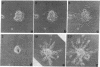
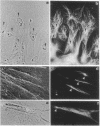
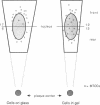
Abstract
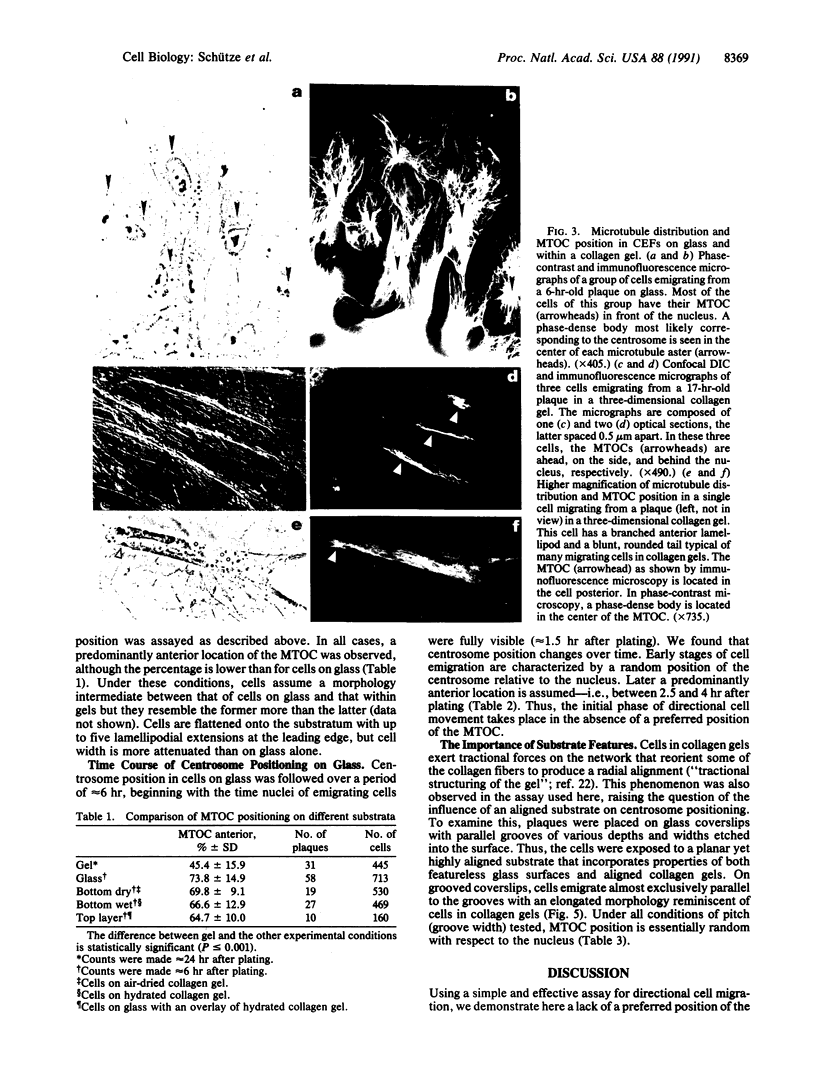
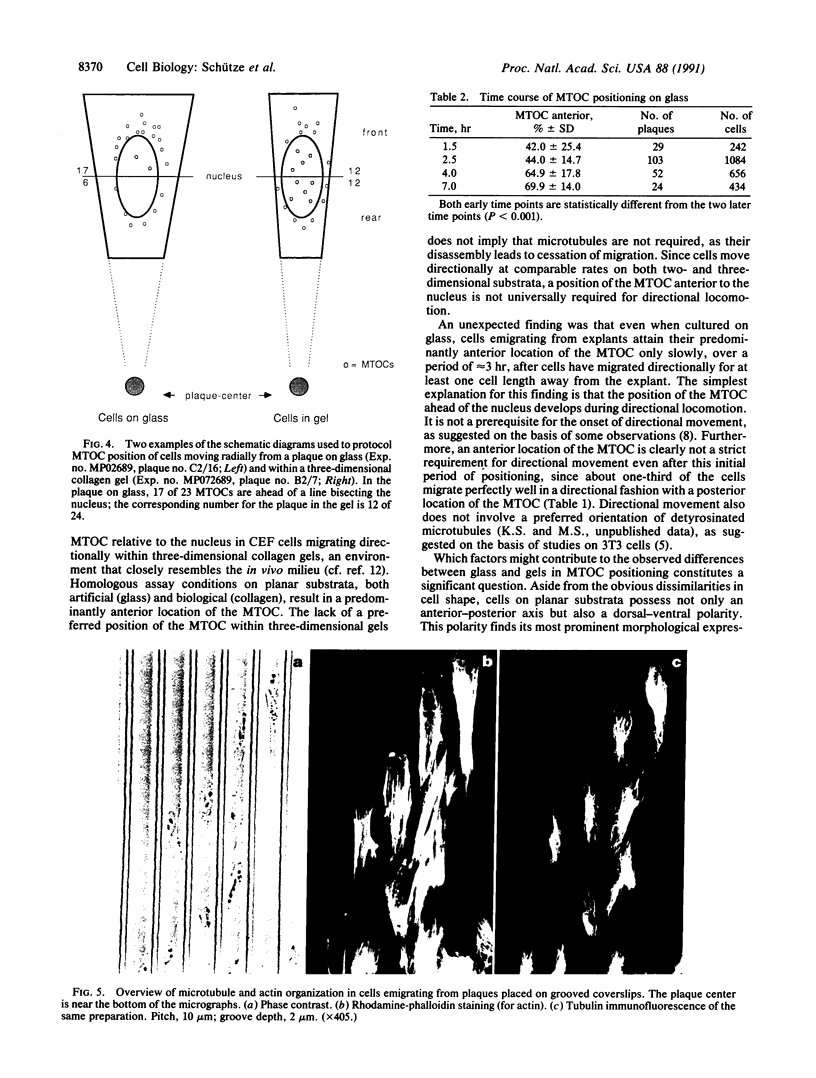
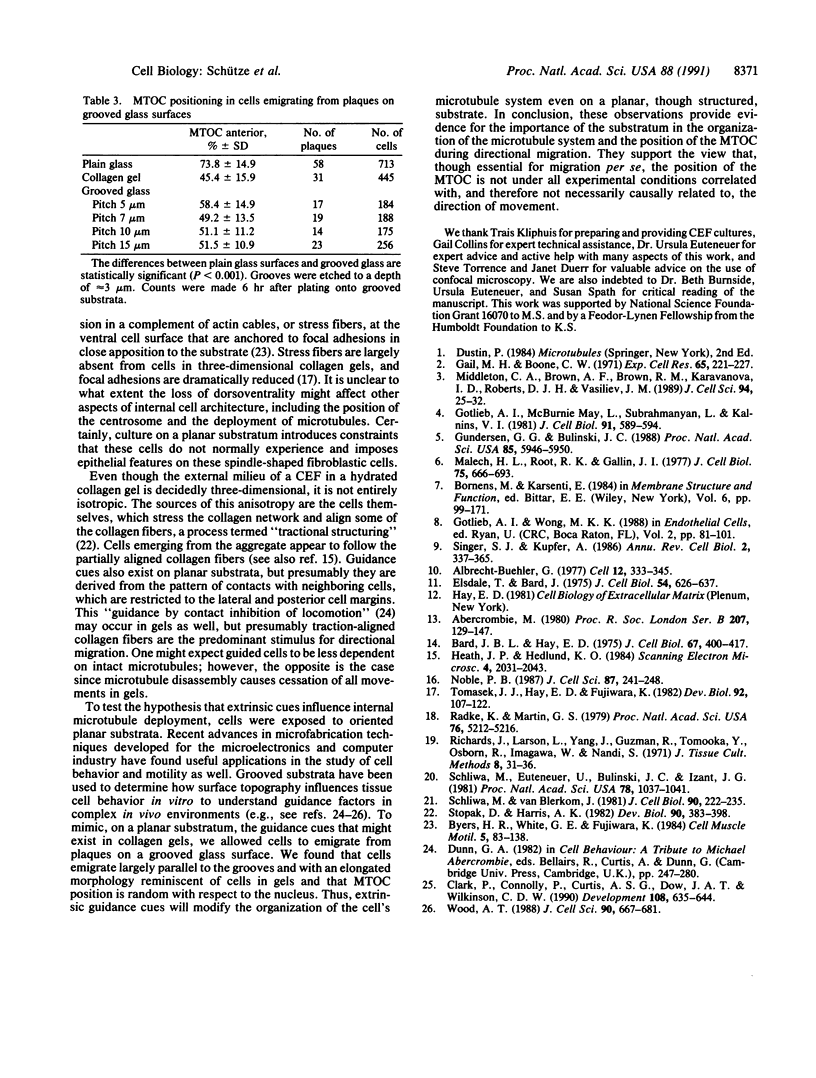
Immunofluorescence and confocal microscopy were used to monitor the positioning of microtubule-organizing centers (MTOCs) during directional migration of chicken embryo fibroblasts on planar substrata and within three-dimensional collagen gels. Homologous assay conditions based on the radial emigration of cells from cell aggregates were used in both cases. Whereas approximately 70% of the cells migrating directionally on glass and at least 60% on other planar substrata have their MTOCs anterior to the nucleus, MTOCs are randomly distributed around the nucleus in cells within collagen gels. The anterior location of the MTOC in cells on glass is attained gradually during the first 4 hr of directional migration. Cells on oriented planar substrata, manufactured by photolithographic etching of narrow parallel grooves into the glass surface, also have a random position of the MTOC, although the cells themselves assume a highly polarized cell shape parallel to the grooves. This environment mimics the partial orientation of the collagen fibers produced by the tractive forces of the cells within collagen networks. These findings demonstrate a difference in MTOC positioning between fibroblasts on planar substrata and within a quasinatural environment.
Full text
PDF
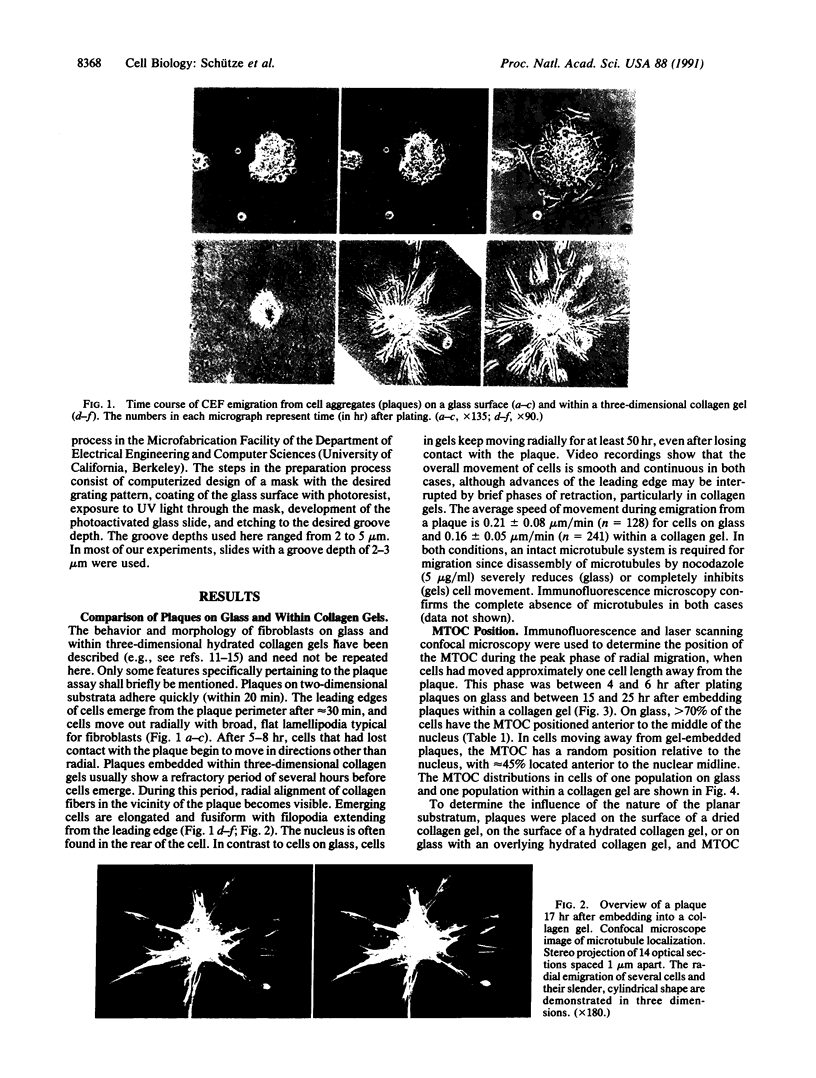
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Buehler G. Phagokinetic tracks of 3T3 cells: parallels between the orientation of track segments and of cellular structures which contain actin or tubulin. Cell. 1977 Oct;12(2):333–339. doi: 10.1016/0092-8674(77)90109-x. [DOI] [PubMed] [Google Scholar]
- Bard J. B., Hay E. D. The behavior of fibroblasts from the developing avian cornea. Morphology and movement in situ and in vitro. J Cell Biol. 1975 Nov;67(2PT1):400–418. doi: 10.1083/jcb.67.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers H. R., White G. E., Fujiwara K. Organization and function of stress fibers in cells in vitro and in situ. A review. Cell Muscle Motil. 1984;5:83–137. doi: 10.1007/978-1-4684-4592-3_2. [DOI] [PubMed] [Google Scholar]
- Clark P., Connolly P., Curtis A. S., Dow J. A., Wilkinson C. D. Topographical control of cell behaviour: II. Multiple grooved substrata. Development. 1990 Apr;108(4):635–644. doi: 10.1242/dev.108.4.635. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gail M. H., Boone C. W. Effect of colcemid on fibroblast motility. Exp Cell Res. 1971 Mar;65(1):221–227. doi: 10.1016/s0014-4827(71)80070-8. [DOI] [PubMed] [Google Scholar]
- Gotlieb A. I., May L. M., Subrahmanyan L., Kalnins V. I. Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol. 1981 Nov;91(2 Pt 1):589–594. doi: 10.1083/jcb.91.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G., Bulinski J. C. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5946–5950. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath J. P., Hedlund K. O. Locomotion and cell surface movements of fibroblasts in fibrillar collagen gels. Scan Electron Microsc. 1984;(Pt 4):2031–2043. [PubMed] [Google Scholar]
- Malech H. L., Root R. K., Gallin J. I. Structural analysis of human neutrophil migration. Centriole, microtubule, and microfilament orientation and function during chemotaxis. J Cell Biol. 1977 Dec;75(3):666–693. doi: 10.1083/jcb.75.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton C. A., Brown A. F., Brown R. M., Karavanova I. D., Roberts D. J., Vasiliev J. M. The polarization of fibroblasts in early primary cultures is independent of microtubule integrity. J Cell Sci. 1989 Sep;94(Pt 1):25–32. doi: 10.1242/jcs.94.1.25. [DOI] [PubMed] [Google Scholar]
- Noble P. B. Extracellular matrix and cell migration: locomotory characteristics of MOS-11 cells within a three-dimensional hydrated collagen lattice. J Cell Sci. 1987 Mar;87(Pt 2):241–248. doi: 10.1242/jcs.87.2.241. [DOI] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., Euteneuer U., Bulinski J. C., Izant J. G. Calcium lability of cytoplasmic microtubules and its modulation by microtubule-associated proteins. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1037–1041. doi: 10.1073/pnas.78.2.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J. Structural interaction of cytoskeletal components. J Cell Biol. 1981 Jul;90(1):222–235. doi: 10.1083/jcb.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Kupfer A. The directed migration of eukaryotic cells. Annu Rev Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- Stopak D., Harris A. K. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev Biol. 1982 Apr;90(2):383–398. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]
- Tomasek J. J., Hay E. D., Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: distribution of actin, alpha-actinin, and myosin. Dev Biol. 1982 Jul;92(1):107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Wood A. Contact guidance on microfabricated substrata: the response of teleost fin mesenchyme cells to repeating topographical patterns. J Cell Sci. 1988 Aug;90(Pt 4):667–681. doi: 10.1242/jcs.90.4.667. [DOI] [PubMed] [Google Scholar]