Abstract
This report describes the use of an oligonucleotide macroarray to profile the expression of 375 genes in Lactococcus lactis subsp. lactis IL1403 during heat, acid, and osmotic stress. A set of known stress-associated genes in IL1403 was used as the internal control on the array. Every stress response was accurately detected using the macroarray, compared to data from previous reports. As a group, the expression patterns of the investigated metabolic genes were significantly altered by heat, acid, and osmotic stresses. Specifically, 13 to 18% of the investigated genes were differentially expressed in each of the environmental stress treatments. Interestingly, the methionine biosynthesis pathway genes (metA-metB1 and metB2-cysK) were induced during heat shock, but methionine utilization genes, such as metK, were induced during acid stress. These data provide a possible explanation for the differences between acid tolerance mechanisms of L. lactis strains IL1403 and MG1363 reported previously. Several groups of transcriptional responses were common among the stress treatments, such as repression of peptide transporter genes, including the opt operon (also known as dpp) and dtpT. Reduction of peptide transport due to environmental stress will have important implications in the cheese ripening process. Although stress responses in lactococci were extensively studied during the last decade, additional information about this bacterium was gained from the use of this metabolic array.
Cheese flavor compounds are derived from the metabolic activities of the microbial community living inside the cheese matrix, especially the starter culture Lactococcus lactis (32). The recent publication of the genome sequence of L. lactis subsp. lactis IL1403 opened many new research opportunities to explore the metabolic capacities of this bacterium (2). The draft sequence of Lactococcus lactis subsp. cremoris SK11 (http://genome.jgi-psf.org/draft_microbes/laccr/laccr.home.html)adds another dimension for study of this genus during dairy fermentations.
Lactococci experience a variety of stresses, including acid, temperature, and osmotic shocks during cheese production and ripening (33). Gene regulation induced by these stresses directly influences the fermentation process and flavor compound generation in cheese. Hence, it is important to systematically evaluate the impact of environmental stress on expression of central metabolic genes. Before the existence of DNA microarray technology, two-dimensional gel electrophoresis was the only tool available for studying global effects in this microorganism (9, 12, 13, 16). With the availability of whole genome sequences, DNA array technology has emerged as the primary high-throughput tool for simultaneously monitoring the expression patterns (29). The major barriers preventing the implementation of array technology are the high cost, the technical complexity associated with the current generation of DNA microarrays, and the complex data analysis of the patterns. Recently, we developed a low-cost oligonucleotide-based filter DNA macroarray that greatly reduces these barriers (35, 36).
In this study, we designed a metabolic gene-focused DNA macroarray, using 22- to 24-mer probes to study expression of the metabolic genes in L. lactis subsp. lactis IL1403 during stress conditions associated with dairy fermentations. Several known stress-associated genes were used as controls for each stress condition. We found that expression of the control set of genes matched previously published results. This result was used as a control to ensure data integrity for other observations. As a group, the expression patterns of investigated metabolic genes were significantly altered by heat, acid, and osmotic stresses (P < 0.01). Specifically, 13 to 18% of the investigated genes were differentially expressed during each of the environmental stress treatments.
MATERIALS AND METHODS
Bacterial strains.
L. lactis subsp. lactis IL1403 (courtesy of Larry McKay, University of Minnesota) is a plasmid-cured strain that lacks lactose transport and utilization genes, the cell envelope protease, a citrate transporter, restriction-modification systems, and other plasmid-encoded features. IL1403 was grown at 30°C overnight without shaking in M17 broth (Difco, Sparks, Md.) supplemented with 0.5% glucose (M17G).
Design and fabrication of an oligonucleotide-based DNA macroarray.
A DNA macroarray was designed based on the published L. lactis subsp. lactis IL1403 genome sequence (2). A modified version of Primer3 was used to design the oligonucleotide probes (27). The criteria used for oligonucleotide candidate selection were length (22- to 24-mer), melting temperature (63 to 65°C, calculated based on a 50 mM salt concentration and a 50 nM DNA concentration), GC percentage (40 to 60%), absence of significant secondary structures, and at least four mismatches with any sequence in the genome. The sequences and some characteristics of 384 oligonucleotide probes (including controls) used in this study can be found at a supplemental web site (http://labgenome.usu.edu/macroarray/). Among 384 oligonucleotides, 375 probes selected for their corresponding gene targets were annotated to have roles in protein degradation or in carbohydrate, fatty acid, nucleic acid, and amino acid metabolism (2). The additional nine probes included four positive control probes designed from the spiked Arabidopsis thaliana genes and five negative control probes, including an empty spot, a random 15-mer probe, a probe designed from a reverse complementary strand of dnaK, and probes derived from the plasmid-encoded genes prtP and prtM. Probes were synthesized with standard desalting purification (Sigma-Genosys, Austin, Texas). The synthesized oligonucleotides were resuspended in nuclease-free water to a normalized concentration of 100 μM.
A polyinosine tail was added to each oligonucleotide by using a terminal transferase reaction (35, 36). Each tailing reaction mixture (volume, 10 μl) was composed of 100 pmol of oligonucleotide, 100 nmol of dITP (Roche Applied Science, Indianapolis, Ind.), 20 U of terminal transferase (New England BioLabs, Beverly, Mass.), 5 mM CoCl2, 0.2 M potassium cacodylate, 0.25 mg of bovine serum albumin/ml, and 25 mM Tris-HCl (pH 6.6). The tailing reactions were incubated at 37°C for 2 h. Tailed oligonucleotides were spotted onto a positively charged nylon membrane (Millipore, Bedford, Mass.) with a manual arrayer (VP Scientific, San Diego, Calif.) and fixed by baking the membrane at 80°C for 30 min. The printed arrays were stored at room temperature in plastic bags until used.
Stress treatments.
Cultures of L. lactis subsp. lactis IL1403 grown overnight in M17G broth were harvested by centrifugation at 5,000 × g for 10 min at 4°C, washed in 0.85% NaCl, and refreshed with fresh M17G broth for 10 min at 30°C. The cells were re-collected by centrifugation at 5,000 × g for 10 min at 4°C and resuspended in fresh broth or stress conditioning media as specified below. The cell densities in the treatment media were adjusted to an optical density at 600 nm of 1.5.
The medium for the control (no stress treatment) and the heat shock treatment was sterile M17G broth. The acid stress medium was sterile M17G broth adjusted to pH 5.5 with lactic acid, and the osmotic shock medium was sterile M17G broth supplemented with NaCl to a final concentration of 4%. For all treatments, except heat shock, cultures were incubated at 30°C. Heat shock samples were incubated at 42°C. The total RNA was collected (as described below) after 30 min of incubation in each stress condition. The cell density (optical density at 600 nm) and pH of the medium for each condition were determined at the time of RNA isolation. The experiments, including the control, were biologically replicated twice. In addition, each biological replicate also contained two technical duplicates on each array.
Total RNA isolation.
Cells from 1.5-ml cultures were collected by centrifugation at 16,000 × g for 2 min at room temperature, resuspended in 50 mM EDTA (pH 8.0) containing 50 mg of lysozyme (Sigma, St. Louis, Mo.)/ml, and incubated for 10 min at 25°C. After centrifugation at 5,000 × g for 1 min, the pellet was resuspended in 300 μl of the Lysis/Binding solution supplied in the RNAqueous kit (Ambion Inc., Austin, Tex.). The total RNA was subsequently extracted according to the manufacturer's recommendations. Additional contaminating genomic DNA and protein were removed by using the RNeasy kit (QIAGEN, Valencia, Calif.) according to the manufacturer's recommendations with an additional LiCl precipitation after using the kit. With this protocol, we routinely isolated about 100 to 200 μg of bacterial total RNA with an A260/A280 ratio of 2.0 to 2.2. The mRNA transcripts (1 ng each) of RCP1 and XCP2 genes from A. thaliana (Stratagene, La Jolla, Calif.) were added before isolation of total RNA and used as the positive controls for RNA extraction.
Labeling of total RNA with biotin.
Biotin was incorporated into the cDNA from the RNA transcripts with an indirect labeling procedure (35). Briefly, 100 μg of total RNA was reverse transcribed into cDNA with random hexamers and Superscriptase II (Invitrogen, Carlsbad, Calif.). The reverse transcription procedure described by the manufacturer was used, except that the deoxynucleoside triphosphate (dNTP) was replaced with an aminoallyl-dNTP mixture (aa-dNTP). The 20× aa-dNTP stock mixture was composed of 10 mM dATP, dGTP, dCTP, 4 mM dTTP, 0.1 mM ddATP (Invitrogen), and 6 mM aminoallyl-dUTP (Sigma). Once reverse transcription was complete, the enzyme was heat inactivated and the RNA templates were degraded with RNaseH (Epicentre, Madison, Wis.). The reaction mixture was cleaned by using the Qiaquick-PCR purification kit (QIAGEN) according to the kit instructions, except that the Tris-containing washing buffer was replaced with 75% ethanol. The purified single-strand cDNA was eluted from the columns twice with a total of 100 μl of phosphate-buffered saline buffer (pH 7.2). Two hundred micrograms of Sulfo-NHS-LC-biotin (Pierce, Rockford, Ill.) was added to the purified amino-labeled single-strand-cDNA solution to reach a final concentration of 2 μg/μl, and the mixture was incubated at 25°C for 1 h. The reaction was terminated by adding 24 μmol of hydroxylamine. After incubation with hydroxylamine at 25°C for 15 min, the labeled DNA was purified by using a Qiaquick-PCR purification kit. The total 100-μl eluted DNA mixture was heated at 100°C for 5 min and snap-cooled on ice for 5 min. All the labeled target cDNA was added to 10 ml of freshly prepared prehybridization buffer (see below) and hybridized with a printed array. The mRNA transcripts (1 ng each) of the LTP4 and LTP6 genes from A. thaliana (Stratagene) were spiked into the purified total RNA mixture before the RNA labeling reaction and used as the positive controls for the labeling reaction.
Hybridization and detection.
The DNA macroarrays were prehybridized for at least 1 h at 52°C in a 150-ml prehybridization solution composed of 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% N-laurosylsarcosine, 0.02% sodium dodecyl sulfate (SDS), and 1× blocking reagent (VectorLab, San Diego, Calif.). After prehybridization, 10 ml of fresh hybridization solution was mixed with labeled DNA targets and added to the hybridization tube to replace the prehybridization solution. After hybridization at 52°C overnight, the membrane was washed twice at 50°C for 5 min with a washing solution containing 2× SSC and 0.01% SDS. A stringent wash was conducted twice with a wash solution (0.5× SSC and 0.01% SDS) at 50°C for 15 min. The biotin-based chemiluminescence detection step was conducted using the North2South hybridization and detection kit (Pierce) according to the kit instructions, except that the blocking time was extended from 15 min to 1 h. Each membrane array was exposed to chemiluminescent films (Roche Applied Science) with an exposure time of 60 min.
Data analysis of DNA macroarray results.
The exposed films were digitized into 16-bit TIFF images with a desktop scanner at 800 dots per in. (Expression 1600; Espon, Long Beach, Calif.). Subsequently, the images were processed with an ImageJ plugin using a semiautomatic gridding procedure and a bivariate Gaussian distribution model-based spot segmentation algorithm (35). Based on spot diffusion and spatial correlation of pixel intensities in a spot, this algorithm estimated the spot intensity and background from a spot even when the spot was saturated (36). Practically, this spot-finding algorithm was found to extend the dynamic range of array data from 2 orders of magnitude in the original film data to 4 orders of magnitude or more (35, 36). The practical limitation of this algorithm resulted from overlapping between neighboring spots and was therefore determined by both the spot size and the pitch of the array. The smaller the spot size and the larger the pitch size, the higher the dynamic range could be extended.
Statistical analysis.
We utilized an adapted locally weighted regression scatter plot smoothing (LOWESS)-based procedure to normalize background-corrected DNA macroarray data. Although the LOWESS-based normalization procedure was originally developed for normalization of two-color DNA microarray data (37), it has been widely adopted in the data normalization of “single-color” array formats, even in Affymetrix array data normalization procedures (8). The reason for the widespread applicability of this normalization procedure was that it is based on an assumption that was held for any array formats: specifically, that the majority of genes were not differentially expressed between treatments. Briefly, background-corrected DNA macroarray data were used as the intensity in one channel and normalized to a common reference in another channel by following a global LOWESS procedure. The common reference used in normalization was generated via averaging the expression intensities of each gene over the control and treatment conditions. The statistical significance of differential gene expression was calculated with a statistical package called SAM (31).
RESULTS
Design of oligonucleotide probes for DNA array.
Proper design of oligonucleotide probes is critical to the success of open reading frame-specific oligonucleotide array analysis. Because of the small genome size of L. lactis subsp. lactis IL1403 (2.4 Mb), a very stringent criterion for probe specificity was applied in oligonucleotide design (i.e., at least four mismatches in a 21- or 22-mer probe with any nontarget sequence in the genome). An exhaustive genome-wide search for homologs was conducted for each oligonucleotide candidate to ensure its specificity for the targeted gene and to reduce cross-hybridization with non-target gene sequences.
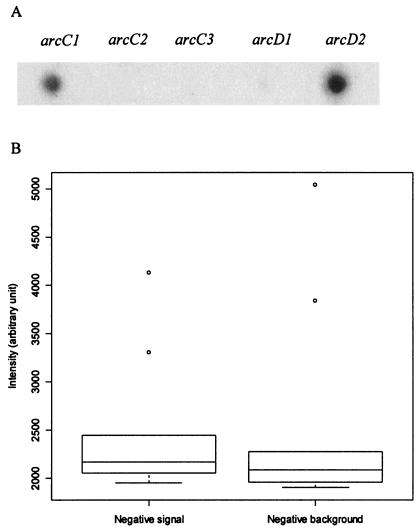
The specificity of the oligonucleotide probes was demonstrated experimentally from DNA macroarray hybridization results, using a subset of the genes that are duplicated and highly homologous (Fig. 1). Lack of cross-hybridization between these sequences was illustrated in an array hybridization result with the duplicate copies of the arcC and arcD probes (Fig. 1A). These genes have three and two homologs in the IL1403 genome, respectively (2). The oligonucleotide probes designed in this study differentiated the expression of each gene from that of its paralogues. The specificity of this DNA macroarray was also reflected in the absence of nonspecific hybridization signals in each of the negative control spots (Fig. 1B). Except for the random 15-mer spots, all negative spots had signals in the range of background intensity levels.
FIG. 1.
Probe specificity during hybridization. L. lactis subsp. lactis IL1403 is analyzed after 30 min of growth in M17G at pH 7.0 (i.e., control condition as described in Materials and Methods). (A) Hybridization image demonstrated no cross-hybridization between genes with homologous sequences in the IL1403 genome. The arcA, arcB, arcC1, arcC2, arcD1, and arcD2 genes encoded proteins involved in the arginine deiminase pathway and belonged to arc gene clusters containing multiple promoter regions (4). (B) Box plot demonstrates low nonspecific hybridization in negative control probes (i.e., an empty spot, a random 15-mer, a probe designed from reverse complementary strand of dnaK, and probes from the plasmid-borne genes prtP and prtM). Box plot group 1 consisted of the signal medians in negative spots (before background correction), while group 2 consisted of the background medians in negative spots.
L. lactis subsp. lactis IL1403 under environmental stresses.
Gene expression profiles of L. lactis subsp. lactis IL1403 during heat, acid, and osmotic stresses were determined. The array contained 375 oligonucleotide probes that corresponded to a set of genes that are differentially expressed during stress conditions. These probes were used as an internal control for reliability of the resulting data. Another portion of the oligonucleotide probes corresponded to genes involved in carbohydrate, fatty acid, nucleic acid, and amino acid metabolism and protein degradation pathways in L. lactis subsp. lactis IL1403 (2, 15).
During the 30-min incubation under the stress conditions, the cell density of the nonstressed control culture increased ∼26%, while cell densities during heat, acid, and salt stresses increased 27, 20, and 10%, respectively. The pH of the media dropped from 7.2 to 6.2 in the stress control culture to 6.4 during salt stress and to 6.1 during heat stress. The pH of lactic acid stress conditions started at pH 5.5 and decreased to 5.1 during 30 min. For comparison, an overnight culture of L. lactis IL1403 grown in M17G had a pH of ∼5.5. Our observations indicate that treatment with 4% NaCl induced osmotic stress and had a larger effect on the growth and acid production than did the other two stress treatments.
(i) Expression of the control set of stress genes.
The control set of genes that are regulated by environmental stresses and assessed with the DNA array in this study are listed in Table 1. Expression of the heat shock gene dnaK was induced 8-fold during heat stress, and its expression increased ∼2-fold with acid stress. This is strikingly similar to previously published results, where Northern blots or two-dimensional gel electrophoresis showed that dnaK was induced ∼8- to 10-fold after 30 min of heat shock (11, 16) and ∼2-fold by acid treatment (13). In addition, citrate and malate fermentation genes in acid stress (citE-citF and mleS), glutamine transporter genes in acid stress (glnP-glnQ operon), and the compatible solute glycine-betaine transporter (busAA-busAB) in both osmotic and heat stresses were also expressed, which was consistent with reported results under the stress conditions (10, 19, 21-24).
TABLE 1.
Consistency of stress responses of L. lactis subsp. lactis IL1403 from expression profiling with data from previous reports
| Gene | Expression with stress treatment
|
Comment(s) | Reference(s) | |||||
|---|---|---|---|---|---|---|---|---|
| Heat
|
Acid
|
Salt
|
||||||
| Responsea | Matchb | Responsea | Matchb | Responsea | Matchb | |||
| citE | + | √ | − | NA | Physiological data, but no citP in IL1403 | 19 | ||
| citF | + | √ | Physiological data, but no citP in IL1403 | 19 | ||||
| mleS | − | NA | + | √ | − | NA | Physiological data | 23 |
| busAA | + | √ | + | √ | Physiological and genetic data | 10, 21, 22 | ||
| busAB | + | √ | + | √ | Physiological and genetic data | 10, 21, 22 | ||
| glnP | − | NA | − | √ | High stress resistance from insertion mutant in MG1363 | 24 | ||
| glnQ | − | NA | − | √ | High stress resistance from insertion mutant in MG1363 | 24 | ||
| dnaK | + | √ | + | √ | Northern blot, 2D gelc | 1, 16 | ||
+, in this study, positive regulation of gene expression was detected under the specified stress; the −, negative regulation of gene expression was detected.
√, the result from this study was consistent with previous work. NA, no previous study was available for result verification.
2D, two-dimensional.
(ii) Stress responses during heat shock.
During the 30-min, 42°C heat treatment, 64 genes were differentially expressed relative to the control stress condition (Table 2). Thirty-four of those genes were positively regulated, and the other 30 genes were negatively regulated. Expression of a putative amino acid ABC transporter (yjgC-yjgD-yjgE) increased significantly (α < 0.001), along with that of several other amino acid transporters (ydcC, ydgC, and yvdF), while several peptide transport genes (dtpT, optB, optC, optD, and optF) in the lactococcal proteolytic system were repressed ∼20-fold. In addition, heat shock changed the expression of genes involved in the nucleotide salvage pathway, methionine biosynthesis, glutamine uptake and biosynthesis, arginine catabolism, and betaine uptake. Induction of betaine transporter was consistent with a previous observation that the betaine transport activity in L. cremoris NCDO763 increased with a high growth temperature (10). This might be attributed to the thermoprotectant function of betaine (3). The dnaK gene, which was used as an internal positive control, was expectedly induced eightfold in this treatment.
TABLE 2.
Responses of L. lactis subsp. lactis IL1403 during different stress treatmentsa
| Function | Gene | Fold changeb with:
|
Description of gene productf | ||||
|---|---|---|---|---|---|---|---|
| Heatc | Acidd | Salte | |||||
| PTS | ptsH | −2.9 | Phosphocarrier protein Hpr | ||||
| ptsI | −2.2 | Phosphotransferase system, enzyme I (EC 2.7.3.9) | |||||
| yedE | −340.1 | −14.2 | PTS system, beta-glucosides-specific IIA component, putative (EC 2.7.1.69) | ||||
| yedF | −2.4 | −83.5 | −6.8 | Beta-glucoside-specific PTS system IIBC component (EC 2.7.1.69) | |||
| Sugar metabolism | pmi | −5.6 | Mannose-6-phosphate isomerase (EC 5.3.1.8) | ||||
| uxuB | +14.9 | Fructuronate reductase (EC 1.1.1.57) | |||||
| yrcA | −4.6 | −2.2 | Phospho-beta-glucosidase (EC 3.2.1.86) | ||||
| Glycolysis/gluconeogenesis | enoA | −2.2 | Enolase (EC 4.2.1.11) | ||||
| enoB | +3.3 | 2-Phosphoglycerate dehydratase (EC 4.2.1.11) | |||||
| fbp | −14.1 | −21.5 | Fructose-1,6-bisphosphatase (EC 3.1.3.11) | ||||
| gapB | −3.3 | −3.8 | Glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.12) | ||||
| Pentose phosphate pathway | gnd | −5.2 | −3.6 | 6-Phosphogluconate dehydrogenase; decarboxylating (EC 1.1.1.44) | |||
| tkt | −2.6 | Transketolase (EC 2.2.1.1) | |||||
| zwf | +4.0 | Glucose-6-phosphate 1-dehydrogenase (EC 1.1.1.49) | |||||
| Pyruvate metabolism | citE | +2.8 | −14.7 | Citrate lyase beta chain (EC 4.1.3.6) | |||
| citF | +14.3 | Citrate lyase alpha chain (EC 4.1.3.6 2.8.3.10) | |||||
| ldh | −2.0 | +2.3 | l-lactate dehydrogenase (EC 1.1.1.27) | ||||
| mae | −9.7 | Malate oxidoreductase (EC 1.1.1.38) | |||||
| mleS | −30.6 | +2.9 | −2.8 | Malolactic enzyme (EC 1.1.1.38) | |||
| Fatty acid biosynthesis | accA | −3.2 | −30.8 | Acetyl-CoA carboxylase carboxyl transferase subunit alpha (EC 6.4.1.2) | |||
| accB | −4.7 | Acetyl-CoA carboxylase biotin carboxyl carrier protein | |||||
| accC | −51.2 | Biotin carboxylase (EC 6.3.4.14) | |||||
| fabD | −2.3 | Malonyl CoA-acyl carrier protein transacylase (EC 2.3.1.39) | |||||
| fabG1 | −3.7 | 3-Oxoacyl-[acyl-carrier protein] reductase (EC 1.1.1.100) | |||||
| fabH | −6.0 | 3-Oxoacyl-[acyl-carrier-protein] synthase III (EC 2.3.1.41) | |||||
| fabZ2 | −5.8 | (3R)-Hydroxymyristoyl-[acyl carrier protein] dehydratase (EC 4.2.1.-) | |||||
| Fatty acid catabolism | fadA | +4.2 | +24.7 | Acetyl coenzyme A acetyltransferase (EC 2.3.1.9) | |||
| lplL | −18.5 | Lipoate-protein ligase (EC 6.-.-.-) | |||||
| thiL | +3.8 | Acetyl coenzyme A acetyltransferase (EC 2.3.1.9) | |||||
| RNA polymerase | rpoE | +2.9 | DNA-directed RNA polymerase delta chain (EC 2.7.7.6) | ||||
| RNA reaction | truA | +19.1 | tRNA pseudouridine synthase A (EC 4.2.1.70) | ||||
| truB | +2.7 | tRNA pseudouridine synthase B (EC 4.2.1.70) | |||||
| yljE | +4.5 | Putative RNA methyltransferase (EC 2.1.1.-) | |||||
| Aminoacyl-tRNA biosynthesis | alaS | +5.7 | Alanyl-tRNA synthetase (EC 6.1.1.7) | ||||
| argS | +3.0 | Arginyl-tRNA synthetase (EC 6.1.1.19) | |||||
| fmt | +5.3 | Methyonyl-tRNA formyltransferase (EC 2.1.2.9) | |||||
| gatA | +2.9 | Glu-tRNA amidotransferase subunit A (EC 6.3.5.-) | |||||
| gltX | +3.5 | Glutamyl-tRNA synthetase (EC 6.1.1.17) | |||||
| glyT | −2.8 | −8.7 | Glycyl-tRNA synthetase beta chain (EC 6.1.1.14) | ||||
| ileS | −3.4 | −10.2 | Isoleucyl-tRNA synthetase (EC 6.1.1.5) | ||||
| leuS | +6.5 | Leucyl-tRNA synthetase (EC 6.1.1.4) | |||||
| Deoxyribonucleotide and ribonucleotide interconversions | nrdE | −9.0 | +4.1 | Ribonucleoside-diphosphate reductase alpha chain (EC 1.17.4.1) | |||
| nrdF | −7.8 | Ribonucleoside-diphosphate reductase beta chain (EC 1.17.4.1) | |||||
| Deoxyribonucleotide biosynthesis | dukA | +21.9 | +4.3 | +6.2 | Deoxypurine kinase (EC 2.7.1.113) | ||
| dut | +10.3 | Deoxyuridine 5′-triphosphate nucleotidhydrolase (EC 3.6.1.23) | |||||
| thyA | 2.8 | +5.7 | Thymidylate synthase (EC 2.1.1.45) | ||||
| yeaB | +14.7 | dTMP kinase (EC 2.7.4.9) | |||||
| Ribonucleotide biosynthesis | purA | −28.0 | Adenylosuccinate synthase (EC 6.3.4.4) | ||||
| purL | −6.6 | −24.9 | Phosphoribosylformylglycinamidine synthase II (EC 6.3.5.3) | ||||
| purN | +8.8 | Phosphoribosylglycinamide formyltransferase (EC 2.1.2.2) | |||||
| pydA | +4.5 | Dihydroorotate dehydrogenase A (EC 1.3.3.1) | |||||
| Salvage of nucleosides and nucleotides | add | +3.3 | −12.5 | Adenosine deaminase (EC 3.5.4.4) | |||
| deoB | −2.3 | +3.5 | Phosphopentomutase (EC 5.4.2.7) | ||||
| prsA | +2.4 | Ribose-phosphate pyrophosphokinase (EC 2.7.6.1) | |||||
| prsB | +8.2 | Ribose-phosphate pyrophosphokinase (EC 2.7.6.1) | |||||
| udp | +22.7 | Uridine phosphorylase (EC 2.4.2.3) | |||||
| upp | +4.9 | Uracil phosphoribosyltransferase (EC 2.4.2.9) | |||||
| yfiG | +9.4 | +6.4 | +6.2 | Thymidine kinase (EC 2.7.1.21) | |||
| Other ribonucleotide metabolism | pnpA | −7.8 | −4.0 | Polyribonucleotide nucleotidyltransferase (EC 2.7.7.8) | |||
| relA | +2.1 | GTP pyrophosphokinase (EC 2.7.6.5) | |||||
| ytfB | +9.9 | ADP-ribose pyrophosphatase (EC 3.6.1.13) | |||||
| Protease | yueE | +3.5 | Protease | ||||
| yuhB | +4.7 | Protease | |||||
| Peptidase | gcp | +4.4 | O-sialoglycoprotein endopeptidase (EC 3.4.24.57) | ||||
| pepC | −35.6 | Aminopeptidase C (EC 3.4.22.40) | |||||
| pepDA | +3.8 | Dipeptidase (EC 3.4.-.-) | |||||
| pepP | −7.8 | Aminopeptidase P (EC 3.4.11.9) | |||||
| pepXP | −4.6 | X-prolyl dipeptidyl aminopeptidase (EC 3.4.14.11) | |||||
| Peptide transporter | dtpT | −22.6 | −11.7 | Di-/tripeptide transporter | |||
| optB | −19.4 | −3.3 | −3.5 | Oligopeptide ABC transporter permease protein | |||
| optC | −17.5 | Oligopeptide ABC transporter permease protein | |||||
| optD | −23.3 | −5.6 | Oligopeptide ABC transporter ATP binding protein | ||||
| optF | −13.7 | −4.1 | −5.8 | Oligopeptide ABC transporter ATP binding protein | |||
| Aromatic amino acid biosynthesis | aroA | −6.4 | 3-Phosphoshikimate 1-carboxyvinyltransferase (EC 2.5.1.19) | ||||
| aroD | +3.2 | 3-Dehydroquinate dehydratase (EC 4.2.1.10) | |||||
| Function | Gene | Fold changeb with:
|
Description of gene productf | ||||
| Heatc | Acidd | Salte | |||||
| aroF | +3.3 | Phospho-2-dehydro-3-deoxyheptonate aldolase (EC 4.1.2.15) | |||||
| trpF | +19.5 | Phosphorybosyl-anthranilate isomerase (EC 5.3.1.24) | |||||
| trpG | +6.1 | Anthranilate synthase component II (EC 4.1.3.27) | |||||
| Aspartate family amino acid biosynthesis | ansB | +16.6 | l-asparaginase (EC 3.5.1.1) | ||||
| asnH | +9.4 | Asparagine synthase (EC 6.3.5.4) | |||||
| dapA | +4.1 | Dihydrodipicolinate synthase (EC 4.2.1.52) | |||||
| dapB | +2.6 | Dihydrodipicolinate reductase (EC 1.3.1.26) | |||||
| hom | −3.6 | Homoserine dehydrogenase (EC 1.1.1.3) | |||||
| lysA | +2.4 | Diaminopimelate decarboxylase (EC 4.1.1.20) | |||||
| metA | +25.4 | Homoserine O-succinyltransferase (EC 2.3.1.46) | |||||
| metB1 | +24.0 | Cystathionine gamma-synthase (EC 4.2.99.9) | |||||
| metB2 | +5.7 | Cystathionine beta-lyase (EC 4.4.1.8) | |||||
| metE | −3.9 | 5-Methionine synthase (EC 2.1.1.14) | |||||
| thrC | −3.1 | Threonine synthase (EC 4.2.99.2) | |||||
| ychH | −8.4 | 2,3,4,5-tetrahydropyridine-2-carboxylate N-succinyltransferase (EC 2.3.1.117) | |||||
| Branch chain amino acid biosynthesis | ilvC | +6.6 | Ketol acid reductoisomerase (EC 1.1.1.86) | ||||
| Glutamate family amino acid metabolism | glnA | −18.3 | −3.7 | Glutamine synthetase (EC 6.3.1.2) | |||
| gltB | +4.0 | Glutamate synthase (NADPH) large chain (EC 1.4.1.13) | |||||
| gltD | −2.8 | +2.7 | Glutamate synthase (NADPH) small chain (EC 1.4.1.13) | ||||
| Histidine biosynthesis | hisH | −6.3 | Amidotransferase (EC 2.4.2.-) | ||||
| Serine family amino acid biosynthesis | cysE | +5.5 | Serine acetyltransferase (EC 2.3.1.30) | ||||
| cysK | +5.1 | Cysteine synthase (EC 4.2.99.8) | |||||
| Amino acid catabolism | araT | −2.6 | Aromatic amino acid specific aminotransferase (EC 2.6.1.-) | ||||
| arcA | −6.5 | −61.1 | Arginine deiminase (EC 3.5.3.6) | ||||
| glmS | +10.3 | Glucosamine-fructose-6-phosphate aminotransferase (isomerizing) (EC 2.6.1.16) | |||||
| metK | +2.3 | S-adenosylmethionine synthetase (EC 2.5.1.6) | |||||
| sdaB | +6.0 | l-serine dehydratase beta subunit (EC 4.2.1.13) | |||||
| yeiG | +3.7 | Putative aminotransferase (EC 2.6.1.-) | |||||
| ytjE | +33.4 | Aminotransferase (EC 2.6.1.-) | |||||
| Amino acid transporter | arcD1 | −23.8 | Arginine/ornitine antiporter | ||||
| arcD2 | −3.1 | −2.4 | −2.9 | Arginine/ornitine antiporter | |||
| brnQ | −27.4 | Branch chain amino acid transporter | |||||
| glnP | −14.6 | −24.1 | Glutamine ABC transporter permease and substrate binding protein | ||||
| glnQ | −6.5 | −16.3 | Glutamine ABC transporter ATP-binding protein | ||||
| lysQ | −81.3 | Lysine-specific permease | |||||
| ydcC | +7.0 | Amino acid ABC transporter permease protein | |||||
| ydgC | +3.1 | Amino acid permease | |||||
| yjgC | +25.2 | Amino acid ABC transporter substrate binding protein | |||||
| yjgD | +4.5 | −6.1 | Amino acid ABC transporter permease protein | ||||
| yjgE | +86.8 | Amino acid ABC transporter ATP binding protein | |||||
| yvdF | +2.5 | Amino acid ABC transporter substrate binding protein | |||||
| ABC transporters, prokaryotic | busAA | +26.9 | +60.0 | Betaine ABC transporter ATP binding protein | |||
| busAB | +13.8 | +49.9 | Betaine ABC transporter permease and substrate binding protein | ||||
| choQ | +7.8 | Choline ABC transporter ATP binding protein | |||||
| choS | +24.7 | +2.5 | Choline ABC transporter permease and substrate binding protein | ||||
| potA | −2.6 | Spermidine/putrescine ABC transporter ATP-binding protein | |||||
| potB | −8.4 | Spermidine/putrescine ABC transporter permease protein | |||||
| Peptidoglycan and polysaccharide biosynthesis | dltA | +2.5 | d-alanine activating enzyme (EC 6.3.2.-) | ||||
| murF | +3.3 | UDP-N-acetylmuramoylalanyl-d-glutamyl-2,6-diaminopimelate-d-alanyl-d-alanyl ligase (EC 6.3.2.15) | |||||
| murG | +7.5 | UDP-N-acetylglucosamine-N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol N-acetylglucosamine transferase (EC 2.4.1.-) | |||||
| murI | +3.7 | Glutamate racemase (EC 5.1.1.3) | |||||
| racD | −2.3 | Aspartate racemase (EC 5.1.1.13) | |||||
| Menaquinone and ubiquinone biosynthesis | hmcM | −10.7 | −3.3 | Hydroxymethylglutaryl-CoA synthase (EC 4.1.3.5) | |||
| menD | +4.7 | 2-Oxoglutarate decarboxylase/2-succinyl-6-hydroxy-2,4-Cyclohexadiene-1-carboxylate synthase (EC 4.1.1.71 4.1.3.-) | |||||
| yhdB | +17.2 | O-succinylbenzoate-CoA synthase (EC 4.2.1.-) | |||||
| yhfE | +44.2 | Hypothetical methlytransferase (EC 2.1.1.-) | |||||
| Glutathione and thioredoxin | gshR | +4.8 | Glutathione reductase (EC 1.6.4.2) | ||||
| trxB1 | +3.7 | Thioredoxin reductase (EC 1.6.4.5) | |||||
| One carbon pool by folate | folD | +2.7 | Methylenetetrahydrofolate dehydrogenase (NADP+)/methenyltetrahydrofolate cyclohydrolase (EC 1.5.1.5 3.5.4.9) | ||||
| Folic acid biosynthesis | pabA | −13.8 | para-aminobenzoate synthetase component II (EC 4.1.3.-) | ||||
| pabB | −8.0 | para-aminobenzoate synthase component I (EC 4.1.3.-) | |||||
| Heat shock protein | dnaK | +8.3 | +10.3 | DnaK protein | |||
The threshold for the software package SAM was minimal two-fold change in expression ratio and maximal false discovery rate of 1%.
An empty slot indicated no significant change between the control and the treatment. +, gene expression was induced under the stress condition than in control condition; −, gene expression was repressed under the stress condition.
In heat shock responses, the median number of genes whose expression was falsely called significant was 0.46, with a median false discovery rate of 0.72%. That is, on average we expected less than one false positive in this column.
In acid shock responses, the median number of genes whose expression was falsely called significant was 0.42, with a median false discovery rate of 0.85%. That is, on average we expected less than one false positive in this column.
In osmotic shock responses, the median number of genes whose expression was falsely called significant was 0.45, with a median false discovery rate of 0.66%. That is, on average we expected less than one false positive in this column.
(iii) Stress responses during acid stress.
Lactic acid stress altered the expression profiles of 50 genes compared to the control growth condition after the 30-min treatment (Table 2). Twenty-four genes were induced and another 26 genes were repressed during the stress. As in the heat stress response, the proteolytic system was predominately repressed by acid stress. The expression of the β-glucoside-specific phosphotransferase system (PTS) (yedE-yedF) was repressed more than 100-fold. This was the largest decrease in expression observed in this study. Among the genes induced during acid stress, both the choline transport genes (choQ-choS) and tryptophan biosynthesis genes were induced approximately 20-fold. In addition, the citrate and malate fermentation genes (citE, citF, mleS, and ldh) were induced 2- to 15-fold in this treatment. The exchange of the divalent substrate citrate or malate with the monovalent fermentation product lactate would generate a membrane potential and maintain the pH gradient across the membrane (19, 23). Interestingly, the plasmid-encoded citP citrate/lactate antiporter was missing in L. lactis IL1403. This indicated that an additional transporter system exists in IL1403 that compensates for the transport function of the CitP protein.
We also observed that the expression of glutamine biosynthesis and transport system genes (glnA and the glnP-glnQ operon) was repressed during heat stress. This is significant, since the intracellular glutamine pool is the central control point for gram-positive bacteria to control nitrogen flow during metabolism (30). Disruption of glutamine transporter genes (glnP-glnQ) confers strong lactic acid resistance in lactococci (24). This observation warrants further investigation to determine regulation of nitrogen flux in lactococci.
(iv) Stress responses during osmotic stress.
During the 30 min of osmotic stress with 4% salt, 68 genes were differentially expressed relative to the control growth condition (Table 2). Half of those genes (34 genes) were positively regulated, while the other genes were negatively regulated. As expected, the genes involved in the compatible solute glycine-betaine transport (busAA-busAB) were induced 60-fold (21). The other genes induced during osmotic stress were involved in nucleotide salvage, glutamate biosynthesis, lysine biosynthesis, and peptidoglycan biosynthesis. The heat shock gene dnaK was also induced after osmotic stress treatment, as expected (16). The genes involved in fatty acid biosynthesis, citrate and malate fermentation, and the arginine deiminase pathway were repressed. Repression of fatty acid biosynthesis gene expression may be reflective of the low growth rate during osmotic stress, since the growth rate is commonly correlated with fatty acid synthesis in bacteria (18, 20). In addition, gene expression of related proteolytic enzymes and the β-glucoside-specific PTS system were also repressed during osmotic stress, as in the other two stress responses.
DISCUSSION
Previously, a member of our group used oligonucleotide probes (40-mers) to monitor the expression profiles of genes involved in the arginine deiminase pathway in L. lactis with a protocol similar to a reverse Northern blot (4). In the present study, this approach was expanded to study metabolic gene expression during environmental stress conditions with even shorter oligonucleotide probes. The primary advantage of short oligonucleotide probes in DNA arrays is their high specificity. For example, the expression levels of the alcohol dehydrogenase paralogues with 88% identity (adh1 and adh2) in yeast were indistinguishable with a cDNA array but were specifically detected using a 30-mer oligonucleotide probe array (6). In order to minimize the possibility of cross-hybridization between homologues, a very conservative criterion for probe selection was adopted, that is, at least four mismatches to nonspecific genes in 22- to 24-mer oligonucleotide probes. Other investigators also demonstrated that three mismatches in a 30-mer oligonucleotide probe are sufficient to differentiate the expression of genes with >90% identity (26). The effectiveness of this criterion was clearly demonstrated in this study by the lack of cross-hybridization between arcC and arcD paralogues in the IL1403 genome (Fig. 1).
In this study, we examined the transcriptional responses of L. lactis IL1403 during heat, lactic acid, and osmotic stresses. The stress conditions (30 min at each stress treatment) were derived from previous studies (12, 13) with the goal of verifying the array data with a large set of internal control probes and with multistep events during gene expression. This also offered a mechanism for assessing reproducibility of the array information during the stress treatments. However, it is noteworthy that the intention for these conditions was not to exactly mimic the stress conditions present during cheese production and storage but rather to estimate the impact of stress on cellular responses. For example, both acid and heat stresses occur gradually during cheese making. In the cheese-making process, lactic acid stress occurs over several hours during the initial fermentation period, where lactose is converted into lactic acid by the bacteria added to the milk. After milk coagulation with rennet and curd cutting, heat is gradually applied over a period of 30 min to control the activity of bacteria and to remove whey. Osmotic stress occurred rapidly during salting of milled curds, which was used to stop further acid development, provide an element of flavor, and help preserve the final cheese. Although the stress models used in this study were not intended to exactly replicate these conditions, they simulate the gradual and serial stress treatments as in real-world cheese-making processes. Gene expression data provide a guide for further investigation that does mimic cheese production and ripening. The array data also provide initial insight into the metabolism of lactococci during stress with a system-wide perspective and into how the cell deals with environmental stress with the lack of a battery of sigma factors that are found in other gram-positive organisms.
Studies defining the role of environmental stress responses for L. lactis are described extensively (25, 33). Previous work is especially rich in reports on heat, acid, and salt stresses, since these are the stresses that starter culture would regularly encounter during industrial use (7, 12, 13, 24). The rich source of information provided us an opportunity to compare and contrast array results with those of previous studies done with traditional genetic, physiological, and biochemical approaches. As such, we took this opportunity to compile a list of known stress responses of L. lactis from previous work and used them comparatively with the results from our DNA macroarray to provide a set of control data that can be captured only using arrays (Table 1). In all cases where information was available in the literature, the observations from array expression profiling were consistent with those in previously published studies. This close agreement between our results and those in the broad body of literature provides a high level of confidence in the performance of the DNA macroarray protocol that leads us to make confident observations about new responses that have not been previously described during stress experiments.
The transcriptional regulation of purine metabolism and the upstream pentose-phosphate pathway genes have important implications in the stress resistance in L. lactis subsp. cremoris MG1363 (7, 24). With transposon mutagenesis, disruption of the hpt, relA, guaA, deoB, or tkt gene in MG1363 conferred on the bacterium resistance phenotypes for heat and/or acid stress. This was explained by the alteration in the intracellular (p)ppGpp pool and induction of the stringent response that subsequently led to a multistress resistance phenotype (7, 24). IL1403 followed this regulation scheme with both heat and osmotic stresses but not with acid stress. For example, deoB was repressed with heat stress, and tktA was repressed with osmotic stress. In addition, the ribose-phosphate pyrophosphokinase (prsA-prsB) operon was induced during osmotic stress. Since ribose-phosphate pyrophosphokinase and phosphopentomutase (deoB) share ribose-5-phosphate as a substrate, induction of prsA-prsB would confer a phenotype similar to that with repression of deoB. Differential expression of GTP pyrophosphokinase (relA) under osmotic stress also provided direct evidence for the involvement of stringent response.
IL1403 utilizes acid resistance mechanisms different from those of MG1363. For example, lactic acid resistance for strain MG1363 is chloramphenicol sensitive (25), while it is not for IL1403 (13). Our array data indicated this phenotype might be related to the differential expression pattern of the deoB gene between the two strains. The deoB gene encodes phosphopentomutase, a key enzyme in the purine salvage pathway (24). In IL1403, deoB was induced more than threefold with acid stress (Table 2). In contrast, the transposon insertion in deoB of MG1363 exhibited enhanced survival rates with acid stress treatments (24), which implies that repression of this gene in wild-type MG1363 would lead to a similar acid tolerance response. In conjunction with chloramphenicol sensitivity data, we suspected that while MG1363 relied on induction of a stringent response for acid resistance, IL1403 probably utilized a nonstringent response mechanism to resist the acid damage.
Proteolysis is one of the primary metabolic activities that is required to maintain the growth of lactococci in a high-protein-content medium, such as milk (17). The lactococcal proteolytic system is primarily composed of the following: (i) a cell envelope proteinase (encoded by plasmid-borne prtP), (ii) transport systems for oligopeptides (encoded by the opp operon), and (iii) a multitude of intracellular peptidases (17). In addition to lacking the plasmid-encoded prtP, IL1403 is also defective in expression of the opp operon (corresponding spot intensities in our DNA macroarray were similar to background levels in all examined conditions). However, the opt operon (also known as dpp), encoding a functional di/tri- and tetrapeptide permease system (28), was actively expressed in IL1403. With all three stresses, both opt and dtpT (another di/tri-peptide transporter) were repressed, while several amino acid transporters with unknown specificity were induced during heat stress. Peptidase expression was largely unaffected by any stress condition, with the exception of pepDA and gcp. These data indicate that lactococci reduced its ability to transport large extracellular peptides during stress treatments and increased its ability to transport short peptides and amino acids. Since peptide degradation and downstream amino acid catabolism are critical in cheese flavor production (32), our observations highlight the potential importance of limited autolysis providing substrate access for subsequent flavor development during cheese ripening (5). Alternatively, lactococci require extracellular hydrolysis of large proteins to short peptides and amino acids prior to transport for use as flavor precursors.
Amino acid metabolism in lactococci is one of the key processes for flavor compound formation in cheese (32). One of the most unexpected findings in this study was the transcriptional response of sulfur amino acid metabolic genes during the stress treatments. During heat shock, the metA-metB1 and metB2-cysK operons, which encode proteins used for methionine and cysteine biosynthesis, were induced 25- and 5-fold, respectively. The net result from this induction would lead to accumulation of intracellular methionine during heat shock. In contrast, the methionine synthase (metE) was repressed in acid stress, with a concomitant induction of metK, a gene involved in methionine catabolism. This expression pattern would result in a reduction of the internal methionine pool during acid stress. The concentration of intracellular methionine is an important parameter in cheese flavor production, because it is a precursor to methanethiol, one of the primary flavor compounds found in Cheddar cheese (34), and is used in many other metabolic processes for cell growth. It is unclear how sulfur amino acid metabolism was involved in heat and acid stress resistance, although methionine metabolism is known to play a central role in DNA metabolism, single-carbon metabolism, oxidation/reduction status, and cellular energy.
The arginine deiminase pathway in lactococci is important in the regulation of intracellular pH and in providing energy during carbohydrate starvation (4). Surprisingly, the genes that comprise the arginine deiminase pathway in IL1403 were not significantly induced during acid stress. This is consistent with our physiological data, where IL1403 had diminished abilities to metabolize arginine to produce ammonia and neutralize acids in the media than strain ML3 (L. Chou, Y. Xie, and B. Weimer, unpublished data). Interestingly, arginine deiminase pathway genes were repressed in both heat and osmotic stress treatments. Repression of the arginine degradation pathway during heat shock was also observed in Bacillus subtilis (14).
While the majority of the genes were regulated by individual stress treatments, several genes were simultaneously controlled by two or three stress conditions (Fig. 2). A number of stress responses were common in all the stress treatments, such as repression of several transporter genes (yedE, optB, optF, and arcD2) and induction of two nucleotide kinase genes (dukA and yfiG). In addition, there were also groups of genes coregulated by two different stress treatments (Fig. 2). A total of 10 genes had similar gene expression patterns under both heat and osmotic stress treatments. Since both heat and osmotic stresses induced the stringent response in IL1403, we expected that some of this regulation might belong to the general stress protection mechanism directly or indirectly induced by the stringent response. Similarly, eight genes and six genes were coregulated in both heat and acid stresses and in both osmotic and acid stresses, respectively. Because of the sensitivity and noise issues of DNA arrays, it is not surprising that some of these dual-stress resistance genes are coregulated as multistress resistance groups, such as arcA, dnaK, thyA, pnpA, and yedE. For example, the heat shock protein DnaK is known to be induced in all stress treatments (1, 13, 16).
FIG. 2.
Venn diagram of shared stress responses of L. lactis subsp. lactis IL1403. The gene names were grouped according to its response to each stress treatment and present in each circle. The common stress responses were represented in the intersection area among the circles. The gene induced in a specific stress treatment is boldfaced, while repressed genes are listed in normal font weight.
In conclusion, we successfully used a filter-based oligonucleotide DNA macroarray protocol to profile transcriptional-level stress responses of L. lactis subsp. lactis to several stress conditions commonly found during fermentation. Approximately 13 to 18% of the arrayed genes were differentially expressed in each of the stress treatments. Our array results agreed well with previously reported observations and provided a useful tool as an internal control for the array to increase confidence in the results. More importantly, new insights into the stress responses of L. lactis subsp. lactis IL1403 were observed from a global regulation perspective. For example, the methionine biosynthesis pathway was induced during heat shock, but methionine utilization pathways were induced during acid stress. L. lactis IL1403 remodeled the transporter used for peptide transport during stress treatments, while expression of the peptidase complement remained relatively unchanged. In addition, our data provide an explanation for differences between L. lactis IL1403 and MG1363 acid resistance mechanisms that were unexplained previously.
Acknowledgments
We thank Gregory J. Podgorski of Utah State University for critical reviews and helpful comments on this work.
Y. Xie is grateful for the support of the President's Fellowship and Gandhi Fellowship of Utah State University.
Mention of companies and products does not constitute endorsement by Utah State University or Utah Agricultural Experiment Station.
Footnotes
Utah Agricultural Experiment Station contribution number 7539.
REFERENCES
- 1.Arnau, J., K. I. Sorensen, K. F. Appel, F. K. Vogensen, and K. Hammer. 1996. Analysis of heat shock gene expression in Lactococcus lactis MG1363. Microbiology 142:1685-1691. [DOI] [PubMed] [Google Scholar]
- 2.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldas, T., N. Demont-Caulet, A. Ghazi, and G. Richarme. 1999. Thermoprotection by glycine betaine and choline. Microbiology 145:2543-2548. [DOI] [PubMed] [Google Scholar]
- 4.Chou, L. 2002. Studies of arginine catabolism in Lactococcus lactis spp. lactis ML3 with small-scale DNA array. Ph.D. dissertation. Utah State University, Logan.
- 5.Crow, V. L., T. Coolbear, P. K. Gopal, F. G. Martley, L. L. McKay, and H. Riepe. 1995. The role of autolysis of lactic acid bacteria in the ripening of cheese. Int. Dairy J. 5:855-875. [Google Scholar]
- 6.Dorris, D., R. Ramakrishnan, T. Sendera, and S. Magnuson. 2002. Oligonucleotide array technologies for gene expression profiling, p. 25-38. In E. Grigorenko (ed.), DNA arrays: technologies and experimental strategies. CRC Press, New York, N.Y.
- 7.Duwat, P., S. D. Ehrlich, and A. Gruss. 1999. Effects of metabolic flux on stress response pathways in Lactococcus lactis. Mol. Microbiol. 31:845-858. [DOI] [PubMed] [Google Scholar]
- 8.Gautier, L., L. Cope, B. M. Bolstad, and R. A. Irizarry. 2004. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20:307-315. [DOI] [PubMed] [Google Scholar]
- 9.Guillot, A., C. Gitton, P. Anglade, and M. Y. Mistou. 2003. Proteomic analysis of Lactococcus lactis, a lactic acid bacterium. Proteomics 3:337-354. [DOI] [PubMed] [Google Scholar]
- 10.Guillot, A., D. Obis, and M. Y. Mistou. 2000. Fatty acid membrane composition and activation of glycine-betaine transport in Lactococcus lactis subjected to osmotic stress. Int. J. Food Microbiol. 55:47-51. [DOI] [PubMed] [Google Scholar]
- 11.Hansen, M. C., A. K. Nielsen, S. Molin, K. Hammer, and M. Kilstrup. 2001. Changes in rRNA levels during stress invalidates results from mRNA blotting: fluorescence in situ rRNA hybridization permits renormalization for estimation of cellular mRNA levels. J. Bacteriol. 183:4747-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartke, A., S. Bouch, J. C. Giard, A. Benachour, P. Boutibonnes, and Y. Auffray. 1996. The lactic acid stress response of Lactococcus lactis subsp. lactis. Curr. Microbiol. 33:194-199. [DOI] [PubMed] [Google Scholar]
- 13.Hartke, A., J. Frere, P. Boutibonnes, and Y. Auffray. 1997. Differential induction of the chaperonin GroEL and the co-chaperonin GroES by heat, acid, and UV-irradiation in Lactococcus lactis subsp. lactis. Curr. Microbiol. 34:23-26. [DOI] [PubMed] [Google Scholar]
- 14.Helmann, J. D., M. F. Wu, P. A. Kobel, F. J. Gamo, M. Wilson, M. M. Morshedi, M. Navre, and C. Paddon. 2001. Global transcriptional response of Bacillus subtilis to heat shock. J. Bacteriol. 183:7318-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanehisa, M., S. Goto, S. Kawashima, and A. Nakaya. 2002. The KEGG databases at GenomeNet. Nucleic Acids Res. 30:42-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilstrup, M., S. Jacobsen, K. Hammer, and F. K. Vogensen. 1997. Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Appl. Environ. Microbiol. 63:1826-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunji, E. R., I. Mierau, A. Hagting, B. Poolman, and W. N. Konings. 1996. The proteolytic systems of lactic acid bacteria. Antonie Leeuwenhoek 70:187-221. [DOI] [PubMed] [Google Scholar]
- 18.Li, S. J., and J. E. Cronan, Jr. 1993. Growth rate regulation of Escherichia coli acetyl coenzyme A carboxylase, which catalyzes the first committed step of lipid biosynthesis. J. Bacteriol 175:332-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magni, C., D. de Mendoza, W. N. Konings, and J. S. Lolkema. 1999. Mechanism of citrate metabolism in Lactococcus lactis: resistance against lactate toxicity at low pH. J. Bacteriol. 181:1451-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marini, P. E., C. A. Perez, and D. de Mendoza. 2001. Growth-rate regulation of the Bacillus subtilis accBC operon encoding subunits of acetyl-CoA carboxylase, the first enzyme of fatty acid synthesis. Arch. Microbiol. 175:234-237. [DOI] [PubMed] [Google Scholar]
- 21.Obis, D., A. Guillot, J. C. Gripon, P. Renault, A. Bolotin, and M. Y. Mistou. 1999. Genetic and biochemical characterization of a high-affinity betaine uptake system (BusA) in Lactococcus lactis reveals a new functional organization within bacterial ABC transporters. J. Bacteriol. 181:6238-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obis, D., A. Guillot, and M. Y. Mistou. 2001. Tolerance to high osmolality of Lactococcus lactis subsp. lactis and cremoris is related to the activity of a betaine transport system. FEMS Microbiol. Lett. 202:39-44. [DOI] [PubMed] [Google Scholar]
- 23.Poolman, B., D. Molenaar, E. J. Smid, T. Ubbink, T. Abee, P. P. Renault, and W. N. Konings. 1991. Malolactic fermentation: electrogenic malate uptake and malate/lactate antiport generate metabolic energy. J. Bacteriol. 173:6030-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rallu, F., A. Gruss, S. D. Ehrlich, and E. Maguin. 2000. Acid- and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol. Microbiol. 35:517-528. [DOI] [PubMed] [Google Scholar]
- 25.Rallu, F., A. Gruss, and E. Maguin. 1996. Lactococcus lactis and stress. Antonie Leeuwenhoek 70:243-251. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnan, R., D. Dorris, A. Lublinsky, A. Nguyen, M. Domanus, A. Prokhorova, L. Gieser, E. Touma, R. Lockner, M. Tata, X. Zhu, M. Patterson, R. Shippy, T. J. Sendera, and A. Mazumder. 2002. An assessment of Motorola CodeLink microarray performance for gene expression profiling applications. Nucleic Acids Res. 30:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365-386. [DOI] [PubMed] [Google Scholar]
- 28.Sanz, Y., F. Toldra, P. Renault, and B. Poolman. 2003. Specificity of the second binding protein of the peptide ABC-transporter (Dpp) of Lactococcus lactis IL1403. FEMS Microbiol. Lett. 227:33-38. [DOI] [PubMed] [Google Scholar]
- 29.Schena, M., D. Shalon, R. W. Davis, and P. O. Brown. 1995. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270:467-470. [DOI] [PubMed] [Google Scholar]
- 30.Schreier, H. J. 1993. Biosynthesis of glutamine and glutamate and the assimilation of ammonia, p. 281-298. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. Biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 31.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbach, G. 1995. Contribution of lactic acid bacteria to flavor compound formation in dairy products. Int. Dairy J. 5:877-903. [Google Scholar]
- 33.van de Guchte, M., P. Serror, C. Chervaux, T. Smokvina, S. D. Ehrlich, and E. Maguin. 2002. Stress responses in lactic acid bacteria. Antonie Leeuwenhoek 82:187-216. [PubMed] [Google Scholar]
- 34.Weimer, B., K. Seefeldt, and B. Dias. 1999. Sulfur metabolism in bacteria associated with cheese. Antonie Leeuwenhoek 76:247-261. [PubMed] [Google Scholar]
- 35.Weimer, B., Y. Xie, L. Chou, and A. Cutler. 2004. Gene expression arrays in food, p. 333-343. In J. L. Barredo (ed.), Microbial products and biotransformation. Humana Press, Totowa, N.J.
- 36.Xie, Y. 2003. Determining the influence of extracellular proteinase from Brevibacterium linens on the metabolism of Lactococcus lactis spp. lactis using functional genomics. Ph.D. dissertation. Utah State University, Logan.
- 37.Yang, Y. H., S. Dudoit, P. Luu, and T. P. Speed. 2001. Normalization for cDNA microarray data. Proc. SPIE 4266:141-152. [Google Scholar]