Abstract
Polymeric nanoparticulate carriers play an important role and holding a significant potential for the development of novel immunomodulatory agents as easily they are taken up by antigen presenting cells. They allow an enhanced antigen stability, better immunogenicity and immunostimulatory effect with sustained and controlled release of the antigen to the target sites. Better information and vital understanding of mechanism of action, interaction of such vectors with the APCs and dendritic cells and antigen release kinetics in immunomodulatory effects, and improved knowledge of their in vivo fate and distribution are now needed, those collectively would speed up the rational strategies of nanoparticles as carriers for vaccines and other protein antigens. The evolution of such innovative adjuvants for protein and DNA immunizations are an exciting and growing zone in immunology, which may enhance the clinical outcomes in many infectious and non-infectious diseases. This review summarizes the recent advances in nano-vaccinology with polymeric (especially biodegradable) carriers, their methods of preparation, surface modification, their interaction with antigen presenting cells, release of antigens, its kinetics and mechanism in the delivery of vaccines via non-invasive routes.
Keywords: Nanoparticles, biodegradable polymers, vaccine, noninvasive route, antigen presenting cells, dendritic cells
Introduction
The process of vaccination to encourage an adaptive immunity is thought to be used for many infectious diseases and cancers. The conventional forms of vaccines are primarily composed of live attenuated viruses, inactivated pathogens, or inactivated toxoids. These methodologies are successful for vaccine developments those induce an antigen-antibody and cytotoxic T lymphocyte (CTL) immune responses, and destroy infected host cells with intracellular organisms. The currently developed new generation vaccines are much safe, with defined components, but these vaccine systems have low immunogenicity, hence need some adjuvants and delivery carriers to induce sufficient immune responses [1]. Delivery of vaccines and adjuvants those induce antigen-specific humoral and cellular immunity are much fruitful for efficient vaccine systems development (Figure 1).
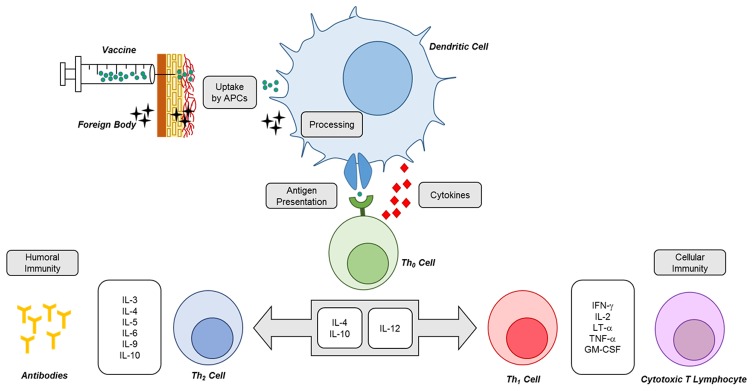
Figure 1.
Simple depiction of immune response mechanisms following nano-sized particulate vaccination. Uptake and processing of particulate antigen by dendritic cells is a crucial step that remarkably dictates the fate of immune response. The high cross-presentation ability of dendritic cells is a major trait that polarizes the ultimate immune response towards cellular or humoral immunity.
Due to the current progress in nano-sciences the nanoparticles (NPs) have become very striking for their applications in the fields of drug delivery, gene delivery, vaccine delivery and other biomedical applications. Nanostructured carriers (1-500 nm) exhibit unique functions and properties due to their particle size, shape and size distribution effects [2].
It is believed that nano-sized carriers would be capable of improved interaction with cell membrane and proteins as most of the bio-active agents and macromolecules like viruses, proteins and membranes are having natural nanostructures. NPs shape and size allow these carriers to be incorporated into a number of biomedical devices for diagnostic and remedial purposes [3].
The inventive progress in nanotechnology is currently being influenced to formulate therapeutic agents in biocompatible and biodegradable nanocarriers like NPs, nanocapsules, some magnetic NP conjugates, various micellar formulation systems and superparamagnetic iron oxide NPs. The small sized magnetite NPs with narrow particle size distribution and high magnetization values had been employed in many biomedical applications [4], such as targeted drug delivery [5], contrast agents in magnetic resonance imaging [6] and hyperthermia treatment of cancers [7].
Recently, biodegradable polymeric carriers have got a great attention by the researchers for various drug delivery systems, the underlying reason behind this superiority of biodegradable carriers have potential advantages over their non-degradable counterparts. As they have reduced toxicity and side effects as well as they do not accumulate in the cells and tissues even after repeated administration. Moreover, degradation of these carriers could be used as a tool for the release of the therapeutic agents and release of plasmid DNA into the cytosol [8,9]. The evolution of NPs has given an immense enthusiasm to the formulators for the development of new immunomodulatory agents, because these nanocarriers can easily deliver and efficiently manipulate the antigens at the target sites [10].
Besides biodegradable some non-biodegradable NPs were also investigated for the delivery of vaccines [11], where carbon, silica and most commonly gold [12] are used to encapsulate antigens and more frequently to provide surface for covalent attachment of antigens. Among these the most frequently used are gold NPs are 2-50 nm size range, Similarly, carbon NPs have been explored for the delivery of vaccines for oral use. Silica NPs have also been investigated as templates which were then carbonized using sucrose as a source of carbon under nitrogen gas. The NPs so obtained were around 400-450 nm size range with surrounded 50 nm mesopores on the surface of particles, within these receptacles an antigen could be sheltered from the gastrointestinal tract’s tough biological environment and encouraging good mucosal immunity after oral administration [13].
A large number of drugs, vaccines and genes are being delivered into the body using nanoparticulate carriers through a number of administration routes. Without any failure, the NPs are being used safely to deliver hydrophilic or hydrophobic drug molecules, proteins, vaccines, and many other biological macromolecules into the body.
These new generation vaccines are safe with well-defined ingredients, but here the antigens are less immunogenic hence require some adjuvants and suitable delivery systems to encourage maximum immune responses [1,14]. Adjuvants in immunology were firstly pronounced by Ramon as “substances used in combination with a specific antigen that produced a more robust immune response than the antigen alone” [15]. Till now, phosphate and hydroxide salts of calcium and aluminum are the main adjuvants approved for human use. However, there are some disadvantages associated in the use of alum-type adjuvants for vaccination [16]. So, the advancement of more effective and safest adjuvants in the delivery of vaccines to achieve a high and long-term immune responses is prime focus for the researchers in this era [17]. Therefore, development of nanocarriers for vaccines in the field of immunogenicity have drawn the attention as some nanocarriers have been found themselves to be antigenic and some have been revealed to increase antigenicity of even the conjugated weak antigens and hence assist as adjuvants. The property of nanocarriers to increase antigenicity depends up on NPs surface property (zeta potential) and their sizes and can expressively contribute to the development of better quality vaccine formulations [18-21].
Even though the previously established alum-based adjuvants, often fails to induce cellular immune responses and was found reactogenic to the host cells, but the nanocarriers afford an alternative for the delivery of antigens, which not only triggers different components of the immune system but also have excellent biocompatibility and biodegradability. Because of their sizes, nanocarriers move and processed as such in to cells through non-classical pathways and easily express various immune responses.
In this manuscript, we reviewed and discussed mainly some biodegradable polymeric NPs containing surface-immobilized and encapsulated antigens as carriers for vaccine delivery via non-invasive routes which are alternatives to the presently used traditional alum carriers for vaccines.
Biodegradable polymeric nanoparticles: introduction and development
Biodegradable polymeric nanoparticles
The biodegradable materials are natural and synthetic in origin and by enzymatic or non-enzymatic actions, can be degraded easily when used in vivo, to produce biocompatible, toxicologically safe by-products which can be easily eliminated by the normal metabolic pathways of the body [22]. Several natural and synthetic polymers like polylactide-co-glycolide, polylactic acid, chitosan, alginate, carbopol, gelatin, dextran etc., have been extensively explored for the effective mucosal delivery of vaccines and substantial results have been obtained [23]. Biodegradable polymeric NPs have been extensively used for site-specific delivery of vaccines. Generally used biodegradable polymers and their matrices for the development of nanoparticle preparations are.
Poly (lactide-co-glycolide)
On the basis of uniform particle size and better encapsulation efficiency, poly (lactide-co-glycolide) (PLGA) is one of the most successfully used biodegradable polymeric matrices. They are hydrolyzed in the body tissues and results in to lactic acid and glycolic acid as monomeric metabolites which are biodegradable in nature. Normally, lactic and glycolic acids are found in the body where they contribute in a number of biochemical and physiological reactions, hence a minimum or no systemic toxicity and side effects are associated in the use of PLGA for the drug delivery and biomedical applications. Moreover, PLGA based nanoparticulate carrier system have been extensively used to encapsulate proteins, peptide, vaccines, and genes for in vivo delivery [24-26].
Polylactic acid (PLA)
It is a biocompatible and biodegradable polymer which is broken in to lactic acid monomeric units in the body tissues. Lactic acid is a natural by-product of anaerobic cellular respiration, in the Cori cycle by the liver it is converted into glucose and is used as an energy source in the body, hence PLA based NPs are safe and non-toxic [25].
Poly-ε-caprolactone (PCL)
Under the normal physiological conditions in the human body, poly-ε-caprolactone is broken down by hydrolysis to its ester forms and has minimum toxic effects. The rate of degradation of PCL is slower than the polylactides, so it is considered as better candidate for making controlled and sustained delivery carriers and implantable devices [27]. A model antigen, tetanus toxoid loaded small sized PCL NPs produced an M1/M2-macrophages polarization of human blood monocyte-derived macrophages and helper T-cells polarization of autologous CD4+ T cells and an efficient CD8+ T-cell responses were also stimulated. In one study, tetanus toxoid loaded small PCL NPs were found to be able to produce persistent and strong cell mediated and humoral immunity against tetanus toxoid two months after single injection in mice and without any booster dose [28].
Chitosan
Chitosan is a natural hydrophilic biopolymers, non-toxic, biocompatible, biodegradable and have adsorption and mucoadhesion properties. It is a biopolymer of residues of glucosamine, obtained from wastes of seafood like; shrimp, crab shell and cell wall of fungi [29]. It has been found to be nontoxic in both experimental animals and humans. Chitosan are more focused than other polysaccharides as it is biocompatible, degrade into non-toxic byproducts in vivo and has capability to open up the intercellular tight junctions of epithelial cells [30]. It has immune-enhancing effects, and has been used as adjuvant for vaccines, carriers for gene delivery [31], carriers for protein and many other drug molecules [32-35]. Chitosan has been used as a carrier for novel nasal vaccine delivery and are found suitable carrier for mucosal drugs and many other vaccines [36]. Chitosan NPs strongly induces immune response against many antigens and enhances uptake of antigen by mucosal lymphoid tissues. As novel adjuvant for vaccines, chitosan has been used as carrier for hepatitis-B vaccine [37], piglet paratyphoid vaccine [38], H3N2-influenza vaccine [39]. Chitosan oligomer as an adjuvant for A/H5N1-influenza vaccine was used and found that it enhanced and stimulated the immune response rate and high titers of autoantibodies in mice [40]. Hence, chitosan NPs are considered as an excellent and potential adjuvant for A/H1N1 influenza vaccines [41]. Moreover chitosan NPs has been extensively investigated as a mucosal gene carrier, and chitosan based DNA NPs have been found effective in the induction of mucosal and systemic immune responses in many studies [42-44], also chitosan NPs as a pneumococcal DNA vaccine carrier has been reported recently [45].
Gelatin
Gelatin is a widely used ingredient in medical and food products and is a good nontoxic biocompatible and biodegradable alternative. Gelatins are nontoxic, biodegradable, bioactive, and economic as well as they are efficient in controlled drug delivery of many actives. Being a poly-ampholyte gelatin consist of cationic and anionic groups along with hydrophilic group, so the swelling behavior and thermal properties of gelatin based NPs, depend up on crosslinking between cationic and anionic groups, hence these properties of gelatin can be exploited to develop a preferred kind of NPs [46,47]. Cationized gelatin NPs have been used as a biodegradable and well tolerated carrier for immunostimulatory CpG oligonucleotides (CpG-ODN) in murine system and was found that NPs enhanced the uptake of CpG-ODN and strongly increase the activation of the immune system [48].
Dextran
Dextrans are hydrophilic polysaccharides with ≥ 1000 Dalton molecular weights, which have a linear backbone of α-linked D-glucopyranosyl repeating units and have high water solubility. The hydroxyl groups present in dextran propose sites for derivatization, and these functionalized glycoconjugates represent a class of biocompatible, biodegradable and environmentally safe compounds for the delivery of drugs and other actives [49]. In a study, a model enzyme, horseradish peroxidase (HRP) was encapsulated in an acid-sensitive, tunable biodegradable polymer, acetylated dextran (Ac-DEX) and the vaccine stability outside cold chain conditions was evaluated. It was found that Ac-DEX particles remained spherical in nature, as compared to PLGA particles, and HRP encapsulation reduces its storage temperature dependence and enhances its stability outside cold chain conditions. This indicates that an Ac-DEX based micro or and nanoparticulate carrier systems has extensive use as vaccines and other drug molecules [50].
Alginates
The anionic polysaccharide, alginate obtained from cell walls of brown algae is a linear copolymer with homo-polymeric blocks of (1-4)-linked β-D-mannuronate and its C-5 epimer α-L-guluronate, respectively and linked covalently with each other in different blocks. The diphtheria toxoid loaded NPs were developed by using alginates and characterized by immunizing guinea pigs and immunization parameters were compared with the conventionally used vaccine, the diphtheria toxoid-loaded alginate NPs showed highest humoral immune response as compared to conventional ones. Thus, alginate based NPs can be considered as a novel promising nanocarrier for vaccine with high immunogenicity adjuvant system [51]. Alginic acid-coated chitosan NPs as carrier for oral delivery of legumain based DNA vaccine was developed to protect against breast cancer [52]. The chitosan NPs coated with alginic acid was found to be a safe and proficient implement for oral delivery of legumain-DNA vaccine and effectively improved the autoimmune responses and protected against breast cancer in mice.
Development of biodegradable polymeric nanoparticles
Previously the NPs and microparticles were developed by using the polyalkyl-cyanoacrylate, but due to the larger size ranges of microparticles the initial zeal for their use in the field of medicine was diminished. The microparticles were not able to cross the intestinal mucosal barrier of the gastrointestinal tract when they were used as a carrier in oral drug delivery system hence were assumed as failed delivery system. On the other hand, due to their sizes NPs have got benefit over microparticles and found better even for intravenous delivery of drugs [26]. Biodegradable polymeric NPs can be synthesized by using a number of materials like proteins, polysaccharides and synthetic decomposable polymers and the choice of these materials and polymers is depend on the designing of delivery carriers and their applications. Number of methods is now available for the preparation of various kind of polymeric NPs, depending upon the nature and property of drugs (solubility and stability) to be encapsulated in the polymer matrix, targeted organ, and route of administration and mechanism of drug release. Biodegradable polymeric NPs for vaccine delivery are generally prepared by using a number of polymers (discussed above): e.g. poly (α-hydroxy acids), poly (amino acids) and polysaccharides to create a vesicular structure which can either accommodate or adsorb antigens.
Double emulsion-solvent evaporation technique
The poly (α-hydroxy acids) used are poly-D-L-lactide-co-glycolide acid (PLGA), and PLA which are generally prepared by double emulsion-solvent evaporation technique [53], where the polymer is dissolved in organic solvent followed by the addition of the antigen then homogenized to get a primary emulsion. With the addition of an emulsifying agent a water-in-oil-in-water multiple emulsion is then formed, which results in the precipitation of the polymer around the antigen [54,55]. The obtained multiple emulsion solution is dried by solvent evaporation technique to prevent the polymer degradation due to ester hydrolysis catalyzed by water [56,57]. However stabilizers are used to prevent protein (antigen) denaturation, but still this method has limitations because of low antigen entrapment efficiency and protein denaturation at the oil-water interface [58]. The problem of degradation and denaturation of protein, as well as low encapsulation of antigens can be solved by the use of stabilizers like, carrier proteins (e.g., albumin) and surfactants at the time of primary emulsion preparation, or by the use of some cryoprotectant like, glucose, trehalose and mannitol to the protein phase. Stability of proteins can also be improved when they will be encapsulated as a solid rather than in solution forms [59].
Moreover, the antigen encapsulation and stability can be enhanced by the use of biocompatible amphiphilic copolymers i.e. poly (amino acids) like poly (γ-glutamic acid) (γ-PGA), poly (ε-lysine), poly (L-arginine) and poly (L-histidine) which do not require an emulsion step for the NPs preparation [60]. Moreover, the common protease enzymes do not easily recognize these γ-linked glutamic acids in γ-PGA, so provides more stability [58]. In this situation dimethyl sulfoxide (DMSO) is used as organic solvent and rather than emulsification, centrifugation process is used to prepare the NPs, resulting a uniform sized with higher percentage of antigen-encapsulation efficiency, which are stable over wide range of acidic pH for a long duration [61]. Hence, it is thought that the γ-PGA linked with L-Phenylalanie based NPs would have greater potential for drug and vaccine carriers. Similarly, alkyl esters of γ-PGA based biodegradable NPs have been investigated and reported as potential drug and protein carriers [62].
Preparation of chitosan nanoparticles prepared by different methods
Polysaccharide like chitosan is generally used to encapsulate antigens. Because of the biocompatibility of chitosan more attention has been given on it for NPs preparation [30]. There are many ways of chitosan NPs preparation but a complex coacervation process is preferred for the encapsulation of the protein antigens where NPs are formed extemporaneously when two or more hydrophilic colloids are mixed, the chitosan precipitates and surround the plasmid-DNA to prevent the nuclease degradation of plasmid-DNA [63]. An ionotropic gelation technique is used for chitosan NPs NPs for vaccine delivery which is grounded on interaction of positively charged amino groups in chitosan molecules and the negatively charged cross linkers like, tripolyphosphate and so obtained colloidal systems can be modified further with the addition of adjuvant like polyethylene glycol on the particles surface to enhance antigen absorption and to attain a sustained and slow release of antigens. Chitosan NPs are prepared by various techniques which are summarized in Table 1.
Table 1.
Preparation of chitosan nanoparticles by different methods
| Methods | Remarks | References |
|---|---|---|
| Self-assembly technique | The homogeneous solvents like, ethanol or hexane are used for the dispersion of NPs. Polyvinyl alcohol (PVA) and polyvinyl pyrrolidone (PVP) are used as stabilizer in this technique. NPs are formed by entropy-driven forces that lead to self-assembly. | [64] |
| Complex coacervation process | Formation of NPs occurs spontaneously when two hydrophilic colloids mix together and electrostatic forces take place e.g. cationic chitosan (CS) precipitates around anionic plasmid DNA. The degradation of DNA by nucleases are also protected by CS. | [63] |
| Emulsion-droplet coalescence technique | This technique is based on the emulsion crosslinking and precipitation of chitosan around the drugs or proteins. Preparation of gadopentetic acid-NPs for the treatment of tumors, showed a sustained release and enhanced retention within the tumors. | [65] |
| Ionic gelation method | Macromolecules those are hydrophilic natural polymers like gelatin, alginate, chitosan and agarose have been used in this technique. NPs obtained by ionic gelation need surface modifications, PEG are used for this to prevent the macrophages activity, as well as to help in the absorption and to provide sustained release of drug. | [66,67] |
| Polymerization methods | Polymerization of monomers in an aqueous medium is the mechanism of NPs formation. Polybutyl cyanoacrylate and poly-alkyl-cyanoacrylate NPs have been prepared by this method. | [68,69] |
| Nanoprecipitation method | This method is generally used for the encapsulation of hydrophobic and hydrophilic drug molecules. The polymers and drugs are dissolved in a polar and water-miscible solvents, drop by drop addition of this solution into aqueous surfactant results desired sized NPs directly by quick diffusion of solvent. | [70] |
| Salting-out method | Polymers are dissolved in water miscible organic phase and then emulsified by salts (e.g. magnesium chloride hexahydrate or magnesium acetate tetrahydrate) which are not soluble in the organic phases. The addition of water to the obtained primary o/w-emulsion under magnetic stirring leads to the migration of the water-miscible organic phase to the aqueous phase, resulting nanospheres formation. NPs are obtained by ultracentrifugation to remove the agents responsible for salting out process. | [71,72] |
Polymeric nanoparticles for vaccines delivery
The researchers in recent era have given much attention to the biodegradable polymeric nanocarriers because of their ability to deliver both hydrophilic and lipophilic therapeutic agents, genes, vaccines etc. and of course they are biodegradable in nature [73]. Biodegradable polymeric NPs have been given much attention as potential vehicles for vaccine antigens which can stabilize antigens as well as act as adjuvants. More important is that some of these NPs are even have ability to enter antigen presenting cells (APCs) by various pathways, hence modulate the immune responses. Different biodegradable polymers have been already used in solid particulate form for vaccine delivery [74]. In these delivery systems the antigens are either encapsulated within and/or adsorbed onto the surface of the NPs. Attachment of antigens onto the surface of NPs allow the immunogen presentation to the immune systems in the similar way that it would be presented by pathogens and hence engender a similar response [58]. Wilson et al. developed a pH-responsive, endosomolytic polymeric NPs carrier for vaccine delivery to enhance cellular and humoral immunity. Such kind of NPs were developed by using amphiphilic diblock copolymers (consisted of ampholytic core-forming block and polycationic corona block fixed with pyridyl disulfide group to empower a dual-delivery of antigens as well as immunostimulatory CpG oligodeoxynucleotide (CpG-ODN) adjuvants. They assembled simultaneous packaging of CpG-ODN and a thiolated protein ovalbumin (OVA) as antigen in to polymeric NPs. The subcutaneous administration of the developed vaccine carrier in to mice, the OVA-NP adjuvant significantly expressed higher CD8+ T-cell response as compared to the group of mice vaccinated with free OVA. Similarly, intradermal injection of OVA-NP adjuvant further amplified the cellular immunity. They concluded that the developed pH-responsive, endosomolytic NPs encouraged antigen cross-presentation and boost cellular and humoral immunity through dual-delivery of antigens and CpG-ODN, hence this carrier system is a promising and have potential for the delivery of protein subunit vaccines [75].
A targeted carrier for vaccine delivery was developed using biodegradable PLGA with superparamagnetic iron oxide particles and the antigen was labeled fluorescently in single particulate system [76]. The targeted delivery of vaccine carriers coated with antibodies to the dendritic cells in vivo was studied, which are primarily responsible in the instigation of adaptive immunity and recently have been exploited in the immunotherapy against cancerous and many infections [77].
Vesicular carriers for oral delivery of vaccines
A number of technologies and vesicular approaches have been designed the delivery of vaccines. The carriers like liposomes [78], non-ionic surfactant based vesicles niosomes [79], the highly flexible and soft membrane transfersomes [80], a novel lipid based and significantly stable than liposomes oral delivery system archaeosomes [81] and virosomes [82,83] are frequently used novel carrier systems for topical applications of therapeutics and vaccines. The tetanus toxoid was applied topically on to the skin of albino Wistar rats through vesicular carriers like liposomes, niosomes and transfersomes and their respective immune responses were compared by analyzing the antibody serum IgG, and they found that the immune responses of such non-invasive immunization by vesicular carriers were almost similar to that of the invasive intramuscular injection of the same dose of tetanus toxoid [84]. Topical use of non-invasive vaccination on skin through the above vesicular carriers would advance the vaccination curricula as there would not be a trained personnel requirement and needle pricking could be escaped. A number of immune cells are present on skin, when it comes in contact with immunogens, they provoke a robust specific as well as non-specific immunological responses by various mechanisms. The immunogens from such vesicular carriers can transfer effectively across the skin via follicular pathway, lamellar lipids and through corneocytes as well [85].
The versatility and plasticity are the two major advantages of liposomes, archaeosomes and virosomes-based carriers for vaccine delivery. On the basis of chemical properties, hydrophilic antigens like, proteins, peptides, nucleic acids and carbohydrates can encapsulated in the aqueous inner space of liposomes and hydrophobic moieties like antigens, lipopeptides, linker molecules and adjuvants are introduced in the lipid bilayer [78,86]. Liposomes have been studied for their comparative potential in non-invasive delivery of tetanus toxoid [84]. Modified liposomes as cationic adjuvant liposomal preparations for vaccine delivery have been developed with an adjuvant CAF01 consisting of mycobacterial cord factor glycolipid trehalose dibehenate as immunomodulator, and dimethyl dioctadecylammonium to give positively charged surface. Similarly cationic liposome-DNA complex have been developed to establish an effective targeting of human papilloma virus (HPV) type-16 for cancer immunotherapy [87] and muramyl dipeptide, monophosphoryl lipid-A and listeriolysin-O as adjuvants have been developed also for vaccine delivery [88].
To activate the systemic and mucosal immune systems, the immunity stimulating effects of archaeosomes as carrier for oral administration have been found to be superior as compared to liposomes and other conventional formulations in providing antigen specific systemic as well as mucosal immune responses. Moreover, archaeosomes efficiently facilitates antigen specific CD8+ T-cell proliferation [89], however the mechanisms of action is not well known [90]. The study of archaeosomes indicated that it had good stability in simulated intestinal and gastric fluids, showed prolonged residence in the GI tract after oral administration, and immunity against ovalbumin (OVA) antigen were effectively enhanced by stimulating the considerable systemic (IgG) as well as mucosal (IgA) responses [81]. Therefore, archaeosomes might be considered as a potential carrier for vaccines and adjuvant for efficient oral immunization and may hold a great promise in developing oral vaccines for weak antigens [91].
Advances in surface modifications
NPs have different problems as delivery carriers and have very short circulating life span within the body after intravenous administration and rapidly cleared from the body due to cellular phagocytosis, hence the therapeutic efficiency of actives was slow down and could not be sustained for a long duration. However, by the surface modification technique for NPs, the complexity of their phagocytic elimination has been resolved, because the surface modified NPs are protected well from the cellular phagocytosis and their early removal from the vascular systems when delivered intravenously [92].
When nanoparticulate carriers are used through intravenous route the macrophages detects as they are foreign body and remove them immediately in the body. Therefore, there is need of surface modification of the injected NPs in such a way that the macrophages did not recognize as foreign bodies and will not be engulfed by macrophages from the circulation [93].
Biodegradable NPs loaded with antigen and surface modified with bacterial lipopolysaccharides were found to have dynamic improvement in immune response against the encapsulated antigen. Such kind of vaccine systems are thought to be good as they are flexible in nature, allowing easy addition of antigen, adjuvant, and potentiators for immunity [94]. The surface modification of NPs and mechanisms involved in this process allow the specific moieties or antigens to be incorporated into the modular system without any significant alteration of their transfection efficacy and safety [95]. By taking a lead from the modular aspect of a virus, a modular DNA-delivery system has been developed by using DNA, dendrimer and silica NPs and cellular fate of the modular DNA delivery were studied [96].
Modification of NPs surface with biomolecules those are normally present in human body will be capable to be present in the body for a long duration, so that there will more chances for the carrier system to reach safely, rapidly and effectively to the target organ as compared to non-surface modified NPs. Researchers have developed many methods for NPs surface modification, among them the most common practiced method is surface coating of prepared NPs with poly-ethylene glycol (PEG), this approach enhances the biological half-life of such nano-carriers in the blood circulation. The steric repulsion caused by PEG on the surface of NPs are thought to be the reason to prevent the opsonization of NPs and hence longer retention in the circulation [97].
Protamine is also used as surface modifying agent [98], encapsulated ovalbumin (OVA) antigen into PLGANPs coated with protamine. They used with and without protamine coated OVA-NPs to stimulate murine bone marrow-derived dendritic cells (BMDCs) and found an increase in endocytosis of protamine-coated NPs by BMDCs. Protamine coated OVA-NPs caused significant upregulation of CD80, CD86, and CD83, increased secretion of IL-12p70, and decreased production of IL-4 as compared to non-protamine coated OVA-NPs by BMDCs. The results of the study concluded that protamine-coated OVA-NPs improve the cross-presentation of encapsulated exogenous antigen by helping in uptake of the antigen and lysosomal escape, therefore protamine coated OVA-PLGA-NPs are potent adjuvant and vector for cellular vaccines [98].
Streptococcus equi enzymatic extract adsorbed poly (lactic acid) NPs were developed and characterized as well as surface of NPs was modified by using glycolchitosan (GCS). GCS treated surface modified NPs improved the sustained release profile of the adsorbed enzyme as even after 30 days release study, 20% of protein were found to be attached on the surface of NPs. Desorption studies on developed NPs shown initially a burst release profile, followed by a sustained release [56]. Other than PEG, protamine and glycolchitosan some more hydrophilic polymers like, Pluronics, Polysorbates, polysaccharides (dextran) are also being used effectively as surface modifying agents for antigen coated biodegradable NPs [99].
Noninvasive routes of vaccine delivery
Administration of vaccines through needle and syringe causes acceptability issues especially in children and infants, moreover in some developing countries the syringes and needles are reused inappropriately which may cause a high risk of infection. Therefore, a noninvasive approach for oral vaccination would appear a fruitful alternative. Till date, live attenuated vaccines are only being used orally in human beings and the efficacy of such vaccines greatly depends on the spread of these vaccines in the gastrointestinal tract and their subsequent. Sometimes, the reversion of the vaccines to virulent forms might occur that limits the use of live attenuated vaccines for many infectious diseases [100]. Development of orally administered vaccines with replication-lacking viral vectors could be a better substitute for the live attenuated vaccines.
With the consideration of more than 90% of infections occur through mucosal surfaces, vaccination at mucosal sites is an important objective for the researchers, as mucosal or oral vaccination resulted in mucosal IgA-immune responses in the respiratory tract [101] in contrast to the current parenteral vaccinations which provide virus-neutralizing antibodies (IgG) in the lungs which might protect influenza infections [102]. On the basis of such findings, Tumpey et al. verified that mucosal IgA responses exhibit cross-protective immunity against antigenic viruses, and concluded that induction of mucosal immune response by oral vaccination might offer wide and better safety against drifted, heterologous strains [103].
The stimulation of mucosal immune responses needed the presence of lymphoid tissues associated with mucosa that provide a source of B-cells and T-cells [104]. Inductive sites in the respiratory and gastro-intestinal tracts are well defined which are consisted of aggregated lymphoid tissues (like, gut, nasal, and bronchial-associated lymphoid tissues) and mucosa-associated lymph nodes (like, mesenteric and mediastinal lymph nodes). Whereas, the vaginal mucosa is lacking of histologically perceptible mucosa-associated lymphoid tissue so at this site the role of inductive site is played by draining iliac lymph nodes. Additionally, antigen-uptake through the vaginal mucosa and immune responses in the genital tract are regulated by the estrus phase and hormonal status in female, so the female genital tract has some unique features that could be considered in the development of mucosal vaccination [105,106].
In an attempt the T-cell priming was studied after immunization through vaginal route in hormone synchronized mice, and found an effective activation of CD4+ T-cells, but antigen-specific CD4+ T-cell clonal growth was even detected in iliac lymph nodes, and proliferated T-cells scattered towards spleen and distal lymph nodes, same as that has been observed after immunization through nasal route [107]. They concluded that vaginal immunization through vaginal route is effective in stimulating CD4+ T-cell priming even though in the genital tract there is no any organized mucosa-associated inductive sites [108]. An early clonal growth of antigen-specific CD4+ T-cells were found in cervical and mediastinal lymph nodes as well as in the nasal-associated lymphoid tissues in three days of post immunization via nasal route by Streptococcus gordonii as recombinant vaccine vector [109], and the divided T-cells were detected five days post priming in the respiratory, intestinal and genital tracts in the dispersed [110].
Generally lungs are considered as the major site of infections and pathology, hence immunity in this organ has shown a significant effect in controlling the primary infections caused by Mycobacterium tuberculosis and immunity against this microorganism majorly depends up on stimulation of cell-mediated type of responses, and an essential role in this process has been played by gamma interferon in human and animal models. Doherty compared oral immunization to subcutaneous immunization in mice by using a subunit vaccine, loaded with two immunodominant proteins of M. tuberculosis, in a blend of detoxified monophosphoryl lipid-A (MPL) based adjuvants. His result indicated a significant induction in type 1 responses after oral boosting comparatively to subcutaneous immunization protocols [111].
Mucosal immunization
In mucosal immunization process, vaccination can be done by oral or intranasal routes by nano-sized particle aerosol. In one study, a Rift Valley fever (RVF) MP-12 vaccine was given to monkeys by intranasal drops, they developed about 80% plaque reduction neutralization titers of ≥ 1:40 and the developed neutralizing antibodies were found to protect RVF in monkeys when tested with the virulent RVF [112]. They concluded that immunization with RVF MP-12 live attenuated vaccine via intranasal route (aerosol) provided an effective immunization against RVF and better alternative route of administration as compared to the traditional intramuscular route.
Diphtheria toxoid was encapsulated in to poly-epsilon-caprolactone (PCL) based polymeric NPs and subjected for their potential as a mucosal vaccine delivery and compared with the PLGA based polymeric NPs carrying the same toxoid. They used Caco-2 cells for in vitro study and found satisfactorily higher uptake of hydrophobic PCL NPs as compared to PLGA based nanocarriers and this similar higher uptake of PCL NPs was also found in case of in vivo experiments following nasal administration. PCL based NPs induced diphtheria serum specific IgG antibody responses sufficiently as compared to PLGA-NPs and significant positive correlation was found between hydrophobicity of PCL-NPs and the immune responses following intranasal as well as intramuscular administration of the nanocarriers. The cytokine assays exhibited the serum IgG antibody response induction was different through different routes of administration, which was designated by the varying IL-6 and IFN-gamma levels. They concluded that the nanocarriers motivating the highest IgG antibody responses did not essentially provoke the higher cytokines IL-6 and IFN-gamma levels [113].
Oral spray immunization
Globally, there is a great challenge in the health sector scientists and formulators in developing the non-invasive needle-free vaccines [114]. In an experiment by Stahl et al. a non-replicating viral vector vaccines was developed and tested whether a natural pathway to the inductive site of the mucosa-associated lymphatic tissues can be used for noninvasive immunizations [100]. On to the tonsils of rhesus macaques, they sprayed the developed replication-deficient viral vector vaccines and named the method tonsillar immunization, this method of immunization caused the cellular and humoral immune responses induced by simian immunodeficiency virus (SIV) antigens [115,116]. A reduced level of viral-RNA after a stringent SIV challenge was found and a protection level almost equivalent after systemic immunization with same vaccine was observed. Therefore, the oral immunization with viral vectors against systemic and predominantly respiratory tract infections can be considered as a novel approach to avoid the difficulties associated with syringe and needle. Moreover, such replication-deficient vectors can be used to deliver vaccines to mucosal lymphatic tissues, can sweep over the epithelial barrier and can prevent any tolerance induction. Similarly, a subtopical delivery of Ankara vaccines into the palatine tonsils of rhesus macaques through needle-free injection device induced a considerable responses and suppression of viral burden post challenge of SHIV89.6P [117].
Oral influenza vaccine
The oral influenza vaccines can be targeted in the upper or lower part of the gastro-intestinal tract to give an effective immune responses, the same strategy has been tried in mice [118]. The study of Koping-Hoggard et al., 2005, revealed that without adjuvant significant systemic but little respiratory mucosal immune responses were encouraged in mice after influenza subunit vaccine delivery to the upper (intragastric) and the lower (intracolonically) gastro-intestinal tract. They observed that these responses were expressively enhanced when the same vaccine was adjuvanted with the heat-labile enterotoxin of Escherichia coli. The delivery of the vaccine with adjuvant in to the lower gastro-intestinal tract i.e. intracolonic delivery, induced better cellular immune responses and the preferred Th1-skewing of the responses and the intragastric delivery of the same adjuvanted vaccine were also found to augment T-helper responses but no Th1-skewing was found in this case. Hence, it can be concluded that, the accurate blend of strong mucosal adjuvant like, enterotoxin of Escherichia coli and of course the site of antigen delivery, e.g. in the lower part of the gastro-intestinal tract potentiate effective vaccination by means of oral route.
Nasal delivery of vaccines
The potential use of nanocarriers for mucosal vaccine delivery has found considerable advantages in the field of nanotechnology. In fact most of the vaccines including protein antigens and DNA-vaccines are very unstable in biological environment and limited efficacy due to poor crossing capacity to biological barriers, hence needs protection against degradation and design of appropriate antigen carriers overcome these challenges respectively [119]. The design of polymeric nanocarriers for nasal delivery of vaccines is one of the appropriate solution for these problems. Due to an effective and significant physical and chemical defensive barriers the nasal delivery of naked plasmid-DNA vaccine induces very weak immune responses [120]. Therefore, to improve the immunogenicity by mucosal delivery of DNA vaccines, chitosan, a natural biodegradable polymer based NPs as a carrier could be used. Recently, chitosan NPs was used as a pneumococcal DNA-vaccine carrier [45]. Xu et al., 2011 immunized the BALB/c mice through intranasal route by chitosan encapsulated psaA-NPs, and evaluated its efficacy in developing the mucosal and systemic immune responses and protection against nasopharyngeal colonization. They found that the mucosal, systemic, and cellular immune responses were encouraged with boosted level with chitosan-psaA vaccines by intranasal immunization. Also, the nasopharyngeal carriage was found to be decreased effectively in the immunized mice, suggesting that nasal delivery of such vaccines could be a noninvasive, efficient, convenient and easy route for DNA-vaccines delivery against pneumococcal infections.
Delivery of antigen from nanocarriers and its interaction with antigen presenting cells (APC)
Nanocarriers can deliver antigens in various ways, which has a considerable and thoughtful impact on the immune responses, whether the antigen is adsorbed on to the surface of NPs to attach with the APCs or entrapped in to the core of nanocarriers for sustained/controlled release and enhanced the exposure duration to the immune systems. NPs are adaptable and flexible which can be modified easily with immunostimulatory components to boost the intensity of the immune responses and with the surface modifiers to enhance their in vivo stability and steadiness [58].
The biodegradable polymeric NPs carrying antigens represent an exciting approach related to antigen-specific cellular and humoral immunity through the targeting of antigens to antigen presenting cells (APCs) and consequently induce maturation and cross presentation of antigens to initiate the strong immune responses [21,121]. Dendritic cells (DCs) and macrophages frequently targeted in designing of vaccines, are specific APCs considered as initiators and modulators of immune responses which competently uptake and process the antigens [122]. The DCs and macrophages both process antigens via major histocompatibility complex (MHC class-I and MHC class-II) pathways. The immature DCs come across the antigens, nanocarriers, and pathogens (bacteria and virus) at the site of administration and phagocytosed them and then presenting antigens on MHC class-II or MHC class-I molecules [123,124]. Hence, delivery of antigens to DCs is having significant meaning to develop efficient vaccines. The safety, efficacy and specificity of antigen delivery to DCs, DC-targeting approach is the latest technique which comprising a fusion antibody against DC-specific antigen receptors where heavy chains are conjugated with an antigen and a systemic administration of adjuvants were given for the growth of DCs [125], this technique has already been used and validated in different diseases in many experiments, e.g. LcrV is a virulence protein obtained from Yersinia pestis was targeted to DCs in to mice which protected them against pneumonic plague [126], similarly HER2/neu is a non-mutated tumor antigen was targeted to mature DCs for the induction of a combined immunity in mice that protected them against breast cancer [127]. Even though DC-targeting approach seems to be a useful implication but still having some limitations like, size and solubility of antigens to the DC-targeting antibodies, need of mammalian cell transfection to produce well-designed antibodies, and adjuvants may cause adverse reactions or hyper activations due to systemic administration [128,129]. Use of nanotechnology to develop nanosized protein cage for the advancement in DCs-based vaccine strategy could solve these challenges, as protein cages were found to have better choice for accommodation of varied sizes of antigens and their solubility, cost effective as they are produced easily in E. coli, and addition of selective adjuvants and antigens together are possible in such cages. Lumazine synthase protein cages as efficient DCs-based vectors for vaccines have been analyzed for its functional roles in various diseases [130].
The physicochemical characteristics like size, shape, and zeta-potential exert an important function for the interaction of NPs and APCs. In association with the targeting strategy the size and shape, zeta-potentials and differences in material properties of nanocarriers display substantial part for the uptake of antigens. NPs were effectively taken up by macrophages as compared to microparticles [131]. The comparable size of NPs to pathogens can easily be recognized and subsequently taken up by APCs to induce immunity. The virus sized NPs in size ranges 20-200 nm are easily taken up by DCs and the particles in micron size (0.5-5 μm) are preferentially taken up by macrophages [132]. PLGA-NPs of 300 nm sized showed good internalization and high activation of DCs as compared to 1 μm particles and similarly, higher acceptance of 200-600 nm sizes PLA-NPs as compared to 2-8 μm sized particles were observed by macrophages [131,133]. But there are differences in optimum size of nanoparticulate carriers for vaccine, as DCs were found to have higher uptake efficiency of an amphiphilic poly (amino acid) based NPs (30 nm) as compared to 200 nm sized NPs of the same polymer, while there were no differences in macrophages uptake of polyacrylamide hydrogel NPs (35 nm) and microparticles (3.5 μm). These differences in uptake of nanocarriers might be due to the differences in the materials properties, as different polymers used as vaccine carriers have potency to initiate an effective immunity at a particular optimum size range [134].
In addition to the size, shape of NPs significantly affects the interaction of APCs with the vectors. The shape of nanocarriers has a leading role in phagocytosis by macrophages because engulfing of such carriers is strongly reliant on their shape at the interface of APCs and vectors. The solid spherical particles have been found to have higher phagocytosis as compared to other shaped particles as, gold NPs (spherical in shape and 40 nm) as compared to cubic and rod shape (20 nm) were induced effective immunity, but in case of APCs uptake behavior, the rod shaped gold NPS were found more efficient as compared to the cubical and spherical gold NPs [121,135]. The above findings indicated that the NPs uptake by APCs intensely governed by the confined geometry at the boundary between cells and NPs.
In addition to size and shape of NPs, the surface charge also significantly affects the activation of immunity. Due to anionic nature of cell membranes, the cationic NPs and other positively charged nanocarriers have been found to induce higher phagocytosis action of APCs, which is due to electrostatic interactions of the carriers with the anionic cell membranes [136]. In some in vitro experiments, it was found that a cationic surface considerably increases the micron sized polystyrene particles (but not for NPs) uptake by DCs and macrophages as compared to anionic and non-ionic surfaces [137,138]. Conversely, in vivo experiments with positively and negatively charged liposomes proved themselves as effective adjuvants for cell-mediated immunity induction [139,140]. But there are some adverse effects in the use of positively charged nanocarriers, as cationic NPs induced platelet aggregation and hemolysis which might be due to their electrostatic interaction with anionic cell membranes. Similarly, in many experiments it was found that the hydrophobic NPs induced higher immunity as compared to hydrophilic NPs, which was proved in a study where tetanus toxoid was encapsulated in to PLGA and PLA hydrophobic NPs. The immune responses of hydrophobic polymeric NPs were found much better than that of the hydrophilic polymeric NPs as well as than those of the conventional alum adsorbed tetanus toxoids after single dose vaccination of each by intramuscular injection in to Wistar rats [141].
Release kinetics and mechanism
The release kinetics of the antigen plays an important role in the humoral immunological responses. The mechanism of release of protein antigen from polymeric nanocarriers might be through diffusion and bio-erosion. Release of antigens from nanocarriers may occur through the pores of polymeric matrix by means of diffusion. The release of antigens can also be controlled and regulated by slower dissolution of dispersed antigen within the matrix as the aqueous phase diffuses to the inner part of the polymer matrix and might be due to polymer swelling in soluble carriers. The antigens are equivalently dispersed in antigen-impermeable glassy polymer in the case of swelling mediated release carriers so through the dry matrices no any diffusion occurs. When such carriers are administered in to the body through any route, the aqueous phases from the biological environment causes swelling and penetration which in turn causes relaxation of polymer chain and diffusion of antigens take place. In the carriers those follow swelling process; dissolution of matrix largely depends up on chemical structure and physical states of the polymers. The amorphous and linear polymers dissolve themselves in a faster rate as compared to the completely or partially crystalline and cross-linked polymers. Conclusively, we may say that the release of antigens from such carriers follow a Fickian diffusion kinetics or case-II transport. When the carriers are constituted from the bio-erodible matrices in which antigens are present in dispersed manner. When such carriers come in contact with aqueous phases, sustained release of antigens in a controlled way due to the bio-erosion of the matrices takes place. Generally in bio-erodible carriers, the release of antigens or drugs occur through bulk or surface hydrolysis catalyzed by some enzymes or chemicals, causes solubilization and degradation of the polymer matrix in to smaller aqueous soluble molecules. The mechanism of release from bio-erodible carriers exhibit a zero-order kinetics when they undergo surface hydrolysis if the carrier maintain its constant surface geometry and a poorly water soluble active agent is encapsulated in to such carriers [142].
The release kinetics of loaded active agents or antigens from polymeric NPs can be organized by changing the polymer compositions [73]. The NPs prepared from a variety of polymers like, polysaccharides, poly (α-hydroxy acids) or poly (amino acids) for the formation of vesicles which can either accommodate the antigens inside it or adsorb on its surfaces. PLGA and PLA are commonly used poly (α-hydroxy acids) for NPs preparation by means of double emulsion-solvent evaporation method [53].
In general the polymer based NPs for the delivery of vaccines or drug shows a biphasic release pattern of the active moiety, initially a burst release within the initial hours after administration then a slow, basal plateau phase which simulated the more sustained release of the actives over the later hours, which might be due to the protein antigen encapsulated within the interior core of polymer matrix of the PLGA-NPs and sheltered from the outer biological microenvironment [143]. Similar results were observed in experiments where the antigen was released from tumor antigen-containing PLGA-NPs in a clinically applicable eight day period [144]. Moreover, to explore the effect of release kinetics on immunological responses, investigated to two nanocarriers, namely PLGA-NPs and liposomes carrying ovalbumin (OVA) were investigated. Mice were vaccinated with the two nanocarriers to compare the efficiency of each in a long-term immunization study. Immunization with PLGA-NPs exhibited prolonged and higher circulating IgG antibody titers as compared to liposomal formulation, and the extent of cellular immune responses were highest in those animals vaccinated with PLGA-NPs, which furthermore indicated a high occurrence of effector-like memory responses on CD8+ T-cell phenotype, efficacy in pathogen recall and recovery from pathogen infections, finally causing an enhanced clearance of intracellular bacteria [143]. They concluded that the different performance of the above two nanocarriers are not only due to their compositional differences but also due to the release kinetics of the said antigen delivery.
Magnetic nanoparticles by some biodegradable polymers
The use of magnetic NPs needs some surface modifications to be more functional and biocompatible. The magnetic NPs generally have a large surface area/volume ratio and have a propensity to agglomerate and adsorb various kinds of plasma proteins. Normally these NPs are rapidly engulfed and cleared by macrophages in the body tissues when they are in agglomerate state or are enveloped with the plasma proteins. Hence, surface modifications of magnetic NPs are needed in drug delivery systems and in biomedical applications that could stabilize and functionalize the magnetite NPs in the physiological conditions [4]. For example, the magnetic NPS were surface-modified with poly ethylene glycol (PEG) and folic acid, respectively, to enhance their intracellular uptake and capability to specific cells targeting [145].
The most popularly used method of surface modification of magnetic NPs is its encapsulation in a polymeric matrix, like polylactic acid or polylactic-co-glycolic acid [146], which can be used as a targeted drug delivery system. Similarly, magnetic-thermoresponsive NPs can be obtained by using some temperature-sensitive polymers, like poly N-isopropyl acrylamide, Pluronics F-68, Pluronics F-127, and these Pluronics also acts as cryoprotectant and prevents NP agglomeration [147]. Another method of surface modification of magnetic NPs is the polymer chains grafting with a number of functional groups present on their surfaces. The polymer chains stearic effect stabilizes the magnetic NPs in the physiological condition, and thus magnetic NPs get the new functionality by polymer chains functional groups [148]. The uncharged hydrophilic residues and high surface mobility of poly-ethylene glycol (PEG) which is non-toxic and non-immunogenic, causes high stearic omission to stabilize the surface of magnetic NPs in the physiological conditions, and thus the adsorption of proteins and adhesion of cells are effectively prevented on their surfaces. Hence, the immobilized surface of magnetic NPs by PEG is anticipated the improvement in terms of their stabilization and biocompatibility [149].
Future remarks and alternative directions
Biodegradable nanoparticulate carrier systems may be exploited either to improve or obstruct the immunity or elude recognition of immune system. These carriers with encapsulated antigens like, DNA, peptides and proteins were found to have sufficient prospective for the delivery of vaccines via non-invasive routes of administration. These adjuvants serves three mechanical supports like, antigen stabilization, delivering the antigen, and innate immunity, moreover the prolonged and sustained delivery of antigens provides effective immunity. There are some nano formulations in clinical trials and many are in different phases of preclinical trials or toxicity studies. Still such carrier systems needed mechanistic observations for their immunostimulatory and immunosuppressive effects on the immune system, and advances in such carriers as non-viral vectors to enhance their transduction efficiency remains a critical area for upcoming exploration of targeting and specificity of the vectors, to regulate the expression of genes, and to find out the synergistic effects of gene-based entities and other therapeutics like anticancer agents. The safety and efficacy to deliver plasmid DNA in to human beings still remains a major barrier to pledge immunity and their prospective for fighting in a number of infections. Specific self-assembling synthetic non-viral carriers to deliver DNA have been developed to act various biological functions, like the delivery of their genetic load to the target tissues specifically and shielding them from biodegradation by immune or metabolic pathways in to the body tissues. Furthermore, they must exhibit no or negligible toxicity and be assured that they are safe enough for therapeutic applications as well as they must show their competence to express an antigen or gene for a defined duration in proper way. Here we conclude that all these progression offers a number of obstructions at tissue and cellular levels. Hence, these barriers could be overcome, which is the primary aim, with the development of an effective biodegradable polymer-based DNA therapeutics, proteins and peptides as antigens.
The presumed cytotoxicity of such biodegradable NPs must be removed for their use in pharmaceutical and other biomedical fields. Recently, cationic polymers are the choice for the formulators as non-viral antigen and gene carriers due to ease of its preparation and efficient delivery of the actives. But the low transfection efficacy and unwanted toxicity of the cationic polymers are the most puzzling phases; hence these demerits can be removed by different modifications, like surface modifications and especially for cationic polymers, hydrophobic modifications received highest consideration. Moreover, various inorganic nano-sized carriers are in progress, like magnetic NPs, colloidal gold, semiconductor quantum dots, calcium phosphate etc. The application of nanocarriers in vaccine delivery permits not only an enhanced stability and immunogenicity to antigen, but also provides sustained and controlled release at targeted site, which suggest paths for forthcoming research using NPs to develop the specific and much targeted vaccine preparations for the management of several infections. In conclusion, it can be summarized that the nanotechnology will continue to offer outstanding visions to the immunologist for future to use this technology for the induction of efficient immune responses. The application and advantage of nanotechnology in the field of immunology would definitely affect new approaches for the prophylactic use, prevention, in the treatment and management of human ailments.
Acknowledgements
The authors are grateful to the financial and logistic support of King Abdullah Institute for Nanotechnology and Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia.
Disclosure of conflict of interest
None.
References
- 1.O’Hagan DT, Rappuoli R. Novel approaches to vaccine delivery. Pharm Res. 2004;21:1519–30. doi: 10.1023/B:PHAM.0000041443.17935.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu T, Zhang N, Nichols HL, Shi D, Wen X. Modification of nanostructured materials for biomedical applications. Mater Sci Eng C. 2007;27:579–594. [Google Scholar]
- 3.Mohanraj VJ, Chen Y. Nanoparticles-A review. Trop J Pharm Res. 2006;5:561–573. [Google Scholar]
- 4.Sun L, Huang C, Gong T, Zhou S. A biocompatible approach to surface modification: Biodegradable polymer functionalized super-paramagnetic iron oxide nanoparticles. Mater Sci Eng C. 2010;30:583–589. [Google Scholar]
- 5.Chertok B, Moffat BA, David AE, Yu F, Bergemann C, Ross BD, Yang VC. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29:487–96. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60:1252–65. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikumori T, Kobayashi T, Sawaki M, Imai T. Anti-cancer effect of hyperthermia on breast cancer by magnetite nanoparticle-loaded anti-HER2 immunoliposomes. Breast Cancer Res Treat. 2009;113:435–441. doi: 10.1007/s10549-008-9948-x. [DOI] [PubMed] [Google Scholar]
- 8.Luten J, van Nostrum CF, De Smedt SC, Hennink WE. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J Control Release. 2008;126:97–110. doi: 10.1016/j.jconrel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Bolhassani A, Javanzad S, Saleh T, Hashemi M, Aghasadeghi MR, Sadat SM. Polymeric nanoparticles: potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum Vaccin Immunother. 2014;10:321–32. doi: 10.4161/hv.26796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DM, Simon JK, Baker JR Jr. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee PW, Hsu SH, Tsai JS, Chen FR, Huang PJ, Ke CJ, Liao ZX, Hsiao CW, Lin HJ, Sung HW. Multifunctional core-shell polymeric nanoparticles for transdermal DNA delivery and epidermal Langerhans cells tracking. Biomaterials. 2010;31:2425–34. doi: 10.1016/j.biomaterials.2009.11.100. [DOI] [PubMed] [Google Scholar]
- 12.Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res. 2007;24:1415–26. doi: 10.1007/s11095-007-9257-9. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Zou M, Jiang H, Ji Z, Gao P, Cheng G. Synthesis of a novel kind of carbon nanoparticle with large mesopores and macropores and its application as an oral vaccine adjuvant. Eur J Pharm Sci. 2011;44:653–659. doi: 10.1016/j.ejps.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60:915–28. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramon G. Sur la toxine et surranatoxine diphtheriques. Ann Inst Pasteur. 1924;38:1–10. [Google Scholar]
- 16.Brewer JM. (How) do aluminium adjuvants work? Immunol Lett. 2006;102:10–5. doi: 10.1016/j.imlet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Akagi T, Baba M, Akashi M. Biodegradable nanoparticles as vaccine adjuvants and delivery systems: Regulation of immune responses by nanoparticle-based vaccine. Adv Pol Sci. 2012;247:31–64. [Google Scholar]
- 18.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173:3148–54. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 19.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–64. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 20.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–13. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 21.Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology. 2010;151:458–65. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel) 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg NK, Mangal S, Khambete H, Tyagi RK. Mucosal delivery of vaccines: role of mucoadhesive/biodegradable polymers. Recent Pat Drug Deliv Formul. 2010;4:114–28. doi: 10.2174/187221110791185015. [DOI] [PubMed] [Google Scholar]
- 24.Alshamsan A, Haddadi A, Hamdy S, Samuel J, El-Kadi AO, Uludag H, Lavasanifar A. STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response. Mol Pharm. 2010;7:1643–54. doi: 10.1021/mp100067u. [DOI] [PubMed] [Google Scholar]
- 25.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnology. 2011;9:55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SY, Lee YM. Taxol-loaded block copolymer nanospheres composed of methoxy poly (ethylene glycol) and poly (epsilon-caprolactone) as novel anticancer drug carriers. Biomaterials. 2001;22:1697–704. doi: 10.1016/s0142-9612(00)00292-1. [DOI] [PubMed] [Google Scholar]
- 28.Prashant CK, Bhat M, Srivastava SK, Saxena A, Kumar M, Singh A, Samim M, Ahmad FJ, Dinda AK. Fabrication of nanoadjuvant with poly-epsilon-caprolactone (PCL) for developing a single-shot vaccine providing prolonged immunity. Int J Nanomedicine. 2014;9:937–50. doi: 10.2147/IJN.S55892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31:603–632. [Google Scholar]
- 30.Sonaje K, Chuang EY, Lin KJ, Yen TC, Su FY, Tseng MT, Sung HW. Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: microscopic, ultrastructural, and computed-tomographic observations. Mol Pharm. 2012;9:1271–1279. doi: 10.1021/mp200572t. [DOI] [PubMed] [Google Scholar]
- 31.Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf B Biointerfaces. 2005;44:65–73. doi: 10.1016/j.colsurfb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 32.De Campos AM, Sanchez A, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int J Pharm. 2001;224:159–68. doi: 10.1016/s0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- 33.Sarmento B, Ribeiro A, Veiga F, Ferreira D. Development and characterization of new insulin containing polysaccharide nanoparticles. Colloids Surf B Biointerfaces. 2006;53:193–202. doi: 10.1016/j.colsurfb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Chen XG, Li YY, Liu CS. Self-assembled nanoparticles based on hydrophobically modified chitosan as carriers for doxorubicin. Nanomedicine. 2007;3:258–265. doi: 10.1016/j.nano.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y, Yang W, Wang C, Hu J, Fu S, Dong L, Wu L, Shen X. Nanoparticles based on the complex of chitosan and polyaspartic acid sodium salt: preparation, characterization and the use for 5-fluorouracil delivery. Eur J Pharm Biopharm. 2007;67:621–631. doi: 10.1016/j.ejpb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51:81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 37.Borges O, Cordeiro-da-Silva A, Tavares J, Santarem N, de Sousa A, Borchard G, Junginger HE. Immune response by nasal delivery of hepatitis B surface antigen and codelivery of a CpG ODN in alginate coated chitosan nanoparticles. Eur J Pharm Biopharm. 2008;69:405–16. doi: 10.1016/j.ejpb.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Chen J, Li H, Wang Y, Xie Z, Wu M, Zhang H, Zhao Z, Chen Q, Fu M, Wu K, Chi C, Wang H, Gao R. Porcine interleukin-2 gene encapsulated in chitosan nanoparticles enhances immune response of mice to piglet paratyphoid vaccine. Comp Immunol Microbiol Infect Dis. 2007;30:19–32. doi: 10.1016/j.cimid.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Amidi M, Romeijn SG, Verhoef JC, Junginger HE, Bungener L, Huckriede A, Crommelin DJ, Jiskoot W. N-trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: biological properties and immunogenicity in a mouse model. Vaccine. 2007;25:144–53. doi: 10.1016/j.vaccine.2006.06.086. [DOI] [PubMed] [Google Scholar]
- 40.Hiep LV, Thanh MT, Van DTH, Khanh VTH, Dzung NA. Chitosan oligomer as a hopeful adjuvant for H5n1 influenza vaccine. J Chitin Chitosan. 2008;13:6–8. [Google Scholar]
- 41.Dzung NA, Ngoc Hà NT, Van DTH, Phuong NTL, Thi N, Quynh N, Hiep DM, Hiep LV. Chitosan nanoparticle as a novel delivery system for A/H1n1 influenza vaccine: safe property and immunogenicity in mice. World Acad Sci Eng Technol. 2011;60:1824–1846. [Google Scholar]
- 42.Xu W, Shen Y, Jiang Z, Wang Y, Chu Y, Xiong S. Intranasal delivery of chitosan-DNA vaccine generates mucosal SIgA and anti-CVB3 protection. Vaccine. 2004;22:3603–3612. doi: 10.1016/j.vaccine.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Zhang X, Kang Y, Jin H, Du X, Zhao G, Yu Y, Li J, Su B, Huang C, Wang B. Interleukin-15 enhance DNA vaccine elicited mucosal and systemic immunity against foot and mouth disease virus. Vaccine. 2008;26:5135–5144. doi: 10.1016/j.vaccine.2008.03.088. [DOI] [PubMed] [Google Scholar]
- 44.Yuan X, Yang X, Cai D, Mao D, Wu J, Zong L, Liu J. Intranasal immunization with chitosan/pCETP nanoparticles inhibits atherosclerosis in a rabbit model of atherosclerosis. Vaccine. 2008;26:3727–3734. doi: 10.1016/j.vaccine.2008.04.065. [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Dai W, Wang Z, Chen B, Li Z, Fan X. Intranasal vaccination with chitosan-DNA nanoparticles expressing pneumococcal surface antigen a protects mice against nasopharyngeal colonization by Streptococcus pneumoniae. Clin Vaccine Immunol. 2011;18:75–81. doi: 10.1128/CVI.00263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zillies JC, Zwiorek K, Hoffmann F, Vollmar A, Anchordoquy TJ, Winter G, Coester C. Formulation development of freeze-dried oligonucleotide-loaded gelatin nanoparticles. Eur J Pharm Biopharm. 2008;70:514–521. doi: 10.1016/j.ejpb.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 47.Ofokansi K, Winter G, Fricker G, Coester C. Matrix-loaded biodegradable gelatin nanoparticles as new approach to improve drug loading and delivery. Eur J Pharm Biopharm. 2010;76:1–9. doi: 10.1016/j.ejpb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Zwiorek K, Bourquin C, Battiany J, Winter G, Endres S, Hartmann G, Coester C. Delivery by cationic gelatin nanoparticles strongly increases the immunostimulatory effects of CpG oligonucleotides. Pharm Res. 2008;25:551–562. doi: 10.1007/s11095-007-9410-5. [DOI] [PubMed] [Google Scholar]
- 49.De Geest BG, De Koker S, Demeester J, De Smedt SC, Hennink WE. Pulsed in vitro release and in vivo behavior of exploding microcapsules. J Control Release. 2009;135:268–73. doi: 10.1016/j.jconrel.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 50.Kanthamneni N, Sharma S, Meenach SA, Billet B, Zhao JC, Bachelder EM, Ainslie KM. Enhanced stability of horseradish peroxidase encapsulated in acetalated dextran microparticles stored outside cold chain conditions. Int J Pharm. 2012;431:101–10. doi: 10.1016/j.ijpharm.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 51.Sarei F, Dounighi NM, Zolfagharian H, Khaki P, Bidhendi SM. Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Indian J Pharm Sci. 2013;75:442–9. doi: 10.4103/0250-474X.119829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z, Lv D, Liu S, Gong J, Wang D, Xiong M, Chen X, Xiang R, Tan X. Alginic acid-coated chitosan nanoparticles loaded with legumain DNA vaccine: effect against breast cancer in mice. PLoS One. 2013;8:e60190. doi: 10.1371/journal.pone.0060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, Chen C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9:325–41. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamdy S, Elamanchili P, Alshamsan A, Molavi O, Satou T, Samuel J. Enhanced antigen-specific primary CD4+ and CD8+ responses by codelivery of ovalbumin and toll-like receptor ligand monophosphoryl lipid A in poly (D,L-lactic-co-glycolic acid) nanoparticles. J Biomed Mater Res A. 2007;81:652–62. doi: 10.1002/jbm.a.31019. [DOI] [PubMed] [Google Scholar]
- 55.Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine. 2008;26:5046–57. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 56.Florindo HF, Pandit S, Goncalves LM, Videira M, Alpar O, Almeida AJ. Antibody and cytokine-associated immune responses to S. equi antigens entrapped in PLA nanospheres. Biomaterials. 2009;30:5161–9. doi: 10.1016/j.biomaterials.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 57.Harikrishnan R, Balasundaram C, Heo MS. Poly D,L-lactide-co-glycolic acid (PLGA)-encapsulated vaccine on immune system in Epinephelus bruneus against Uronema marinum. Exp Parasitol. 2012;131:325–32. doi: 10.1016/j.exppara.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 58.Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol. 2013;3:13. doi: 10.3389/fcimb.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panyam J, Dali MM, Sahoo SK, Ma W, Chakravarthi SS, Amidon GL, Levy RJ, Labhasetwar V. Polymer degradation and in vitro release of a model protein from poly (D,L-lactide-co-glycolide) nano- and microparticles. J Control Release. 2003;92:173–87. doi: 10.1016/s0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 60.Holowka EP, Sun VZ, Kamei DT, Deming TJ. Polyarginine segments in block copolypeptides drive both vesicular assembly and intracellular delivery. Nat Mater. 2007;6:52–7. doi: 10.1038/nmat1794. [DOI] [PubMed] [Google Scholar]
- 61.Portilla-Arias JA, Camargo B, Garcia-Alvarez M, de Ilarduya AM, Munoz-Guerra S. Nanoparticles made of microbial poly (gamma-glutamate)s for encapsulation and delivery of drugs and proteins. J Biomater Sci Polym Ed. 2009;20:1065–79. doi: 10.1163/156856209X444420. [DOI] [PubMed] [Google Scholar]
- 62.Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, August JT, Leong KW. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70:399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 63.Devi LB, Mandal AB. Self-assembly of Ag nanoparticles using hydroxypropyl cyclodextrin: synthesis, characterisation and application for the catalytic reduction of p-nitrophenol. RSC Adv. 2013;3:5238. [Google Scholar]
- 64.Tokumitsu H, Ichikawa H, Fukumori Y. Chitosan-gadopentetic acid complex nanoparticles for gadolinium neutron-capture therapy of cancer: preparation by novel emulsion- droplet coalescence technique and characterization. Pharm Res. 1999;16:1830–1835. doi: 10.1023/a:1018995124527. [DOI] [PubMed] [Google Scholar]
- 65.Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm Res. 1997;14:1431–6. doi: 10.1023/a:1012128907225. [DOI] [PubMed] [Google Scholar]
- 66.Xu Y, Du Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm. 2003;250:215–226. doi: 10.1016/s0378-5173(02)00548-3. [DOI] [PubMed] [Google Scholar]
- 67.Boudad H, Legrand P, Lebas G, Cheron M, Duchene D, Ponchel G. Combined hydroxypropyl-beta-cyclodextrin and poly (alkylcyanoacrylate) nanoparticles intended for oral administration of saquinavir. Int J Pharm. 2001;218:113–24. doi: 10.1016/s0378-5173(01)00622-6. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Q, Shen Z, Nagai T. Prolonged hypoglycemic effect of insulin-loaded polybutylcyanoacrylate nanoparticles after pulmonary administration to normal rats. Int J Pharm. 2001;218:75–80. doi: 10.1016/s0378-5173(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 69.Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Control Release. 1999;57:171–85. doi: 10.1016/s0168-3659(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 70.Eley JG, Pujari VD, McLane J. Poly (lactide-co-glycolide) nanoparticles containing coumarin-6 for suppository delivery: in vitro release profile and in vivo tissue distribution. Drug Deliv. 2004;11:255–61. doi: 10.1080/10717540490467384. [DOI] [PubMed] [Google Scholar]
- 71.Zweers ML, Engbers GH, Grijpma DW, Feijen J. In vitro degradation of nanoparticles prepared from polymers based on dl-lactide, glycolide and poly (ethylene oxide) J Control Release. 2004;100:347–356. doi: 10.1016/j.jconrel.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Li X, Deng X, Yuan M, Xiong C, Huang Z, Zhang Y, Jia W. In vitro degradation and release profiles of poly-DL-lactide-poly (ethylene glycol) microspheres with entrapped proteins. J Appl Polym Sci. 2000;78:140–148. [Google Scholar]
- 73.Saroja C, Lakshmi P, Bhaskaran S. Recent trends in vaccine delivery systems: a review. Int J Pharm Investig. 2011;1:64–74. doi: 10.4103/2230-973X.82384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C, Convertine A, Stayton PS. pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7:3912–25. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cruz LJ, Tacken PJ, Bonetto F, Buschow SI, Croes HJ, Wijers M, de Vries IJ, Figdor CG. Multimodal imaging of nanovaccine carriers targeted to human dendritic cells. Mol Pharm. 2011;8:520–31. doi: 10.1021/mp100356k. [DOI] [PubMed] [Google Scholar]
- 76.Drbohlavova J, Chomoucka J, Adam V, Ryvolova M, Eckschlager T, Hubalek J, Kizek R. Nanocarriers for anticancer drugs-new trends in nanomedicine. Curr Drug Metab. 2013;14:547–64. doi: 10.2174/1389200211314050005. [DOI] [PubMed] [Google Scholar]
- 77.Henriksen-Lacey M, Korsholm KS, Andersen P, Perrie Y, Christensen D. Liposomal vaccine delivery systems. Expert Opin Drug Deliv. 2011;8:505–19. doi: 10.1517/17425247.2011.558081. [DOI] [PubMed] [Google Scholar]
- 78.Rentel CO, Bouwstra JA, Naisbett B, Junginger HE. Niosomes as a novel peroral vaccine delivery system. Int J Pharm. 1999;186:161–7. doi: 10.1016/s0378-5173(99)00167-2. [DOI] [PubMed] [Google Scholar]
- 79.Gupta PN, Vyas SP, Singh Gour Vishwidyalaya H. Transfersomes for vaccine delivery: a potential approach for topical immunization. Med Chem Res. 2004;13:414–426. [Google Scholar]
- 80.Li Z, Zhang L, Sun W, Ding Q, Hou Y, Xu Y. Archaeosomes with encapsulated antigens for oral vaccine delivery. Vaccine. 2011;29:5260–6. doi: 10.1016/j.vaccine.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 81.Gluck R, Moser C, Metcalfe IC. Influenza virosomes as an efficient system for adjuvanted vaccine delivery. Expert Opin Biol Ther. 2004;4:1139–45. doi: 10.1517/14712598.4.7.1139. [DOI] [PubMed] [Google Scholar]
- 82.Gargett T, Grubor-Bauk B, Miller D, Garrod T, Yu S, Wesselingh S, Suhrbier A, Gowans EJ. Increase in DNA vaccine efficacy by virosome delivery and co-expression of a cytolytic protein. Clin Trans Immunol. 2014;3:e18. doi: 10.1038/cti.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta PN, Mishra V, Rawat A, Dubey P, Mahor S, Jain S, Chatterji DP, Vyas SP. Non-invasive vaccine delivery in transfersomes, niosomes and liposomes: a comparative study. Int J Pharm. 2005;293:73–82. doi: 10.1016/j.ijpharm.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 84.Singh M, O’Hagan DT. Recent advances in vaccine adjuvants. Pharm Res. 2002;19:715–728. doi: 10.1023/a:1016104910582. [DOI] [PubMed] [Google Scholar]
- 85.Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vaccines. 2014;2:159–82. doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mizuuchi M, Hirohashi Y, Torigoe T, Kuroda T, Yasuda K, Shimizu Y, Saito T, Sato N. Novel oligomannose liposome-DNA complex DNA vaccination efficiently evokes anti-HPV E6 and E7 CTL responses. Exp Mol Pathol. 2012;92:185–90. doi: 10.1016/j.yexmp.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 87.Patravale V, Prabhu P. Potential of nanocarriers in antigen delivery: the path to successful vaccine delivery. Nanocarriers. 2014:1. [Google Scholar]
- 88.Foged C, Hansen J, Agger EM. License to kill: Formulation requirements for optimal priming of CD8(+) CTL responses with particulate vaccine delivery systems. Eur J Pharm Sci. 2012;45:482–91. doi: 10.1016/j.ejps.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 89.Krishnan L, Sad S, Patel GB, Sprott GD. Archaeosomes induce long-term CD8+ cytotoxic T cell response to entrapped soluble protein by the exogenous cytosolic pathway, in the absence of CD4+ T cell help. J Immunol. 2000;165:5177–85. doi: 10.4049/jimmunol.165.9.5177. [DOI] [PubMed] [Google Scholar]
- 90.Sprott GD, Sad S, Fleming LP, DiCaire CJ, Patel GB, Krishnan L. Archaeosomes varying in lipid composition differ in receptor-mediated endocytosis and differentially adjuvant immune responses to entrapped antigen. Archaea. 2003;1:151–164. doi: 10.1155/2003/569283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6:319–327. [Google Scholar]
- 92.Kim D, El-Shall H, Dennis D, Morey T. Interaction of PLGA nanoparticles with human blood constituents. Colloids Surf B Biointerfaces. 2005;40:83–91. doi: 10.1016/j.colsurfb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 93.Demento S, Steenblock ER, Fahmy TM. Biomimetic approaches to modulating the T cell immune response with nano- and micro- particles. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:1161–6. doi: 10.1109/IEMBS.2009.5332625. [DOI] [PubMed] [Google Scholar]
- 94.Gemeinhart RA, Luo D, Saltzman WM. Cellular fate of a modular DNA delivery system mediated by silica nanoparticles. Biotechnol Prog. 2005;21:532–7. doi: 10.1021/bp049648w. [DOI] [PubMed] [Google Scholar]
- 95.Luo D, Han E, Belcheva N, Saltzman WM. A self-assembled, modular DNA delivery system mediated by silica nanoparticles. J Control Release. 2004;95:333–41. doi: 10.1016/j.jconrel.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 96.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Muller RH. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 97.Han R, Zhu J, Yang X, Xu H. Surface modification of poly (D,L-lactic-co-glycolic acid) nanoparticles with protamine enhanced cross-presentation of encapsulated ovalbumin by bone marrow-derived dendritic cells. J Biomed Mater Res A. 2011;96:142–9. doi: 10.1002/jbm.a.32860. [DOI] [PubMed] [Google Scholar]
- 98.Torchilin VP, Trubetskoy VS. Which polymers can make nanoparticulate drug carriers long-circulating? Adv Drug Del Rev. 1995;16:141–155. [Google Scholar]
- 99.Stahl-Hennig C, Kuate S, Franz M, Suh YS, Stoiber H, Sauermann U, Tenner-Racz K, Norley S, Park KS, Sung YC, Steinman R, Racz P, Uberla K. Atraumatic oral spray immunization with replication-deficient viral vector vaccines. J Virol. 2007;81:13180–90. doi: 10.1128/JVI.01400-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Avtushenko SS, Sorokin EM, Zoschenkova NY, Zacharova NG, Naichin AN. Clinical and immunological characteristics of the emulsion form of inactivated influenza vaccine delivered by oral immunization. J Biotechnol. 1996;44:21–8. doi: 10.1016/0168-1656(95)00105-0. [DOI] [PubMed] [Google Scholar]
- 101.Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59:1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 102.Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces b-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001;75:5141–5150. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 104.Pavot V, Rochereau N, Genin C, Verrier B, Paul S. New insights in mucosal vaccine development. Vaccine. 2012;30:142–54. doi: 10.1016/j.vaccine.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 105.Ciabattini A, Pettini E, Medaglini D. CD4(+) T cell priming as biomarker to study immune response to preventive vaccines. Front Immunol. 2013;4:421. doi: 10.3389/fimmu.2013.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marks E, Helgeby A, Andersson JO, Schon K, Lycke NY. CD4(+) T-cell immunity in the female genital tract is critically dependent on local mucosal immunization. Eur J Immunol. 2011;41:2642–53. doi: 10.1002/eji.201041297. [DOI] [PubMed] [Google Scholar]
- 107.Pettini E, Prota G, Ciabattini A, Boianelli A, Fiorino F, Pozzi G, Vicino A, Medaglini D. Vaginal immunization to elicit primary T-cell activation and dissemination. PLoS One. 2013;8:e80545. doi: 10.1371/journal.pone.0080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Medaglini D, Ciabattini A, Cuppone AM, Costa C, Ricci S, Costalonga M, Pozzi G. In vivo activation of naive CD4+ T cells in nasal mucosa-associated lymphoid tissue following intranasal immunization with recombinant Streptococcus gordonii. Infect Immun. 2006;74:2760–6. doi: 10.1128/IAI.74.5.2760-2766.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ciabattini A, Pettini E, Andersen P, Pozzi G, Medaglini D. Primary activation of antigen-specific naive CD4+ and CD8+ T cells following intranasal vaccination with recombinant bacteria. Infect Immun. 2008;76:5817–25. doi: 10.1128/IAI.00793-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Doherty TM, Olsen AW, van Pinxteren L, Andersen P. Oral vaccination with subunit vaccines protects animals against aerosol infection with mycobacterium tuberculosis. Infect Immun. 2002;70:3111–3121. doi: 10.1128/IAI.70.6.3111-3121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morrill JC, Peters CJ. Mucosal immunization of rhesus macaques with Rift Valley Fever MP-12 vaccine. J Infect Dis. 2011;204:617–25. doi: 10.1093/infdis/jir354. [DOI] [PubMed] [Google Scholar]
- 112.Singh J, Pandit S, Bramwell VW, Alpar HO. Diphtheria toxoid loaded poly-(epsilon -caprolactone) nanoparticles as mucosal vaccine delivery systems. Methods. 2006;38:96–105. doi: 10.1016/j.ymeth.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 113.Varmus H, Klausner R, Zerhouni E, Acharya T, Daar AS, Singer PA. Grand challenges in global health. Science. 2003;302:398–399. doi: 10.1126/science.1091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuate S, Stefanou D, Hoffmann D, Wildner O, Uberla K. Production of lentiviral vectors by transient expression of minimal packaging genes from recombinant adenoviruses. J Gene Med. 2004;6:1197–205. doi: 10.1002/jgm.623. [DOI] [PubMed] [Google Scholar]
- 115.Stolte-Leeb N, Sauermann U, Norley S, Fagrouch Z, Heeney J, Franz M, Hunsmann G, Stahl-Hennig C. Sustained conservation of CD4+ T Cells in multiprotein triple modality-immunized rhesus macaques after intrarectal challenge with simian immunodeficiency virus. Viral Immunol. 2006;19:448–457. doi: 10.1089/vim.2006.19.448. [DOI] [PubMed] [Google Scholar]
- 116.Earl PL, Americo JL, Wyatt LS, Eller LA, Montefiori DC, Byrum R, Piatak M, Lifson JD, Amara RR, Robinson HL, Huggins JW, Moss B. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunodeficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology. 2007;366:84–97. doi: 10.1016/j.virol.2007.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amorij JP, Westra TA, Hinrichs WL, Huckriede A, Frijlink HW. Towards an oral influenza vaccine: comparison between intragastric and intracolonic delivery of influenza subunit vaccine in a murine model. Vaccine. 2007;26:67–76. doi: 10.1016/j.vaccine.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 118.Koping-Hoggard M, Sanchez A, Alonso MJ. Nanoparticles as carriers for nasal vaccine delivery. Expert Rev Vaccines. 2005;4:185–96. doi: 10.1586/14760584.4.2.185. [DOI] [PubMed] [Google Scholar]
- 119.Hobson P, Barnfield C, Barnes A, Klavinskis LS. Mucosal immunization with DNA vaccines. Methods. 2003;31:217–24. doi: 10.1016/s1046-2023(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 120.Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg AP. Nanoparticle vaccines. Vaccine. 2014;32:327–37. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 121.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5:487–95. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alshamsan A. Induction of tolerogenic dendritic cells by IL-6-secreting CT26 colon carcinoma. Immunopharmacol Immunotoxicol. 2012;34:465–9. doi: 10.3109/08923973.2011.625034. [DOI] [PubMed] [Google Scholar]
- 123.Alshamsan A, Hamdy S, Das S, Lavasanifar A, Samuel J, El-Kadi AO. Validation of bone marrow derived dendritic cells as an appropriate model to study tumor-mediated suppression of DC maturation through STAT3 hyperactivation. J Pharm Pharm Sci. 2010;13:21–6. doi: 10.18433/j37598. [DOI] [PubMed] [Google Scholar]
- 124.Trumpfheller C, Longhi MP, Caskey M, Idoyaga J, Bozzacco L, Keler T, Schlesinger SJ, Steinman RM. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Internal Med. 2012;271:183–192. doi: 10.1111/j.1365-2796.2011.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Do Y, Koh H, Park CG, Dudziak D, Seo P, Mehandru S, Choi JH, Cheong C, Park S, Perlin DS, Powell BS, Steinman RM. Targeting of LcrV virulence protein from Yersinia pestis to dendritic cells protects mice against pneumonic plague. Eur J Immunol. 2010;40:2791–6. doi: 10.1002/eji.201040511. [DOI] [PubMed] [Google Scholar]
- 126.Wang B, Zaidi N, He LZ, Zhang L, Kuroiwa JM, Keler T, Steinman RM. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res. 2012;14:R39. doi: 10.1186/bcr3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trumpfheller C, Finke JS, López CB, Moran TM, Moltedo B, Soares H, Huang Y, Schlesinger SJ, Park CG, Nussenzweig MC, Granelli-Piperno A, Steinman RM. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Batista-Duharte A, Lindblad EB, Oviedo-Orta E. Progress in understanding adjuvant immunotoxicity mechanisms. Toxicol Lett. 2011;203:97–105. doi: 10.1016/j.toxlet.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 129.Ra JS, Shin HH, Kang S, Do Y. Lumazine synthase protein cage nanoparticles as antigen delivery nanoplatforms for dendritic cell-based vaccine development. Clin Exp Vaccine Res. 2014;3:227–34. doi: 10.7774/cevr.2014.3.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kanchan V, Panda AK. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials. 2007;28:5344–5357. doi: 10.1016/j.biomaterials.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 131.Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Plebanski M. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40:1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 132.Joshi VB, Geary SM, Salem AK. Biodegradable particles as vaccine delivery systems: size matters. AAPS J. 2013;15:85–94. doi: 10.1208/s12248-012-9418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yan S, Gu W, Xu ZP. Re-considering how particle size and other properties of antigen-adjuvant complexes impact on the immune responses. J Colloid Interface Sci. 2013;395:1–10. doi: 10.1016/j.jcis.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 134.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26:244–9. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298:315–22. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 136.Thiele L, Merkle HP, Walter E. Phagocytosis and phagosomal fate of surface-modified microparticles in dendritic cells and macrophages. Pharm Res. 2003;20:221–8. doi: 10.1023/a:1022271020390. [DOI] [PubMed] [Google Scholar]
- 137.Wischke C, Borchert HH, Zimmermann J, Siebenbrodt I, Lorenzen DR. Stable cationic microparticles for enhanced model antigen delivery to dendritic cells. J Control Release. 2006;114:359–68. doi: 10.1016/j.jconrel.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 138.Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, Nakanishi M, Tanaka K, Mayumi T. Positively charged liposome functions as an efficient immunoadjuvant in inducing cell-mediated immune response to soluble proteins. J Control Release. 1999;61:233–240. doi: 10.1016/s0168-3659(99)00097-8. [DOI] [PubMed] [Google Scholar]
- 139.Yotsumoto S, Aramaki Y, Kakiuchi T, Tsuchiya S. Induction of antigen-dependent interleukin-12 production by negatively charged liposomes encapsulating antigens. Vaccine. 2004;22:3503–9. doi: 10.1016/j.vaccine.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 140.Raghuvanshi RS, Katare YK, Lalwani K, Ali MM, Singh O, Panda AK. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. Int J Pharm. 2002;245:109–21. doi: 10.1016/s0378-5173(02)00342-3. [DOI] [PubMed] [Google Scholar]
- 141.Heller J. Controlled release of biologically active compounds from bioerodible polymers. Biomaterials. 1980;1:51–7. doi: 10.1016/0142-9612(80)90060-5. [DOI] [PubMed] [Google Scholar]
- 142.Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM, Fahmy TM. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33:4957–4964. doi: 10.1016/j.biomaterials.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Prasad S, Cody V, Saucier-Sawyer JK, Fadel TR, Edelson RL, Birchall MA, Hanlon DJ. Optimization of stability, encapsulation, release, and cross-priming of tumor antigen-containing PLGA nanoparticles. Pharm Res. 2012;29:2565–2577. doi: 10.1007/s11095-012-0787-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Y, Kohler N, Zhang M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 145.Zhou X, Zhang X, Yu X, Zha X, Fu Q, Liu B, Wang X, Chen Y, Chen Y, Shan Y, Jin Y, Wu Y, Liu J, Kong W, Shen J. The effect of conjugation to gold nanoparticles on the ability of low molecular weight chitosan to transfer DNA vaccine. Biomaterials. 2008;29:111–117. doi: 10.1016/j.biomaterials.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 146.Liu TY, Hu SH, Liu DM, Chen SY, Chen IW. Biomedical nanoparticle carriers with combined thermal and magnetic responses. Nano Today. 2009;4:52–65. [Google Scholar]
- 147.Diwan M, Park TG. Pegylation enhances protein stability during encapsulation in PLGA microspheres. J Control Release. 2001;73:233–44. doi: 10.1016/s0168-3659(01)00292-9. [DOI] [PubMed] [Google Scholar]
- 148.Zhou S, Liao X, Li X, Deng X, Li H. Poly-D,L-lactide-co-poly (ethylene glycol) microspheres as potential vaccine delivery systems. J Control Release. 2003;86:195–205. doi: 10.1016/s0168-3659(02)00423-6. [DOI] [PubMed] [Google Scholar]
- 149.Laurent S, Mahmoudi M. Superparamagnetic iron oxide nanoparticles: promises for diagnosis and treatment of cancer. Int J Mol Epidemiol Genet. 2011;2:367–390. [PMC free article] [PubMed] [Google Scholar]