Abstract
Background:
Inverse relationship between helminths infection and immune-mediated diseases has inspired researchers to investigate therapeutic potential of helminths in allergic asthma. Helminth unique ability to induce immunoregulatory responses has already been documented in several experimental studies. This study was designed to investigate whether excretory/secretory (ES) and somatic products of Marshallagia marshalli modulate the development of ovalbumin-induced airway inflammation in a mouse model.
Methods:
This study was carried out at the laboratories of Immunology and Parasitology of Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran during spring and summer 2015. Allergic airway inflammation was induced in mice by intraperitoneal (IP) injection with ovalbumin (OVA). The effects of ES and somatic products of M. marshalli were analyzed by inflammatory cell infiltration in bronchoalveolar lavage fluid (BALF), pathological changes and IgE response.
Results:
Treatment with ES and somatic products of M. marshalli decreased cellular infiltration into BALF when they were administered during sensitization with allergen. Pathological changes were decreased in helminth-treated group, as demonstrated by reduced inflammatory cell infiltration, goblet cell hyperplasia, epithelial lesion and smooth muscle hypertrophy. However, no significant differences were observed in IgE serum levels, cytokines and eosinophil counts between different groups.
Conclusion:
This study provides new insights into anti-inflammatory effects of ES and somatic products of M. marshalli, during the development of non-eosinophilic model of asthma. Further study is necessary to characterize immunomodulatory molecules derived from M. marshalli as a candidate for the treatment of airway inflammation.
Keywords: Asthma, Excretory/secretory, Helminth therapy, Marshallagia marshalli
Introduction
Allergic asthma is an inflammatory disease characterized by aberrant TH2 responses such as IL-4, IL-5, IL-9 and IL-13 associated with airway hyper-responsiveness (AHR), coughing, chest tightening, mastocytosis, eosinophilia and antibody class-switching to produce IgE (1, 2). The incidence of allergic asthma appears to depend on both genetic and environmental factors (3, 4). The dramatic increase of allergic asthma over the recent decades in developed countries intimates the involvement of other factors than predisposing genetic factors to the development of allergic asthma (5). This phenomenon can be explained by hygiene hypothesis, which proposes that exposure to pathogens reduce the risk of inflammatory diseases, a notion that might help explain the global rise of allergic asthma (6, 7). Several experimental and cross-sectional studies show helminth infection ameliorates immune-mediated diseases such as multiple sclerosis (MS) (8, 9), inflammatory bowel disease (IBD) (10–13), rheumatoid arthritis (RA), diabetes (14–16) and allergic asthma (17–20).
Inverse relation between immune-mediated diseases and helminth infection in the absent of certain curative drugs (21) have inspired researchers to use helminths as a therapeutic agent towards reducing symptoms of allergic asthma (22, 23). Furthermore, because exposure to allergen may cause life-threatening anaphylaxis in asthmatic individuals, there is a great need to develop prevention strategies for allergic asthma.
Helminths possess immunomodulatory mechanism that not only ensures their own survival in host body, but also respond to non-helminth antigens such as allergen and self-antigens (24). This capability of helminths has introduced them as a successful organism that has ever been around from ancient period to now. Establishment of helminths parasites in the host for long period are associated with anergy, increased activity of regulatory T and B cells, alternatively activated macrophages (AAM) and production of immunoregulatory cytokines such as IL-10 and TGF-ß. Moreover, helminths promote B cells to produce IgG4 antibodies, which counteract IgE responses (25–28).
In the last few years, there have been developments in the field of helminths-therapy that have led to new strategy in the treatment of immune-mediated diseases. Using Trichuris suis ova (TSO) for treatment of MS (29, 30) and IBD (31) is an example of treatment based on the concept of hygiene hypothesis.
However, treatment of human immune-mediated diseases with live helminths appears to have several drawbacks such as accidental infection, reduced immune response to viral, bacterial and protozoal infection and increased susceptibility to them, reduced efficacy of vaccine, atopic reaction and poor acceptation with consuming eggs or live worms (24). One solution for this problem would be the use of excretory/secretory (ES) and somatic products of helminths. Although, the mechanism of helminth therapy in inflammatory diseases is not clear, helminths produce soluble antigens that manipulate host immune response and suppress the innate and adaptive immune response to helminths and unrelated antigens (32).
M. marshalli is an Ostertagiine nematode that inhabits in the abomasum of sheep, goat and wild ruminant (33). This nematode has both free-living and host-associated state appeared with the subclinical manifestation (34, 35). Infection with M. marshalli is one of the most prevalent helminth infections in Northeast of Iran, where this study was conducted.
Concerning the extensive body of evidence that shows helminths ameliorate immune-mediated diseases such as allergic asthma, in this study, we investigated that M. marshalli may somehow result in suppression of airway inflammation in a murine model of airway inflammation.
Materials and Methods
Ethical statement
The protocols of animal experimental, animal care and husbandry were reviewed and approved by the guideline of Animal Welfare Committee of Ferdowsi University of Mashhad (permit number: 1392-27816).
Animals
Female BALB/c mice, 6–8 wk old, were purchased from Razi Vaccine and Serum Research Institute, Mashhad, Iran. Animals were housed under non-pathogenic condition at the Animal Facility Laboratory of Faculty of Veterinary Medicine of Ferdowsi University of Mashhad.
Study design
To investigate the effects of M. marshalli in ovalbumin (OVA) induced airway inflammation, mice randomly were divided into four groups each containing five mice: a phosphate buffer saline (PBS) treated group, an OVA-treated group, a group sensitized with OVA and ES products and a group received OVA plus somatic products (Table 1).
Table 1:
Study groups and experimental design (ip= intraperitoneal)
| Study groups | Sensitization at days 0 and 11 | Challenges at days 14,27, 28, 29, 47, 61, 73, 74 and 75 |
|---|---|---|
| PBS group (negative control) | PBS + 200 μl Alum 1.3% (i.p) | PBS by nebulizer inhalation |
| Asthma group (positive control) | 20 μg OVA+ 200 μl Alum 1.3% (i.p) | 1% OVA in PBS by nebulizer inhalation |
| ES group (treatment) | 20 μg OVA+ 200 μl Alum 1.3% with 20 μg ES (i.p) | 1% OVA in PBS by nebulizer inhalation |
| Somatic group (treatment) | 20 μg OVA+ 200 μl Alum 1.3% with 20 μg somatic (i.p) | 1% OVA in PBS by nebulizer inhalation |
Preparation and characterization of helminth ES and Somatic products
To prepare the ES and somatic products, helminths were collected from the abomasum of sheep, at post mortem in the slaughterhouse. Adult worms of M. marshalli were isolated and subjected to evaluate under microscope according to morphological features. The worms that did not show any external and internal damages were chosen and extensively washed several times in sterile PBS.
Somatic products were extracted by homogenization of worms in PBS while on ice followed by centrifugation at 8000 gr for 10 min at 4 °C. For sterilization, the supernatants was passed through a 0.22-μm filter and used as somatic antigen.
For preparation of ES products, adult worms were cultured in 25 cm2 tissue culture flasks containing 5000 worms per 5 ml of serum free DMEM (Bio-Idea, Iran) supplemented with 4.5g/L D-glucose, 2mM L-glutamine, 25mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin and 100 μg/ml gentamicin. The flasks were incubated at 37 °C and 5% CO2, fewer than 95% humidified atmosphere. After 48h of culture, supernatants were collected and centrifuged at low speed to discard large debris and eggs. Afterward Supernatants were concentrated by 3000 MWCO Amicon membrane and sterilized using 0.22-μm filter.
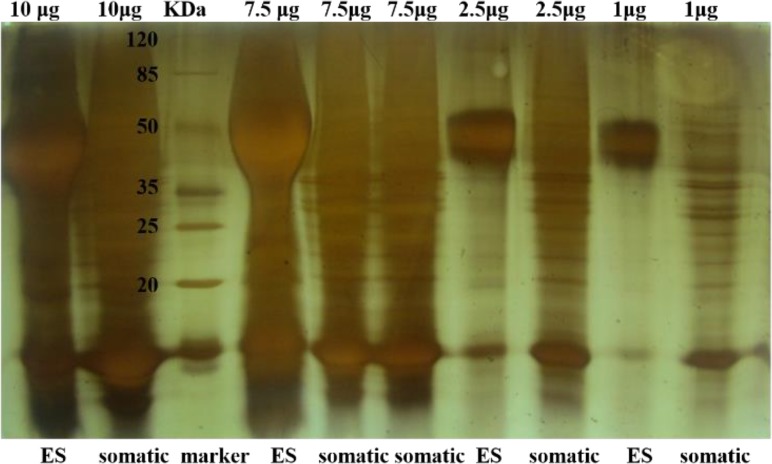
Protein concentration of ES and somatic products was measured by Bradford assay (Bio-Rad, Hercules, CA, USA) and accuracy of them was confirmed by SDS-PAGE (Fig. 1). Concentrated products were stored at −80 °C until use.
Fig. 1:
SDS-PAGE analysis of ES and somatic products of M. marshalli. ES and somatic products from M. marshalli were separated using 12.5% SDS–PAGE according to the method of Laemmli (1970). After electrophoresis, the resolved polypeptide bands were stained with silver staining.
Murine model of airway inflammation
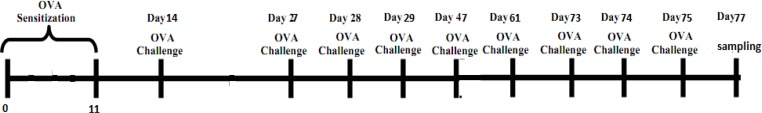
To evaluate the effects of M. marshalli in a chronic model of allergic airway inflammation, induction of allergic airway inflammation were performed in 75 d (36). Briefly, mice sensitized with two IP injections of OVA (grade 3, Sigma Aldrich, ST, Louis, MO, USA) precipitated with aluminum hydroxide (alum, Sigma-Aldrich) on days 0 and 11. The OVA plus ES or somatic groups were sensitized with 20μg ES or somatic with OVA-Alum, respectively and PBS group sensitized with PBS-alum in the same days. Then, mice in OVA, OVA+ES and OVA+somatic group were challenged with an aerosol of 1% OVA protein in PBS for 20 min on days showed in Fig. 2. PBS group were challenged for 15 courses over 75 d with PBS. 24h after the last challenge, the mice were sacrificed and the samples were collected for subsequent analysis. Detailed information regarding the immunization scheme is indicated in Table 1.
Fig. 2:
Schematic diagram of experimental protocol used for sensitization and challenge with OVA
Bronchoalveolar lavage, total, and differential cell counts
Mice were anesthetized one day after the last challenge and their trachea was cannulated. Lung lavage was obtained by 3 injections of 0.4ml PBS into the lungs. A total of 1ml Bronchoalveolar lavage fluid (BALF) collected and centrifuged at 400 gr at 4 °C for 5 min and cell free supernatants were stored at −80 °C for further analysis. The cell pellet of BALF was re-suspended in 100μl PBS and the total number of inflammatory cells infiltrated into BAL fluid was counted using a hemocytometer. For differential cell count, a smear of the cell pellet of BAL fluid was prepared and stained with Giemsa.
In Vitro spleen cell of culture assay
Preparation and culture of a single-cell suspension from mouse spleen were performed as described in previous studies (37). Briefly, single cell suspension from spleen was plated at 5 × 105 cells/ well in 96 well, flat-bottomed plates in RPMI1640 medium supplemented with penicillin, streptomycin, L-glutamine, and 10% FBS. Culturing of cells were undergone in the presence of 50μg/ml OVA, 20 μg/ml ES or somatic antigen for 96h under 37 °C and 5% CO2 in 95% humidified atmosphere. Subsequently, Cell culture supernatants were collected and stored in −80 °C until use.
Measurement of cytokines levels in BAL fluid
Concentration of IFN-γ, IL-4 and IL-10 in BAL fluid and cell culture supernatant was measured by sandwich ELISA assay according to manufacturer’s instructions (eBioscience, Inc, San Diego, CA, USA). The detection limits for each IL-4 and IFN- γ were 4 pg/ml and 15 pg/ml, respectively.
Determination of total IgE in serum
Blood sample was taken from anesthetized mice by cardiac puncture. Blood samples remained at room temperature for 2 h and then were centrifuged at low speed to separate serum. Serum samples were terminally stored in −80 °C until used. The total IgE was measured by commercial ELISA kit, according to manufactures instruction (eBioscience, Inc, San Diego, CA, USA). The detection limit for total IgE was 4 ng/ml.
Lung histology
The lungs dissected from the chest cavity and fixed by pushing the 0.5 ml buffered-formalin 10% via a tracheal tube into them. Further, fixation was performed by incubation of the lungs in the same fixation for 24 h.
The tissues were embedded in paraffin and tissue sections were prepared by cutting tissue into 3–5 μm section. The sections were stained with hematoxylin and eosin for visualizing of inflammatory cells infiltration or with periodic acid-Schiff stain (PAS) for goblet cells hyperplasia. Histopathological changes were observed by light microscope.
Lung sections were evaluated according to a microscopic score, indicating intensity of inflammatory cell infiltration and structural changes such as goblet cell hyperplasia, epithelial lesion, squamous metaplasia and smooth muscle hypertrophy.
The degree of inflammatory cell infiltration was subjectively classified by grading as absent (0), mild (1), moderate (2) or severs (3). In addition, structural changes such as goblet cell hyperplasia were evaluated to scheme for the presence of inflammatory cell.
Statistical Analysis
Data were analyzed using spss 16 (Chicago, IL, USA). Mann-Whitney test or kruskal wallis test was used to verify statistical significance between different groups. P-values<0.05 were considered statistically significant.
Results
ES and somatic products of M. marshalli is required during sensitization to decrease allergic airway inflammation
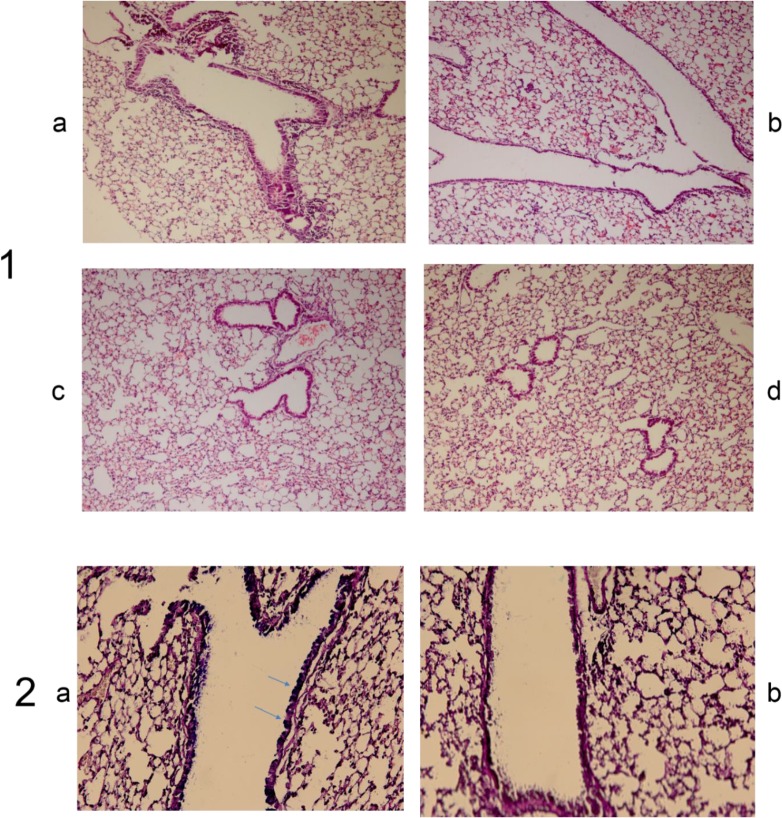
To confirm and extend the studies that are investigating the role of helminths in decreasing of airway inflammation, we evaluated the effects of somatic and ES products of M. marshalli in a murine model of airway inflammation. OVA-induced chronic airway inflammation was associated with recruitment of inflammatory cells, predominantly mononuclear cells and structural changes in airway such as goblet cell hyperplasia, desquamation and increased airway smooth muscle mass (Fig. 3).
Fig. 3:
The suppressive effect of M. marshalli on development of OVA-induced airway inflammation in a mouse model
In contrast, inflammatory cell infiltration and structural changes declined following sensitization with OVA+ somatic and OVA+ ES products (P<0.05). The pathological disorders observed in OVA group were absent in mice sensitized and challenged with PBS. Frequency of pathological scores is shown in Table 2. According to intensity and structural changes shown in more than one area were given a microscopic score from 0 to 3. OVA-induced group exhibited an inflammatory reaction score from 1 to 3.
Table 2:
Frequencies of histopathological scoring (H&E) in all group
| group | number | Intensity of inflammatory cells | squamous metaplasia | goblet cell hypertrophy | epithelial lesions | smooth muscle hypertrophy |
|---|---|---|---|---|---|---|
| OVA | 1 | 1 | 1 | 0 | 0 | 1 |
| 2 | 3 | 2 | 0 | 2 | 2 | |
| 3 | 1 | 1 | 1 | 1 | 1 | |
| 4 | 2 | 2 | 1 | 2 | 2 | |
| 5 | 3 | 2 | 1 | 2 | 1 | |
| PBS | 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 1 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | 0 | 0 | |
| ES | 1 | 1 | 0 | 0 | 0 | 0 |
| 2 | 1 | 1 | 0 | 1 | 0 | |
| 3 | 1 | 1 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | 0 | 0 | |
| Somatic | 1 | 1 | 1 | 0 | 0 | 0 |
| 2 | 1 | 1 | 0 | 0 | 0 | |
| 3 | 2 | 0 | 1 | 0 | 1 | |
| 4 | 1 | 1 | 0 | 0 | 0 | |
| 5 | 1 | 0 | 0 | 0 | 2 |
In contrast, when ES and somatic products were introduced at sensitization phase, inflammatory reaction scores decreased from 0 to 1 and 1 to 2, respectively (P<0.01). Squamous metaplasia showed a score from 1 to 2 in OVA group which was higher than in other groups (P<0.01).
Epithelial lesions such as desquamation, smooth muscle hypertrophy and goblet cell hyperplasia were only observed in OVA group (P<0.01). None of these pathological changes was observed in PBS group except mild infiltration of inflammatory cells in two mice.
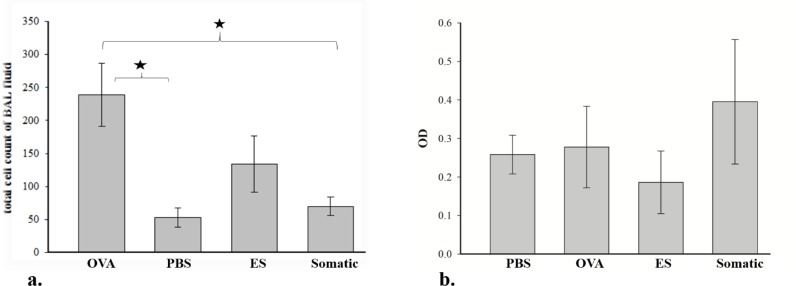
Similar with the finding above, total cell counts in BAL fluid were increased in the OVA group. Mononuclear cells especially lymphocytes were the predominant cells in OVA-induced airway inflammation (Fig. 3). In contrast, following two IP injections of somatic products, there was a tendency to decrease the total cell in BAL fluid but was still elevated compared to total cell counts in PBS group. As shown in Fig. 4, somatic administration did not result in significant decrease of total cell counts. However, total cell counts were significantly (P<0.05) decreased with ES administration.
Fig. 4:
The suppressive effect of ES and somatic products on total cell counts in BAL fluid and total IgE level in serum
Mice were sensitized with PBS or OVA or OVA+ ES or OVA + somatic by IP injection, then challenged with OVA by nebulizer. a. total cell count in BAL fluid. b. total IgE level in serum.
Total serum IgE responses
Total IgE levels were measured in serum by sandwich ELISA. Total IgE level showed a tendency to increase in OVA-induced group. No significant statistical differences were observed between the levels of IgE in sera among the OVA-induced group, ES and somatic treatment groups.
Cytokine analysis in BALF and spleen cell culture supernatants
To investigate the immunological mechanisms associated with the reduced airway inflammation in ES and somatic treated mice, BAL fluid and spleen supernatant were collected and ELISA measured cytokine levels. No significant differences were observed in IL-4, IL-10 and IFN-γ production between different groups (data not shown).
Discussion
Helminths were thought to exert immunoregulatory mechanisms to ensure their own survival in their host. However, recent papers have highlighted a crucial role for helminths as immunomodulators that they could respond to other innocuous antigens such as allergens. These investigations were extended to clinical trials evaluation and at this time using the helminths show a promising therapeutic approach for human IBD (31), MS (29, 30) and lung allergy. A number of clinical trials have been registered using TSO in Crohn’s or ulcerative colitis (UC) patients (38). To date, many commercial companies and private individuals produce helminths for sale with the aim of treatment of inflammatory diseases (39).
ES and somatic products of M. marshalli are effective for suppression of pathological changes of asthma in a murine model of airway inflammation. Co-administration of helminth products with OVA decreased inflammatory cell infiltration in bronchial mucus. In addition, goblet cell hyperplasia, epithelial cell lesion, smooth muscle hypertrophy and epithelial metaplasia were reduced following ES and somatic products treatment. Reduction in pathological changes were not associated with reduction in total IgE levels but there were a tendency in decreasing inflammatory cells infiltration following administration of ES and somatic products. This is the first evidence showing the suppressive effect of M. marshalli on allergic airway inflammation. Surprisingly we found no eosinophilic infiltration in BAL fluid and pathological sections of OVA group. These results may be arbitrated by OVA contamination with endotoxin due to low-grade OVA used in our study. Contaminated OVA with LPS in commercial OVA can decrease eosinophilic inflammation and airway remodeling (40). Moreover, local LPS administration to sensitized mice before allergen challenge has caused lymphocytic inflammation and has inhibited eosinophilic infiltration (41). Furthermore, the dissociation of eosinophilic inflammation from airway hyperactivity (AHR) was observed in some cases of asthma (42).
In the present study, the difference in IgE levels between groups was not significant. Similarly, our result is in agreement with application of Heligosomoides polygyrus (43) and Ascaris pseudo-coelomic fluid (PCF), Fasciola Excretory/Secretory antigen (FES) and hydatid antigen-B (HAgB) (44) on suppressing OVA-induced allergic asthma. However, other investigations have shown a decrease of OVA-specific IgE production in serum by helminthes (12, 18, 19). This discrepancy between our results and other findings may be due to the type of helminthes used. For example, Nippostrongylus brasiliensis had no effect on the amount of IgE in serum but its level decreased in the BAL fluid of mice with helminth infection (45). In addition, protocol used for asthma induction and presence of LPS in commercial OVA could influence IgE levels in our study. Noticeably, systemic administration of LPS before OVA sensitization reduced total IgE and OVA-specific IgE in serum (41).
Unlike other studies, which have shown IL-4, is the major factor regulating Th2 responses, in our result IL-4 did not increase in OVA-induced airway inflammation. This phenomenon could be explained as follows:
First, asthma is a heterogeneous disease with several clinical features that is depending on the intensity and nature of airway inflammation, (46), age, gender, genetic background and environmental influences of the patients (47). Furthermore, cross-sectional studies on asthmatic patients reveal three major patient groups: Th2-high, Th17-high, and Th2/Th17-low. Although, these studies were performed in retrospect to the human, these data might explain the lack of IL-4 in our experimental asthma in mice.
-
Second, while the mechanism of our observation is still unresolved and warrants further investigation, we propose that an alternative way may be involved in induction of asthma.
IL-33 induces classically Th2 cells and IL-5 – producing Th2 cells. In the presence of low OVA, IL-33 induces IL-5 producing Th2 cells independent of IL-4. With a standard dose of OVA, IL-33 enhances both classical and IL-5-producing Th2 cells (48, 49). Therefore, dose of antigen is a principal cause of polarization of naive T cells into classical Th2 or IL-5-producing T cells.
Third, in general, the level of IL-4 is very low in BAL fluid and it is difficult to detect it. Therefore, it is possible that the assay kit applied in this experimental were not sensitive enough to measure such a low concentration of IL-4 in BALF. Furthermore, bronchial epithelial cells have receptor for IL-4 and obviously, they consume it quickly. Therefore, the time of mice killing is crucial factor in detection of cytokines. Furthermore, our failure to measure detectable levels of IL-4 protein in BAL fluid likely related to consumption of this cytokine by receptor expressing cells. The suppressive effects of helminths are related to increased IL-10 and TGF-β production and development of regulatory cells (18, 50).
Available therapeutic drugs are limited to a few drugs that are prescribed to reduce allergic symptoms (21). Because some type of asthma such as non-eosinophilic asthma does not response to therapy with corticosteroids (51), current and new therapeutics are designed to control immunological responses based on asthma phenotype. For example, omalizumab (52) is an anti-IgE therapy and mepolizumab is a therapeutic agent in controlling of eosinophilic asthma exacerbation. However, these treatments proved to be expensive and time-consuming.
The immunomodulatory mechanism of M. marshalli is still unclear but it is possible this nematode acts through the control of host immune responses.
Conclusion
This study provides another example in confirmation of hygiene hypothesis and new insights into prevention and treatment of airway inflammation. Although our experiment did not illustrate eosinophils and IL-4 in BAL fluid, lung sections and total cell count in BAL fluid confirmed airway inflammation. ES and somatic products of M. marshalli were able to suppress airway inflammation independent of asthma phenotypes. Responsiveness to airway inflammation even in non-eosinophilic airway inflammation with poor response to corticosteroids, short lifespan and being non-human pathogenic of M. marshalli, give this nematode a chance to select as a new model for further research in the field of airway inflammation. Further investigations are necessary to identify the parasite-derived molecules involved in immunomodulation. These molecules are important in understanding the cross talk between helminths and host immune responses and development of new asthma treatment strategies.
Acknowledgements
This project was supported by grant number 27816 from Ferdowsi University of Mashhad. We highly appreciate the invaluable assistance and comments of Dr. Hermelijn H. Smits, at the Department of Parasitology, Linden University Medical Center (LUMC) throughout this project. The authors declare that there is no conflict of interest.
References
- 1. Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just TH2 cells. Nat Rev Immunol. 2010; 10 (12): 838– 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012; 18 (5): 716– 25. [DOI] [PubMed] [Google Scholar]
- 3. Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011; 242 (1): 10– 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolterink RGK, Hendriks RW. Type 2 innate lymphocytes in allergic airway inflammation. Curr Allergy Asthma Rep. 2013; 13 (3): 271– 80. [DOI] [PubMed] [Google Scholar]
- 5. Kondrashova A, Seiskari T, Ilonen J, Knip M, Hyöty H. The ‘Hygiene hypothesis’ and the sharp gradient in the incidence of autoimmune and allergic diseases between Russian Karelia and Finland. Apmis. 2013; 121 (6): 478– 93. [DOI] [PubMed] [Google Scholar]
- 6. Bisgaard H, Bønnelykke K, Stokholm J. Immune-mediated diseases and microbial exposure in early life. Clinical & Experimental Allergy. 2014; 44 (4): 475– 81. [DOI] [PubMed] [Google Scholar]
- 7. Kramer A, Bekeschus S, Bröker B, Schleibinger H, Razavi B, Assadian O. Maintaining health by balancing microbial exposure and prevention of infection: the hygiene hypothesis versus the hypothesis of early immune challenge. J Hosp Infect. 2013; 83: S29– S34. [DOI] [PubMed] [Google Scholar]
- 8. Fleming J. Helminth therapy and multiple sclerosis. Int J Parasitol. 2013; 43 (3): 259– 74. [DOI] [PubMed] [Google Scholar]
- 9. Correale J, Farez MF. Parasite Infections in Multiple Sclerosis Modulate Immune Responses through a Retinoic Acid–Dependent Pathway. J Immunol. 2013; 191 (7): 3827– 3837. [DOI] [PubMed] [Google Scholar]
- 10. Lu N, Wang L, Cao H, Liu L, Van Kaer L, Washington MK, et al. Activation of the Epidermal Growth Factor Receptor in Macrophages Regulates Cytokine Production and Experimental Colitis. J Immunol. 2014; 192 (3): 1013– 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferreira I, Smyth D, Gaze S, Aziz A, Giacomin P, Ruyssers N, et al. Hookworm excretory/secretory products induce interleukin-4 (IL-4)+ IL-10+ CD4+ T cell responses and suppress pathology in a mouse model of colitis. Infect Immun. 2013; 81 (6): 2104– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruyssers NE, De Winter BY, De Man JG, Loukas A, Pearson MS, Weinstock JV, et al. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis. 2009; 15 (4): 491– 500. [DOI] [PubMed] [Google Scholar]
- 13. Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, van Rooijen N, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007; 178 (7): 4557– 4566. [DOI] [PubMed] [Google Scholar]
- 14. Zaccone P, Cooke A. Helminth mediated modulation of Type 1 diabetes (T1D). Int J Parasitol. 2013; 43 (3): 311– 8. [DOI] [PubMed] [Google Scholar]
- 15. Mishra P, Patel N, Wu W, Bleich D, Gause W. Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response. Mucosal Immunol. 2012; 6 (2): 297– 308. [DOI] [PubMed] [Google Scholar]
- 16. Lund ME, O’Brien BA, Hutchinson AT, Robinson MW, Simpson AM, Dalton JP, et al. Secreted Proteins from the Helminth Fasciola hepatica Inhibit the Initiation of Autoreactive T Cell Responses and Prevent Diabetes in the NOD Mouse. PloS One. 2014; 9 (1): e86289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamid F, Wiria AE, Wammes LJ, Kaisar MM, Lell B, Ariawan I, et al. A longitudinal study of allergy and intestinal helminth infections in semi urban and rural areas of Flores, Indonesia (ImmunoSPIN Study). BMC Infect Dis. 2011; 11 (1): 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim SE, Kim J-H, Min B-H, Bae YM, Hong S-T, Choi M-H. Crude Extracts of Caenorhabditis elegans Suppress Airway Inflammation in a Murine Model of Allergic Asthma. PLoS One. 2012; 7 (4): e35447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schabussova I, Ul-Haq O, Hoflehner E, Akgün J, Wagner A, Loupal G, et al. Oesophagostomum dentatum Extract Modulates T Cell-Dependent Immune Responses to Bystander Antigens and Prevents the Development of Allergy in Mice. PloS One. 2013; 8 (7): e67544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ebner F, Hepworth M, Rausch S, Janek K, Niewienda A, Kühl A, et al. Therapeutic potential of larval excretory/secretory proteins of the pig whipworm Trichuris suis in allergic disease. Allergy. 2014; 69 (11): 1489– 97. [DOI] [PubMed] [Google Scholar]
- 21. Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009; 31 (3): 438– 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harnett W, Harnett MM. Helminth-derived immunomodulators: can understanding the worm produce the pill?. Nat Rev Immunol. 2010; 10 (4): 278– 84. [DOI] [PubMed] [Google Scholar]
- 23. Weinstock JV. Autoimmunity: The worm returns. Nature. 2012; 491 (7423): 183– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012; 25 (4): 585– 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011; 11 (6): 375– 88. [DOI] [PubMed] [Google Scholar]
- 26. Grencis R. Immunity to Helminths: Resistance, Regulation, and Susceptibility to Gastrointestinal Nematodes. Annu Rev Immunol. 2015; 33: 201– 225. [DOI] [PubMed] [Google Scholar]
- 27. Taylor MD, van der Werf N, Maizels RM. T cells in helminth infection: the regulators and the regulated. Trends Immunol. 2012; 33 (4): 181– 9. [DOI] [PubMed] [Google Scholar]
- 28. Hussaarts L, van der Vlugt LE, Yazdanbakhsh M, Smits HH. Regulatory B-cell induction by helminths: implications for allergic disease. J Allergy Clin Immunol. 2011; 128 (4): 733– 9. [DOI] [PubMed] [Google Scholar]
- 29. Fleming J, Isaak A, Lee J, Luzzio C, Carrithers M, Cook T, et al. Probiotic helminth administration in relapsing–remitting multiple sclerosis: a phase 1 study. Multiple Sclerosis Journal. 2011; 17 (6): 743– 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosche B, Wernecke K-D, Ohlraun S, Dörr JM, Paul F. Trichuris suis ova in relapsingremitting multiple sclerosis and clinically isolated syndrome (TRIOMS): study protocol for a randomized controlled trial. Trials. 2013; 14 (1): 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandborn W, Elliott D, Weinstock J, Summers R, Landry-Wheeler A, Silver N, et al. Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn’s disease. Aliment Pharmacol Ther. 2013; 38 (3): 255– 63. [DOI] [PubMed] [Google Scholar]
- 32. Yatsuda AP, Krijgsveld J, Cornelissen AW, Heck AJ, de Vries E. Comprehensive analysis of the secreted proteins of the parasite Haemonchus contortus reveals extensive sequence variation and differential immune recognition. J Biol Chem. 2003; 278 (19): 16941– 51. [DOI] [PubMed] [Google Scholar]
- 33. Bergstrom R. Prevalence of Marshallagia marshalli (Oriov, 1933) in wild ruminants in Wyoming. Proc 0Ida Acad Sci. 1915; 55: 101– 2. [Google Scholar]
- 34. Moradpour N, Borji H, Razmi G, Maleki M, Kazemi H. Pathophysiology of Marshallagia marshalli in experimentally infected lambs. Parasitology. 2013; 140 (14): 1762– 7. [DOI] [PubMed] [Google Scholar]
- 35. Nabavi R, Eslami A, Shokrani H, Bokaie S, Shayan P, Saadati D. Study on the prevalence, intensity and seasonal dynamics of abomasal helminths in sheep from different climatic zones of Iran. World Appl Sci J. 2011; 12: 441– 5. [Google Scholar]
- 36. Khakzad MR, Mirsadraee M, Mohammadpour A, Ghafarzadegan K, Hadi R, Saghari M, et al. Effect of verapamil on bronchial goblet cells of asthma: An experimental study on sensitized animals. Pulm Pharmacol Ther. 2012; 25 (2): 163– 8. [DOI] [PubMed] [Google Scholar]
- 37. Bayne LJ, Vonderheide RH. Multicolor Flow Cytometric Analysis of Immune Cell Subsets in Tumor-Bearing Mice. Cold Spring Harb Protoc. 2013; 2013: 955– 960. [DOI] [PubMed] [Google Scholar]
- 38. Helmby H. Human helminth therapy to treat inflammatory disorders-where do we stand? BMC Immunol. 2015; 16 (1): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheng AM, Jaint D, Thomas S, Wilson J, Parker W. Overcoming evolutionary mismatch by self-treatment with helminths: current practices and experience. JJEM. 2015. [Google Scholar]
- 40. Oh M-J, Paeng J-W, Lee J-Y, Lee B-J, Choi D-C. The effect of lipopolysaccharide-contaminated ovalbumin on airway inflammation and remodeling in a chronic murine asthma model. Eur Respir J. 2013; 42 (Suppl 57): P1580. [Google Scholar]
- 41. Gerhold K, Blümchen K, Bock A, Seib C, Stock P, Kallinich T, et al. Endotoxins prevent murine IgE production, TH2 immune responses, and development of airway eosinophilia but not airway hyperreactivity. J Allergy Clin Immunol. 2002; 110 (1): 110– 6. [DOI] [PubMed] [Google Scholar]
- 42. Hogan SP, Matthaei KI, Young JM, Koskinen A, Young IG, Foster PS. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J Immunol. 1998; 161 (3): 1501– 9. [PubMed] [Google Scholar]
- 43. Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. J Immunol. 2006; 177 (3): 1628– 35. [DOI] [PubMed] [Google Scholar]
- 44. Mawla MMA, Khalifa KE, Sammour SA, Nazeer JT. Evaluation of the suppressive effects of Ascaris Pseudo-Coelomic Fluid (PCF), Fasciola Excretory/Secretory Antigen (FES) and Hydatid Antigen-B (HAgB) on the development of experimental murine asthma. PUJ. 2012; 5 (1): 41– 48. [Google Scholar]
- 45. Wohlleben G, Trujillo C, Müller J, Ritze Y, Grunewald S, Tatsch U, et al. Helminth infection modulates the development of allergen-induced airway inflammation. Int Immunol. 2004; 16 (4): 585– 96. [DOI] [PubMed] [Google Scholar]
- 46. Chu DK, Al-Garawi A, Llop-Guevara A, Pillai RA, Radford K, Shen P, et al. Therapeutic potential of anti-IL-6 therapies for granulocytic airway inflammation in asthma. Allergy Asthma Clin Immunol. 2015; 11 (1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chung HL. Asthma in childhood: a complex, heterogeneous disease. Korean J Pediatr. 2011; 54 (1): 1– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008; 181 (7): 4780– 90. [DOI] [PubMed] [Google Scholar]
- 49. Guo L, Huang Y, Chen X, Hu-Li J, Urban Jr JF, Paul WE. Innate immunological function of TH2 cells in vivo. Nature Immunol. 2015; 16 (10): 1051– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep. 2010; 10 (1): 3– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007; 62 (12): 1043– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011; 364 (11): 1005– 15. [DOI] [PMC free article] [PubMed] [Google Scholar]