Wijtenburg SA, White MG, Rowland LM, Hong LE. Accelerated white matter aging in schizophrenia: role of white matter blood perfusion. Neurobiology of aging. 2014;35:2411–2418. doi: 10.1016/j.neurobiolaging.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Wilde EA, Bigler ED, Yallampalli R, McCauley SR, Troyanskaya M, Chu Z, Li X, Hanten G, Hunter JV, Levin HS. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. Journal of neurotrauma. 2010;27:303–307. doi: 10.1089/neu.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011:617927. doi: 10.4061/2011/617927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SP, Bae DG, Kang HJ, Gwag BJ, Gho YS, Chae CB. Co-accumulation of vascular endothelial growth factor with beta-amyloid in the brain of patients with Alzheimer’s disease. Neurobiology of aging. 2004;25:283–290. doi: 10.1016/S0197-4580(03)00111-8. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, Xie CW. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, Gorno-Tempini ML, Miller BL, Weiner MW. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain : a journal of neurology. 2009;132:2579–2592. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YZ, Chang C, Wei XE, Fu JL, Li WB. Comparison of diffusion tensor image study in association fiber tracts among normal, amnestic mild cognitive impairment, and Alzheimer’s patients. Neurology India. 2011;59:168–173. doi: 10.4103/0028-3886.79129. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang H, Ting SM, Song S, Gonzales N, Aronowski J. Polymorphonuclear neutrophil in brain parenchyma after experimental intracerebral hemorrhage. Translational stroke research. 2014;5:554–561. doi: 10.1007/s12975-014-0341-2. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Sachdev PS, Trollor JN, Reppermund S, Kochan NA, Brodaty H, Wen W. Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PloS one. 2013;8:e58887. doi: 10.1371/journal.pone.0058887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiology of aging. 2010;31:1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]