Abstract
The ruv operon is induced by treatments that damage DNA and is regulated by the LexA repressor. It encodes two proteins, RuvA and RuvB, that are involved in DNA repair, recombination in RecE and RecF pathways, and mutagenesis. RuvB protein was previously purified and has ATP-binding activity and weak ATPase activity. To study the biochemical properties of RuvA and its interaction with RuvB, we purified RuvA protein to near homogeneity from an over-producing strain. RuvA bound more efficiently to single-stranded DNA than to double-stranded DNA. RuvA bound to DNA greatly enhanced the ATPase activity of RuvB; the enhancing effect of various forms of DNA was in the order of supercoiled DNA greater than single-stranded DNA greater than linear double-stranded DNA. UV irradiation further enhanced the ATPase stimulatory effect of supercoiled DNA dose dependently. The RuvA-RuvB complex has an activity that renatures the cruciform structure in supercoiled DNA. From these experiments and previous work, we infer that the RuvA-RuvB complex may promote branch migration in recombination and may correct irregular structures in DNA, such as cruciforms and hairpins, to facilitate DNA repair using ATP as the energy source.
Full text
PDF

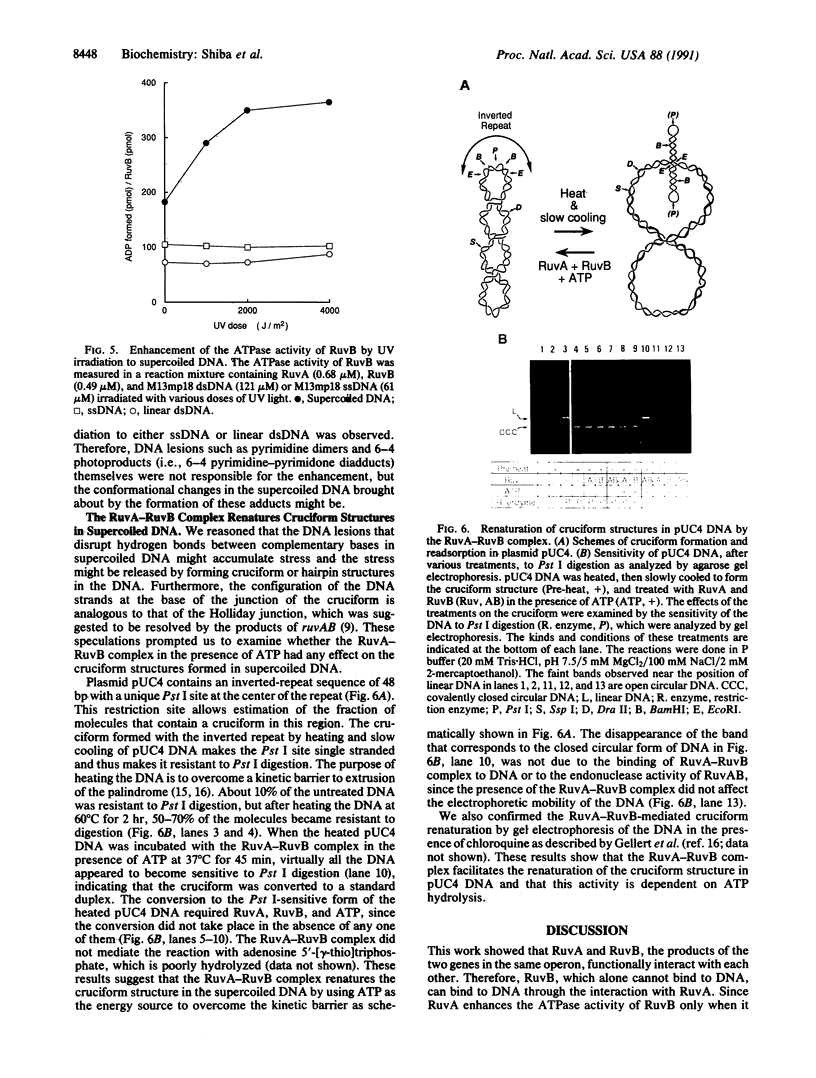

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson F. E., Illing G. T., Sharples G. J., Lloyd R. G. Nucleotide sequencing of the ruv region of Escherichia coli K-12 reveals a LexA regulated operon encoding two genes. Nucleic Acids Res. 1988 Feb 25;16(4):1541–1549. doi: 10.1093/nar/16.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. recA protein-promoted DNA strand exchange. Stable complexes of recA protein and single-stranded DNA formed in the presence of ATP and single-stranded DNA binding protein. J Biol Chem. 1982 Jul 25;257(14):8523–8532. [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Mizuuchi K. Slow cruciform transitions in palindromic DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5545–5549. doi: 10.1073/pnas.80.18.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R. Untwisting B-Z DNA. Trends Genet. 1989 Nov;5(11):355–356. doi: 10.1016/0168-9525(89)90150-9. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Shiba T., Makino K., Nakata A., Shinagawa H. Overproduction, purification, and ATPase activity of the Escherichia coli RuvB protein involved in DNA repair. J Bacteriol. 1989 Oct;171(10):5276–5280. doi: 10.1128/jb.171.10.5276-5280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H., Shiba T., Nakata A., Shinagawa H. Involvement in DNA repair of the ruvA gene of Escherichia coli. Mol Gen Genet. 1989 Oct;219(1-2):328–331. doi: 10.1007/BF00261196. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Benson F. E., Shurvinton C. E. Effect of ruv mutations on recombination and DNA repair in Escherichia coli K12. Mol Gen Genet. 1984;194(1-2):303–309. doi: 10.1007/BF00383532. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Buckman C., Benson F. E. Genetic analysis of conjugational recombination in Escherichia coli K12 strains deficient in RecBCD enzyme. J Gen Microbiol. 1987 Sep;133(9):2531–2538. doi: 10.1099/00221287-133-9-2531. [DOI] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. Binding of the recA protein of Escherichia coli to single- and double-stranded DNA. J Biol Chem. 1981 Aug 25;256(16):8835–8844. [PubMed] [Google Scholar]
- Otsuji N., Horiuchi T., Nakata A., Kawamata J. Strains of Escherichia coli hypersensitive to representative carcinostatic and carcinogenic agents. J Antibiot (Tokyo) 1978 Aug;31(8):794–796. doi: 10.7164/antibiotics.31.794. [DOI] [PubMed] [Google Scholar]
- Otsuji N., Iyehara-Ogawa H. Thermoresistant revertants of an Escherichia coli strain carrying tif-1 and ruv mutations: non-suppressibility of ruv by sfi. J Bacteriol. 1979 Apr;138(1):1–6. doi: 10.1128/jb.138.1.1-6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuji N., Iyehara H., Hideshima Y. Isolation and characterization of an Escherichia coli ruv mutant which forms nonseptate filaments after low doses of ultraviolet light irradiation. J Bacteriol. 1974 Feb;117(2):337–344. doi: 10.1128/jb.117.2.337-344.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargentini N. J., Smith K. C. Role of ruvAB genes in UV- and gamma-radiation and chemical mutagenesis in Escherichia coli. Mutat Res. 1989 Nov;215(1):115–129. doi: 10.1016/0027-5107(89)90224-8. [DOI] [PubMed] [Google Scholar]
- Sharples G. J., Benson F. E., Illing G. T., Lloyd R. G. Molecular and functional analysis of the ruv region of Escherichia coli K-12 reveals three genes involved in DNA repair and recombination. Mol Gen Genet. 1990 Apr;221(2):219–226. doi: 10.1007/BF00261724. [DOI] [PubMed] [Google Scholar]
- Shinagawa H., Makino K., Amemura M., Kimura S., Iwasaki H., Nakata A. Structure and regulation of the Escherichia coli ruv operon involved in DNA repair and recombination. J Bacteriol. 1988 Sep;170(9):4322–4329. doi: 10.1128/jb.170.9.4322-4329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurvinton C. E., Lloyd R. G. Damage to DNA induces expression of the ruv gene of Escherichia coli. Mol Gen Genet. 1982;185(2):352–355. doi: 10.1007/BF00330811. [DOI] [PubMed] [Google Scholar]
- Smolarsky M., Tal M. Novel method for measuring polyuridylic acid binding to ribosomes. Biochim Biophys Acta. 1970 Feb 18;199(2):447–452. doi: 10.1016/0005-2787(70)90087-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman K. F., Mount D. W. Nucleotide sequence binding specificity of the LexA repressor of Escherichia coli K-12. J Bacteriol. 1985 Jul;163(1):376–384. doi: 10.1128/jb.163.1.376-384.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C., Parsons C. A., Picksley S. M. Purification and properties of a nuclease from Saccharomyces cerevisiae that cleaves DNA at cruciform junctions. J Biol Chem. 1987 Sep 15;262(26):12752–12758. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Grossman L. Protein complexes formed during the incision reaction catalyzed by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1986 Mar 25;14(6):2567–2582. doi: 10.1093/nar/14.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]