Abstract
Cannabis use has been associated with an increased risk to develop schizophrenia as well as symptom exacerbation in patients. In contrast, clinical studies have revealed an inverse relationship between the CSF levels of the endocannabinoid anandamide and symptom severity, suggesting a therapeutic potential for endocannabinoid enhancing drugs. Indeed, preclinical studies have shown that these drugs can reverse distinct behavioral deficits in a rodent model of schizophrenia. The mechanisms underlying the differences between exogenous and endogenous cannabinoid administration are currently unknown. Using the phencyclidine (PCP) rat model of schizophrenia, we compared the effects on neuronal activity of systematic administration of delta-9-tetrahydrocannabinol (THC) with the fatty acid amide hydrolase inhibitor URB597. Specifically, we found that the inhibitory response in the prefrontal cortex to THC administration was absent in PCP-treated rats. In contrast, an augmented response to endocannabinoid upregulation was observed in the prefrontal cortex of PCP-treated rats. Interestingly, differential effects were also observed at the neuronal population level, as endocannabinoid upregulation induced opposite effects on coordinated activity when compared to THC. Such information is important for understanding why marijuana and synthetic cannabinoid use may be contraindicated in schizophrenia patients while endocannabinoid enhancement may provide a novel therapeutic approach.
Keywords: THC, schizophrenia, endocannabinoids, electrophysiology, prefrontal cortex, ventral hippocampus
Introduction
Schizophrenia is a debilitating disorder affecting over 1% of the population and characterized by distinct symptoms including hallucinations, delusions, emotional and social withdrawal, as well as cognitive disruptions [Feldman et al., 1997, Association., 2000]. Antipsychotics are usually prescribed to alleviate these symptoms, but given their side effects such as weight gain, dyskinesia, and sexual dysfunction many patients often stop taking their medications [Lieberman et al., 2005]. For these reasons, alternatives to conventional antipsychotics are being pursued.
Endocannabinoid upregulation may be a potential treatment for schizophrenia due to promising clinical and preclinical observations [Leweke et al., 1999, Giuffrida et al., 2004, Seillier et al., 2010, Seillier et al., 2013, Aguilar et al., 2015]. The endocannabinoid system is comprised of lipid messengers, including the prototypic endocannabinoid anandamide (AEA), that are found throughout the brain and activate the cannabinoid type 1 receptor (CB1r). Drug-naïve schizophrenic patients show increased levels of AEA in the cerebrospinal fluid (CSF) [Leweke et al., 1999], and blood levels of AEA are higher in patients experiencing acute schizophrenia than when these same patients are in remission, leading some to hypothesize increased AEA levels contribute to the symptoms of schizophrenia [De Marchi et al., 2003]. Interestingly, across schizophrenia patients, higher CSF levels of AEA have been correlated to less severe psychotic symptoms [Giuffrida et al., 2004], suggesting that AEA upregulation may be a beneficial compensatory response. These findings have inspired research to explore the therapeutic potential of endocannabinoids in animal models of schizophrenia [Seillier et al., 2010, Seillier et al., 2013, Aguilar et al., 2015]. Indeed, recent preclinical studies have shown that AEA upregulation (following blockade of its catabolic enzyme, fatty acid amide hydrolase or FAAH) reverses social withdrawal in the phencyclidine (PCP) rat model of schizophrenia [Seillier et al., 2010] in a CB1 dependent manner [Seillier et al., 2013]. AEA upregulation can also alleviate aberrant dopamine neuron activity in the same model [Aguilar et al., 2015]. However, the clinical literature reveals mixed results for AEA upregulation through FAAH inhibition. Some have found FAAH inhibitors can alleviate positive and negative symptoms in schizophrenia patients [Leweke et al., 2012], while others suggest they have little to no efficacy in these populations [Zuardi et al., 2006, Hallak et al., 2011]. In any case, the neuronal mechanisms underlying the potential therapeutic utility of endocannabinoids are not well understood. Furthermore, disruptions in neuronal activity have been well characterized following acute PCP administration [Suzuki et al., 2002, Jodo et al., 2010], but less so after subchronic PCP treatment [Young et al., 2015] which induces a model of schizophrenia without the confounds associated with acute NMDA receptor blockade [Mouri et al., 2007].
Interestingly, the use of endocannabinoid upregulation as a therapy may appear to be at odds with epidemiological studies on marijuana exposure in schizophrenics. The cannabinoid hypothesis of schizophrenia correlates marijuana use with an increasing risk of psychosis, but causation is not necessarily implied [Moore et al., 2007]. On the other hand, schizophrenia patients who ‘self-medicate’ with cannabis may show superior cognitive functioning compared to their nonsmoking peers [Yucel et al., 2012]. Furthermore, cannabinoids may alleviate some PCP-induced schizophrenia-like symptoms in rodents [Spano et al., 2010, Spano et al., 2013]. Nonetheless, clinical studies of psychotic prone patients revealed that delta-9-tetrahydrocannabinol (THC) can transiently exacerbate positive and negative symptoms of schizophrenia as well as increase learning deficits [D'souza et al., 2005, D'souza et al., 2009, Mason et al., 2009]. Similarly, numerous studies have reported the exacerbation of psychosis in schizophrenia patients [Negrete et al., 1986, Linszen et al., 1994, Degenhardt et al., 2007].
In response to these observations, CB1r antagonists were investigated as a potential antipsychotic therapy [for review see [Roser et al., 2010]]. Preclinical studies had mixed results with CB1r antagonists sometimes alleviating schizophrenia-like positive symptoms [Martin et al., 2003, Tzavara et al., 2003, Madsen et al., 2006, Ferrer et al., 2007, Parolaro et al., 2010], although they were generally more effective at alleviating cannabinoid induced gating deficits [Mansbach et al., 1996, Martin et al., 2003, Dissanayake et al., 2008, Hajos et al., 2008, Parolaro et al., 2010]. Unfortunately, clinical trials failed to demonstrate beneficial effects of these drugs [Meltzer et al., 2004].
Here we compare the effects of the endocannabinoid enhancing drug (FAAH inhibitor) URB597 with exogenous THC administration in the subchronic PCP model of schizophrenia. Specifically, we examined their effects on neuronal activity in brain regions relevant to schizophrenia: the medial prefrontal cortex (mPFC) and ventral hippocampus (vHipp). Given that endocannabinoids are synthesized on demand de novo, we recorded neuronal activity in conscious, freely moving animals [Piomelli, 2003].
Methods and Materials
Animals
All experiments were conducted in accordance with the guidelines outlined by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center.
Chronic Electrode Implantation
Adult male Sprague-Dawley rats (300-400g) were anesthetized with pentobarbital (60mg/kg, i.p.) and treated with atropine (0.1mg/kg, i.p.) to reduce mucus secretion. Rats were placed in a stereotaxic apparatus using blunt atraumatic ear bars and body temperature was maintained at 37°C. Anesthesia was maintained throughout by supplemental administration of pentobarbital as required to maintain suppression of limb compression withdrawal reflex. The surgery was completed under semi-sterile conditions. Microwire polyimide coated stainless steel electrode arrays (Eight wires in a 2x4 array, separated by 0.25mm) were lowered into both the mPFC (A/P +3.0, M/L −0.5, D/V −4.0mm from bregma) and vHipp (A/P −5.6, M/L +5.3, D/V −7.0mm from bregma) in the right hemisphere, and fixed to the skull by dental cement. Stainless steel screws were inserted into the skull and connected to the reference wire of the electrodes. Ketoprofen (5mg/kg) was administered as an analgesic. All rats were singly housed and allowed two weeks to recover with access to food and water ad libitum.
Sub-chronic PCP Treatment
Once recovered from surgery, the rats received an intraperitoneal injection of either PCP (5mg/kg, i.p.) or vehicle (isotonic saline 1ml/kg i.p.) twice daily for 7 days (8 AM and 8 PM), followed by 7 days drug free. This sub-chronic PCP injection schedule induces a well characterized pharmacological model of schizophrenia [Mouri et al., 2007, Neill et al., 2010].
Procedure
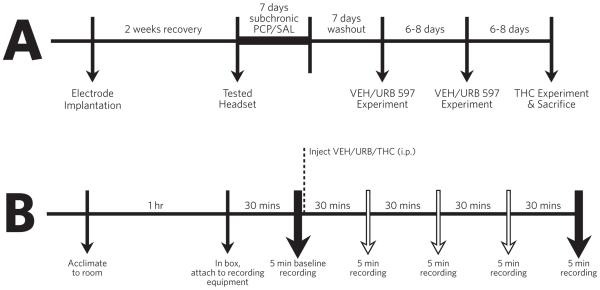
A schematic of the procedure is provided in Figure 1. Animals were moved to the testing room and acclimated for at least one hour. An animal was then given 30 minutes to equilibrate to the testing environment, a Med Associates activity box (17.5” by 17.5”), while connected to recording equipment (Omniplex). Following this period a 5 minute baseline recording was taken. After the baseline an i.p. injection of either vehicle (10% Tween-80, 10% Polyethylene Glycerol, 80% Saline at 65°C), 0.3mg/kg URB597 [fatty acid amide hydrolase (FAAH) inhibitor], or delta-9-tetrahydrocannabinol (THC, 1mg/kg) was given. Next, 5 minute recordings were taken at intervals 30, 60, 90, or 120 minutes after drug injection. Each rat was tested with vehicle, URB597 and THC with at least 6 days between experiments to allow time for the drugs to be completely eliminated. Vehicle and URB597 experiments were given in a counterbalanced order, with the THC experiment always performed last to avoid THC-dependent CB1 receptor tolerance [Breivogel et al., 1999]. Rats were administered URB597 two times in an attempt to examine CB1 and TRPV1 antagonists (antagonists were administered after the 120 minute recording period). However, the results from this approach were inconclusive and a more appropriate paradigm would have been to administer the antagonists prior to the cannabinoids to block their effects. Therefore, these data are not included in the analysis. An experimenter was present throughout all of the recordings and a light tap on the activity box 30 seconds prior to recording was performed to ensure that rats remained awake during the recording session.
Figure 1.
Experimental timeline for surgery, subchronic injections, and recordings (A) as well as the timeline of each experimental recording session (B). The vehicle and URB597 experiments were performed in a counterbalanced order, with the THC experiment always occurring last to minimize potential confounds associated with THC-induced CB1 receptor desensitization.
Histology
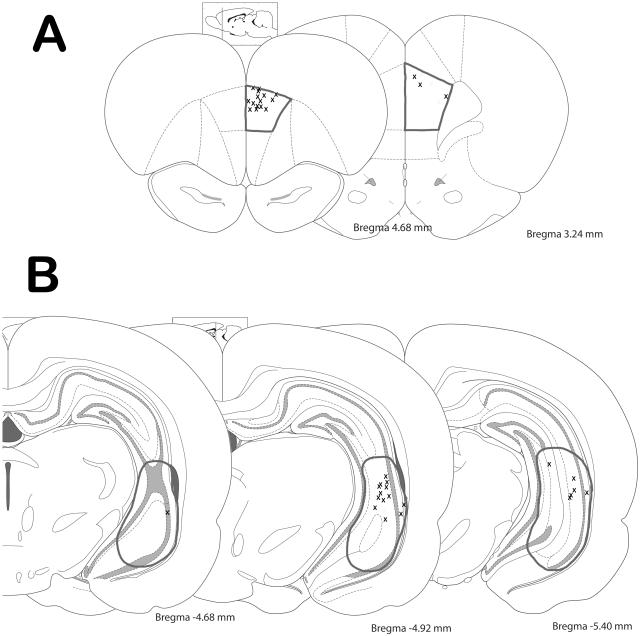
At the cessation of the experiment, rats were deeply anesthetized and decapitated. Their brains were removed and fixed for at least 48 hours (8% w/v paraformaldehyde in phosphate buffered saline) and cryoprotected (25% w/v sucrose in PBS) until saturated. Brains were sectioned (25 micrometer coronal sections), mounted onto gelatin-chrom alum coated slides and processed with a Nissl stain for histochemical verification of electrode sites (Figure 2). All histology was performed with reference to a stereotaxic atlas (Paxinos and Watson, 1986).
Figure 2.
Schematic demonstrating the electrode track placements throughout the medial prefrontal cortex (A) and ventral hippocampus (B). Target areas are outlined in grey. Histology was performed with reference to a stereotaxic atlas (Paxinos and Watson, 1986).
Analysis
Data were recorded using Omniplex software (Plexon Inc, Dallas, TX, USA). Wideband data were recorded in 5 minute intervals via a Plexon Headstage and PBX Preamplifier (HST 8o50-G20, 20x gain, Plexon Inc, Dallas, TX, USA) routing through an Omniplex D system at a 40 KHz sample rate. The recording template was as follows: Gain 6000, pre-threshold time 600 μsec, waveform length 1400 μsec. After the live recording, putative neurons were identified in OfflineSorter V3. First, signals were filtered using a 4th order low-cut Butterworth filter, and the voltage threshold for spike detection was set at −10 to −15% relative to the mean baseline signal. Next, waveforms were aligned by first local extremum after sort start. Cross channel artifacts (i.e. signals that appeared across multiple electrodes simultaneously) were identified by the Offline Sorter software and invalidated. Finally, putative neurons were identified in each electrode channel based on action potential shape. The average neurons recorded per animal are as follows: in SAL animals the PFC had 10.6 ± 2.3 cells while the vHipp had 8.6 ± 1.8 cells; for PCP animals the PFC had 9.75 ± 2.3 cells while the vHipp had 7.9 ± 1.7 cells. Electrophysiological analysis of single unit activity and local field potential activity was performed using commercial computer software (NeuroExplorer 4). All data are represented as the mean ± S.E.M. unless otherwise stated, with n-values representing the number of animals per experimental group. Statistics were calculated and graphs were created using SigmaPlot, Graphpad Prism, and MATLAB.
The firing rates of neurons across the five minute recoding period were averaged into one “animal mean” value per brain region. These averaged firing rates were analyzed by a two-way repeated measure (RM) ANOVA (between variable = subchronic saline / PCP, within variable = baseline / 120mins after acute treatment) for each acute treatment (vehicle, URB597, THC). The Holm-Sidak multiple comparison test was used to examine statistical differences between treatment groups.
In addition, firing rate distributions were fit by Gaussian models while graphical representation of each individual neurons response was plotted in Matlab using Z scores:
| (1) |
where χ represents the average firing rate (calculated in 1 min bins), μ represents the 5 minute baseline firing average, and σ represents the 5 minute baseline standard deviation.
Local field potential (LFP) activity is an electrophysiological measure of the activity of populations of neurons [Pesaran, 2009]. Power spectral density measures the strength of the LFP in a specific frequency band [Einevoll et al., 2013], whereas coherence measures the degree of coordinated LFP activity between the two regions being recorded (vHipp and mPFC) [Robbe et al., 2006]. One LFP measurement was examined per brain region. Coherence value was calculated by comparing the amplitudes of a rodent’s mPFC LFP and its vHipp LFP across various frequency divisions, then summed within each division. LFPs were broken down into the following frequency divisions: Delta (0.3-4 Hz), Theta (4-8 Hz), Alpha (8-13 Hz), Beta (13-30 Hz), and Gamma (30-100 Hz) waves. The power spectral density settings were: maximum frequency 100Hz, 1024 frequency values, normalized to the raw PSD. Power spectral density and coherence were analyzed separately for each frequency division (delta, theta, etc) using two way repeated measure ANOVAs (between = subchronic saline / PCP, within = acute treatment of URB597 or THC against respective baselines). The Holm-Sidak multiple comparison test was used to examine statistical differences between treatment groups.
Materials
Delta-9-tetrahydrocannabinol was obtained from the National Institute for Drug Abuse. URB597 was purchased from Cayman Chemical Company. Phencyclidine, chloral hydrate, atropine, ketoprofen, pentobarbital sodium, and Dulbecco’s phosphate buffered saline were all purchased from Sigma (USA).
Results
The majority of electrode tracks were identified within the mPFC or vHipp. Figure 2 shows representative electrodes in the mPFC and vHipp from each rat used in the analysis. The mPFC electrode array from one rat was outside of the target area and these data were not included for analysis.
Effects of URB597 and THC on Single Unit Activity
The acute effects of URB597 and THC were studied in 7 saline treated animals and 8 PCP treated animals. Each animal had an array of eight electrodes in the mPFC and another array in the vHipp. The vehicle experiment sample sizes were: saline treated animals (n=6 PFC, n=7 vHipp), PCP-treated animals (n=8 PFC, n=7 vHipp). The URB597 experiment sample sizes were: saline treated animals (n=6 PFC, n=7 vHipp), PCP-treated animals (n=8 PFC, n=8 vHipp). The THC experiment sample sizes were: saline treated animals (n=6 PFC, n=6 vHipp), PCP-treated animals (n=5 PFC, n=5 vHipp).
Medial Prefrontal Cortex
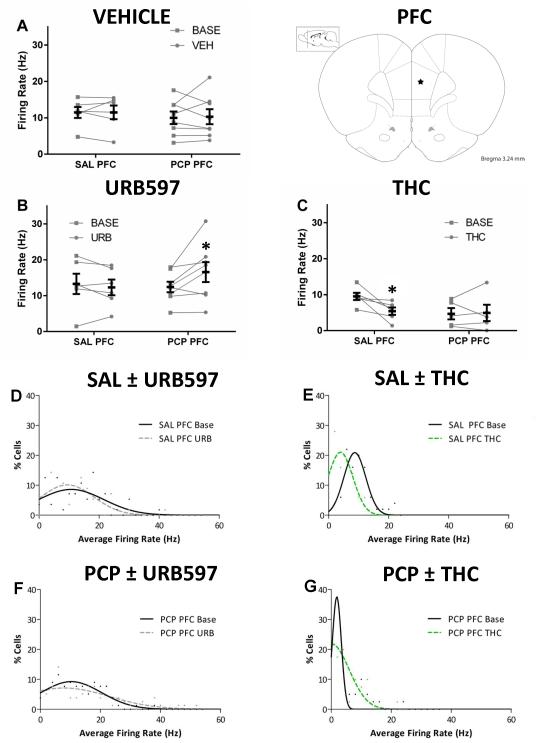
Two-way repeated measures ANOVA were conducted to examine the effects of cannabinoids on mPFC neuronal firing rate in rats treated with subchronic saline or PCP. Rats treated with vehicle were not significantly different from baseline for either saline [Basal 11.5 ± 1.5 Hz –Vehicle 11.5 ± 1.9 Hz; two-way RM ANOVA] or PCP [Basal 10.0 ± 1.7 Hz – Vehicle 10.3 ± 2.1 Hz; two-way RM ANOVA] treated animals (Fig 3A). Endocannabinoid upregulation by URB597 increased mPFC firing rates in PCP [Basal 12.4 ± 1.5 Hz – URB 16.5 ± 2.8 Hz; two-way RM ANOVA; Holm-Sidak; t=2.836; p=0.0298] but not saline [Basal 13.3 ± 2.8 Hz - URB 12.3 ± 2.2 Hz; two-way RM ANOVA; Holm-Sidak; t=0.5782, p=0.57] treated animals (Fig 3B). In contrast, acute THC administration reduced the firing rate of saline treated animals [Basal 9.5 ± 1.0 Hz – THC 5.4 ± 1.0 Hz; two-way RM ANOVA; Holm-Sidak; t=3.068; p=0.0266] without affecting PCP treated animals [Basal 4.7 ± 1.6 Hz – THC 4.9 ± 2.3 Hz; two-way RM ANOVA; Holm-Sidak; t=0.1488; p=0.8850] (Fig 3C).
Figure 3. Single unit activity in the PFC.
Before-after plots of the average mPFC firing rate per animal at baseline and 120 minutes after acute treatment of vehicle (A), URB597 (B), or THC (C). Group means are in foreground (black) while individual animal means are in the background (grey). URB597 increases the average firing rate of mPFC cells in PCP but not saline treated animals (B). THC decreases the average firing rate of mPFC cells in saline but not PCP treated animals (C). Frequency histograms (D-G) were fit by Gaussian models and effects of URB597 or THC can be seen as shifts in the distribution. * represents significant difference from baseline (two-way RM ANOVA p<0.05)
Ventral Hippocampus
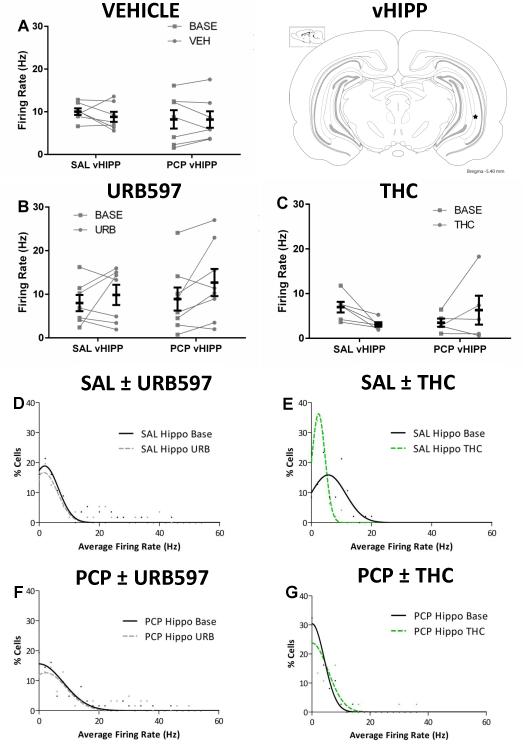
Two-way repeated measures ANOVA were conducted to examine the effects of cannabinoids on vHipp neuronal firing rate in rats treated with subchronic saline or PCP. Rats treated with vehicle were not significantly different than baseline for either saline [Basal 10.0 ± 0.8 Hz – Vehicle 8.8 ± 1.2 Hz; two-way RM ANOVA] or PCP [Basal 8.2 ± 2.1 Hz – Vehicle 8.2 ± 1.9 Hz; two-way RM ANOVA] treated animals (Fig 4A). In contrast to the data obtained in the mPFC, URB597 treatment produced a trend [F(1,13) = 4.307; p=0.0584] for an increase in firing rate in both saline [Basal 8.0 ± 1.9 Hz – URB 9.8 ± 2.3 Hz] and PCP [Basal 8.9 ± 2.6 Hz - URB 12.7 ± 3.1 Hz] treated animals (Fig 4B). THC treatment appeared to produce the opposite response in saline and PCP-treated rats [Fstrain × treatment (1,9) = 6.353; p=0.0327]. However, a Holm-Sidak post hoc test revealed no significant differences in saline [Basal 6.98 ±1.2 Hz – THC 3.1 ± 0.5 Hz; two-way RM ANOVA; Holm-Sidak; t= 2.169; p=0.1130] or PCP [Basal 3.5 ± 0.9 – THC 6.3 ± 3.2 Hz; two-way RM ANOVA; Holm-Sidak; t=1.432; p=0.1858] treated rats (Fig 4C).
Figure 4. Single unit activity in the vHipp.
Before-after plots of the average vHipp firing rate per animal at baseline and 120 minutes after acute treatment of vehicle (A), URB597 (B), or THC (C). Group means are in foreground (black) while individual animal means are in the background (grey). No significant changes in firing rate were evoked by vehicle, URB597, or THC. Nevertheless, URB597 appeared to increase firing rate in both groups (main effect of URB – p<0.06) while THC evokes a significant interaction that suggests a decreased firing rate in saline rats and the opposite response in PCP treated rats. Frequency histograms (D-G) were fit by Gaussian models and effects of URB597 or THC can be seen as shifts in the distribution.
Changes in the distribution of firing frequency induced by acute drug treatment can be visualized with the firing rate histograms which include data from all cells recorded (Figures 3D-G and 4D-G). In addition, single unit changes in neuronal activity are depicted in Supplementary Figures S1 and S2, which permits a more complete visualization of the data presented here.
Effects of URB597 and THC on Coordinated Oscillatory Activity
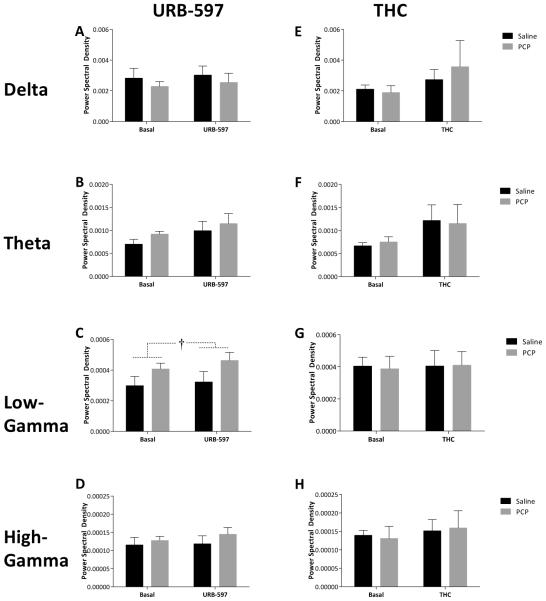
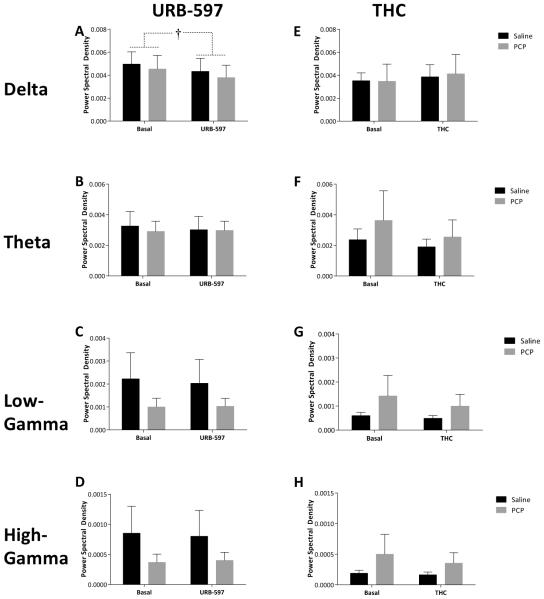
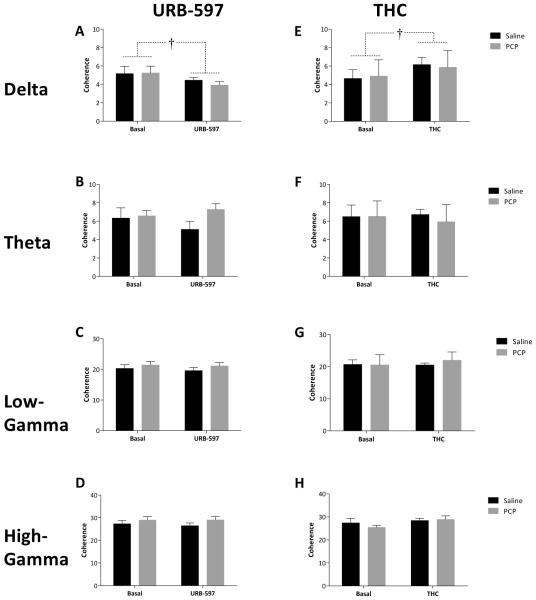
Subchronic PCP treatment had no effect on spontaneous oscillatory activity in either the mPFC or vHipp; however, differences in LFP data were observed following acute URB597 and THC treatment. Specifically, URB597 administration induced a selective increase [F(1,13) = 5.974; p=0.03] in gamma oscillatory activity in the mPFC in both saline [Basal 2.96×10−4 ± 5.59×10−5 dB/Hz – URB 3.23×10−4 ± 5.59×10−5 dB/Hz] and PCP [Basal 4.07×10−4 ± 5.23×10−5 dB/Hz – URB 4.62×10−4 ± 5.23×10−5 dB/Hz] treated rats (Figure 5C). Furthermore, URB597 produced a decrease in vHipp delta band activity [F(1,13) = 10.104; p=0.007] in both saline [Basal 4.96×10−3 ± 1.16×10−3 dB/Hz – URB 4.33×10−3 ± 1.16×10−3 dB/Hz] and PCP [Basal 4.54×10−3 ± 1.09×10−3 dB/Hz – URB 3.79×10−3 ± 1.09×10−3 dB/Hz] treated rats (Figure 6A). Interestingly, THC administration failed to significantly alter oscillatory activity in any group (Figures 5 & 6). Synchrony between the vHipp and mPFC was also measured as the degree of coherence between the LFP’s in these regions (Figure 7). Baseline synchrony between the vHipp and mPFC was unchanged by subchronic PCP treatment; however, URB597 and THC produced opposite effects on low frequency delta synchrony. Specifically, URB597 decreased vHipp-mPFC delta synchrony [F(1,13) = 5.084; p=0.042] in saline [Basal 5.159 ± 0.624 – URB 4.452 ± 0.624] and PCP [Basal 5.246 ± 0.584– URB 3.914 ± 0.584] treated rats while THC increased vHipp-mPFC delta synchrony [F(1,8) = 6.236; p=0.037] in both saline [Basal 4.65 ± 1.134– THC 6.153 ± 1.134] and PCP [Basal 4.9 ± 1.389– THC 5.859 ± 1.389] treated cohorts (Figure 7).
Figure 5. Coordinated activity in the mPFC.
Analysis of power spectral density within the mPFC demonstrates a significant increase in low gamma power (30-55 Hz) in response to URB597 whereas no effects of THC were observed. † depicts main effect of URB597 (two-way RM ANOVA).
Figure 6. Coordinated activity in the vHipp.
Analysis of power spectral density within the vHipp demonstrates a significant decrease in delta rhythms in response to URB597 whereas no effects of THC were observed. † depicts main effect of URB597 (two-way RM ANOVA).
Figure 7. Hippocampal – prefrontal coherence.
Coherence between the vHipp and mPFC is differentially altered by URB597 and THC. Specifically, delta coherence is decreased by URB597 but increased by THC administration. No other effects were observed for either URB597 in THC in any other frequency band. † depicts main effect of drug (two-way RM ANOVA).
Discussion
In this study we report on neurophysiological differences between endogenous (induced by FAAH blockade with URB597) and exogenous (via THC) cannabinoid receptor stimulation in saline and PCP-treated rats. PCP-induced schizophrenia-like symptoms in rats can be rescued by anandamide upregulation [Seillier et al., 2010, Seillier et al., 2013, Aguilar et al., 2015]. However, given the purported differences between the behavioral effects of endogenous and exogenous cannabinoids in human patients [Giuffrida et al., 2004, D'souza et al., 2005, D'souza et al., 2009, Leweke et al., 2012] and rodent models of schizophrenia [Vigano et al., 2009, Seillier et al., 2010], we examined the regulation of neuronal activity in the sub-chronic PCP rat model of schizophrenia. This was recently explored in anesthetized animals [Young et al., 2015], but conscious and freely moving animals are more relevant for studies involving endocannabinoid production, as these lipid messengers are produced on demand.
THC is considered the main psychoactive component in marijuana and is known to induce general decreases in cognitive function [Pope et al., 1996, Morrison et al., 2011, Meijer et al., 2012]. Here we demonstrate that the administration of THC significantly reduced neuronal activity in the mPFC of control rats without significantly affecting mPFC activity in PCP-treated rats. In the vHipp a significant interaction was present such that THC tended to decrease neuron activity in controls and increase neuron activity in PCP-treated rats. Decreases in activity following THC administration are in line with imaging data from clinical observations [Mathew et al., 1999, O'leary et al., 2002, O'leary et al., 2003, Crippa et al., 2004] although these types of studies have yielded inconsistencies ([Mathew et al., 1989, Mathew et al., 1992, Borgwardt et al., 2008, Fusar-Poli et al., 2009], for review see [Martin-Santos et al., 2010]. The results in control animals are also consistent with preclinical data examining the effects of CP55940, a synthetic CB1r agonist that depresses hippocampal and mPFC pyramidal firing rates in vivo [Robbe et al., 2006, Kucewicz et al., 2011]. Interestingly, we observed a dramatically different response in the sub-chronic PCP model. THC increased the activity of a subset of cortical neurons in PCP-treated rats, in contrast to decreases in multiple neurons of saline-treated controls (see Supplementary figures S1 and S2). Offsetting increases and decreases in activity among subsets of neurons likely contributed to this discrepancy. The exact reason for this contrast remains to be elucidated, but is likely associated with the neuropathological alterations (i.e. alterations in dopaminergic, GABAergic or endocannabinoid transmission) induced by the sub-chronic PCP treatment. This altered response to THC may be related to the exacerbation of psychosis that patients with schizophrenia experience when exposed to THC [D'souza et al., 2005]. It is important to note that the baseline firing rate of PCP-treated rats appears to be lower in the THC experiments (Figs 3C, 4C) than in the vehicle (Figs 3A, 4A) and URB597 (Figs 3B, 4B) experiments, so the possibility of a floor effect does exist. However, these baseline data were not significantly different from any other baseline group [two-way RM ANOVA; PCP/saline vs Veh/URB/THC baselines; mPFC data - interaction F(2,18) = 0.3803, p >0.05; vHipp data – interaction F(2,18) = 0.9736, p >0.05].
URB597 is a selective blocker of FAAH and results in region specific increases in AEA and other non-cannabinoid fatty-acid ethanolamides [Desarnaud et al., 1995, Cravatt et al., 2001, Kathuria et al., 2003]. URB597 administration, at a dose previously demonstrated to produce CB1 mediated behavioral effects [Seillier et al., 2010, Seillier et al., 2011, Seillier et al., 2013], failed to alter neuronal activity in either brain region of control rats. We do not believe this to be due to the dose selected, as 0.3mg/kg has been reported to maximally inhibit FAAH enzyme activity in vivo up to six hours following its administration, significantly elevate brain AEA, and induce CB1-dependent behavioral effects in rats [Kathuria et al., 2003]. It is important to note that while THC and AEA induce responses by acting at the same, CB1, receptor, differences in regional specificity of these compounds are likely responsible for the disparate results with these two compounds. Thus, THC activates CB1 receptors throughout the entire neuraxis [Harkany et al., 2003] whereas URB597 only acts in regions with appreciable FAAH activity and will only augment AEA levels in regions where this lipid messenger is being produced on demand de novo (Egertova et al. 1998; Egertova et al. 2003). For this reason, it is not surprising that THC and URB597 produced dramatically different results in control animals and the absence of tonic AEA signaling is one explanation behind the modest effects of URB597. Indeed, previous studies have demonstrated that even at a threefold higher dose URB597 fails to alter hippocampal firing in rats [Coomber et al., 2008]. Another possible explanation arises from the different lipid messengers elevated upon FAAH pharmacological blockade, including molecules that signal at the peroxisome proliferator-activated receptor (PPAR) or transient receptor potential (TRP) families of receptors which might influence neuronal activity [Cravatt et al., 2001, Kathuria et al., 2003]. At high concentrations, AEA can also act as an agonist at the vanilloid receptor 1 (TRPV1) [Zygmunt et al., 1999, Di Marzo et al., 2002] and PPAR receptors [Sun et al., 2007] which further complicates this issue. Although URB597 had no significant effects in control animals, PCP-treated rats receiving URB597 showed a significant increase in mPFC neuronal firing rates. This likely reflects neuropathological alterations induced by the subchronic PCP treatment. Acute PCP treatment can evoke excitation or inhibition in subpopulations of thalamic neurons that project directly to the mPFC [Jodo et al., 2010]. After subchronic PCP treatment these same subpopulations of thalamic neurons might be primed to respond differently to drugs such as cannabinoids, and might have led to the above observation. Taken together, our data indicate differences not only between the actions of exogenous and endogenous cannabinoids, but also dramatically altered responses in PCP-treated vs control rats.
In addition to single-unit activity we also examined the synchronous activity of LFPs to understand neuronal activity from a broader perspective. LFPs were broken down into the following frequency divisions: Delta (0.3-4 Hz), Theta (4-8 Hz), Alpha (8-13 Hz), Beta (13-30 Hz), and Gamma (30-100 Hz) waves. We analyzed delta activity which represents pyramidal cell noise or restful behavior [Anderson et al., 2003, Kiss et al., 2011], theta activity which is present during cognitive tasks [Hyman et al., 2011], and gamma activity which is associated with interneuron activity [Bartos et al., 2002] and working memory tasks [Howard et al., 2003]. We split the gamma activity into low (30-55 Hz) and high (65-99 Hz) before analysis to avoid including electrical noise at 60 Hz. In studies of anesthetized PCP rats, subchronic PCP treatment decreased theta oscillatory power in the mPFC [Young et al., 2015]. In our conscious animals, we observed no differences in baseline oscillatory power at any frequency in saline or PCP treated rats. It is possible that differences in strain (Wistar vs Sprague Dawley), PCP dose (2.0mg/kg vs 5.0mg/kg), or the presence of anesthesia may have contributed to these differences [Young et al., 2015]. In contrast to the divergent results between control and PCP-treated rats observed when examining single unit activity, coordinated activity in the mPFC and vHipp were similarly affected by URB597 administration. Specifically, AEA upregulation by URB597 produced a decrease in hippocampal delta activity and an increase in mPFC gamma in both cohorts. No significant effects on oscillatory activity were observed with 1mg/kg THC which is in contrast to a conscious recording study demonstrating CB1 dependent decreases in hippocampal theta and gamma oscillations following 5mg/kg THC and 0.1mg/kg CP55940 [Robbe et al., 2006]. This discrepancy is likely due to the higher concentration used in previous studies and may be associated with the sedative effects of THC at these high doses.
Using chronically implanted electrodes we found subchronic PCP administration had no effect on baseline single unit or baseline population activity of neurons in the mPFC or vHipp of conscious rats. NMDA receptor antagonists have been consistently reported to increase mPFC activity following acute administration [Suzuki et al., 2002, Jackson et al., 2004]. Subchronic PCP treatment better emulates behavioral symptoms of schizophrenia [Mouri et al., 2007], yet neuronal effects on this time scale are not well known. In agreement with our data, Young et al. [2015] found subchronic PCP treatment did not significantly alter firing rates of single-unit mPFC neurons in anesthetized rats. To our knowledge, this is the first conscious neurophysiological study of the subchronic PCP model. Exposing functional differences between acute and subchronic PCP administration may help future experimental design. Neurophysiological responses to AEA upregulation must be better understood, as FAAH inhibitors are currently being explored as an alternative therapy for patients suffering from schizophrenia [Leweke et al., 2012, Zuardi et al., 2012, Schubart et al., 2014]. These drugs appear to alleviate symptoms of schizophrenia without the side effect profile of antipsychotics [Leweke et al., 2012]. However, conflicting evidence within the clinical literature [Zuardi et al., 2006, Hallak et al., 2011] and details of their mechanism of action must be resolved before moving forward. Local changes in neuronal activity following AEA upregulation cannot be accurately measured in these patients, which emphasize the importance of preclinical studies. Previous preclinical studies in control and PCP-treated rats revealed the degree of AEA upregulation seems to depend on the brain region, pharmacological dose of FAAH inhibitor, and activity the animals are engaged in [Gobbi et al., 2005, Seillier et al., 2010, Seillier et al., 2013, Seillier et al., 2014]. Furthermore, with frequent inconsistencies in human imaging data [Martin-Santos et al., 2010] more invasive and controlled approaches in animal models may finally resolve what these local and global changes may be.
In conclusion, we demonstrate qualitative and quantitative differences between AEA upregulation and exogenous THC administration. Moreover, we demonstrate that the PCP model of schizophrenia displays altered responses to cannabinoids at the single unit levels, while the coordinated activity of the neuronal population within and between regions appear unchanged. These differences provide information relevant to the dichotomy between the effects of THC and endocannabinoid upregulation observed clinically.
Supplementary Material
Supplementary Figure 1. Normalized Single unit activity in the PFC. A) Z score plot depicting normalized firing rate of each individual neuron across time. Bright red colors represent increases while dark blue colors represent decreases in firing rate normalized to a cell’s own baseline firing rate. Recordings spaced 30, 60, 90, and 120 minutes after URB597 administration (x axis) were measured from multiple cells (y axis). URB597 has no net effect on neuronal activity (A), while THC decreases firing rate in saline rats (B). The opposite response is observed in PCP treated rats, i.e. an increased firing of a subset of neurons in response to URB597 (C) and THC (D).
Supplementary Figure 2. Normalized Single unit activity in the vHipp A) Z score plot depicting normalized firing rate of each individual neuron across time. Bright red colors represent increases while dark blue colors represent decreases in firing rate normalized to a cell’s own baseline firing rate. Recordings spaced 30, 60, 90, and 120 minutes after URB597 administration (x axis) were measured from multiple cells (y axis). URB597 has no net effect on neuronal activity (A), while THC appears to decrease firing rate in saline rats (B). The opposite response is observed in PCP treated rats, i.e. an increased firing of a subset of neurons in response to URB597 (C) and THC (D).
Acknowledgements
The authors thank Dr. Alex Seillier for his helpful discussions. This work was supported by the NIH (R01: MH091130) and (R25: GM095480).
Funding: This work was supported by the NIH (R01: MH091130) and (R25: GM095480).
Footnotes
Drs. Lodge and Giuffrida and David Aguilar have no conflicts of interest to declare.
References
- Aguilar DD, Chen L, Lodge DJ. Increasing Endocannabinoid Levels in the Ventral Pallidum Restores Aberrant Dopamine Neuron Activity in the Subchronic Pcp Rodent Model of Schizophrenia. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C, Horne JA. Prefrontal Cortex: Links between Low Frequency Delta Eeg in Sleep and Neuropsychological Performance in Healthy, Older People. Psychophysiology. 2003;40:349–357. doi: 10.1111/1469-8986.00038. [DOI] [PubMed] [Google Scholar]
- Association., A.P. Diagnostic and Statistical Manual of Mental Disorders. Fourth Text Revision (Dsm-Iv-Tr); Washington, DC: 2000. [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, et al. Fast Synaptic Inhibition Promotes Synchronized Gamma Oscillations in Hippocampal Interneuron Networks. Proc Natl Acad Sci U S A. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural Basis of Delta-9-Tetrahydrocannabinol and Cannabidiol: Effects During Response Inhibition. Biol Psychiatry. 2008;64:966–973. doi: 10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic Delta9-Tetrahydrocannabinol Treatment Produces a Time-Dependent Loss of Cannabinoid Receptors and Cannabinoid Receptor-Activated G Proteins in Rat Brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Selley DE, Childers SR. Cannabinoid Receptor Agonist Efficacy for Stimulating [35s]Gtpgammas Binding to Rat Cerebellar Membranes Correlates with Agonist-Induced Decreases in Gdp Affinity. J Biol Chem. 1998;273:16865–16873. doi: 10.1074/jbc.273.27.16865. [DOI] [PubMed] [Google Scholar]
- Coomber B, O'donoghue MF, Mason R. Inhibition of Endocannabinoid Metabolism Attenuates Enhanced Hippocampal Neuronal Activity Induced by Kainic Acid. Synapse. 2008;62:746–755. doi: 10.1002/syn.20547. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to Anandamide and Enhanced Endogenous Cannabinoid Signaling in Mice Lacking Fatty Acid Amide Hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, et al. Effects of Cannabidiol (Cbd) on Regional Cerebral Blood Flow. Neuropsychopharmacology. 2004;29:417–426. doi: 10.1038/sj.npp.1300340. [DOI] [PubMed] [Google Scholar]
- De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid Signalling in the Blood of Patients with Schizophrenia. Lipids Health Dis. 2003;2:5. doi: 10.1186/1476-511X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Tennant C, Gilmour S, Schofield D, Nash L, Hall W, et al. The Temporal Dynamics of Relationships between Cannabis, Psychosis and Depression among Young Adults with Psychotic Disorders: Findings from a 10-Month Prospective Study. Psychol Med. 2007;37:927–934. doi: 10.1017/S0033291707009956. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Cadas H, Piomelli D. Anandamide Amidohydrolase Activity in Rat Brain Microsomes. Identification and Partial Characterization. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Fezza F, Ligresti A, Bisogno T. Anandamide Receptors. Prostaglandins Leukot Essent Fatty Acids. 2002;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- Dissanayake DW, Zachariou M, Marsden CA, Mason R. Auditory Gating in Rat Hippocampus and Medial Prefrontal Cortex: Effect of the Cannabinoid Agonist Win55,212-2. Neuropharmacology. 2008;55:1397–1404. doi: 10.1016/j.neuropharm.2008.08.039. [DOI] [PubMed] [Google Scholar]
- D'souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-Tetrahydrocannabinol Effects in Schizophrenia: Implications for Cognition, Psychosis, and Addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- D'souza DC, Sewell RA, Ranganathan M. Cannabis and Psychosis/Schizophrenia: Human Studies. Eur Arch Psychiatry Clin Neurosci. 2009;259:413–431. doi: 10.1007/s00406-009-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and CB1 cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 2003;119:481–496. doi: 10.1016/s0306-4522(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Egertova M, Giang DK, Cravatt BF. A new perspective in cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc R Soc Lond. 1998;265:2081–2085. doi: 10.1098/rspb.1998.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einevoll GT, Kayser C, Logothetis NK, Panzeri S. Modelling and Analysis of Local Field Potentials for Studying the Function of Cortical Circuits. Nat Rev Neurosci. 2013;14:770–785. doi: 10.1038/nrn3599. [DOI] [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, et al. Faah−/− Mice Display Differential Tolerance, Dependence, and Cannabinoid Receptor Adaptation after Delta 9-Tetrahydrocannabinol and Anandamide Administration. Neuropsychopharmacology. 2010;35:1775–1787. doi: 10.1038/npp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RS, Meyer JS, Quenzer LF. Principles of Neuropsychopharmacology. Sinauer Associates; Sunderland, Mass: 1997. [Google Scholar]
- Ferrer B, Gorriti MA, Palomino A, Gornemann I, De Diego Y, Bermudez-Silva FJ, et al. Cannabinoid Cb1 Receptor Antagonism Markedly Increases Dopamine Receptor-Mediated Stereotypies. Eur J Pharmacol. 2007;559:180–183. doi: 10.1016/j.ejphar.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, et al. Distinct Effects of {Delta}9-Tetrahydrocannabinol and Cannabidiol on Neural Activation During Emotional Processing. Arch Gen Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, et al. Cerebrospinal Anandamide Levels Are Elevated in Acute Schizophrenia and Are Inversely Correlated with Psychotic Symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-Like Activity and Modulation of Brain Monoaminergic Transmission by Blockade of Anandamide Hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Kocsis B. Activation of Cannabinoid-1 Receptors Disrupts Sensory Gating and Neuronal Oscillation: Relevance to Schizophrenia. Biol Psychiatry. 2008;63:1075–1083. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Hallak JE, Dursun SM, Bosi DC, De Macedo LR, Machado-De-Sousa JP, Abrao J, et al. The Interplay of Cannabinoid and Nmda Glutamate Receptor Systems in Humans: Preliminary Evidence of Interactive Effects of Cannabidiol and Ketamine in Healthy Human Subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:198–202. doi: 10.1016/j.pnpbp.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Harkany T, Hartig W, Berghuis P, Dobszay MB, Zilberter Y, Edwards RH, et al. Complementary Distribution of Type 1 Cannabinoid Receptors and Vesicular Glutamate Transporter 3 in Basal Forebrain Suggests Input-Specific Retrograde Signalling by Cholinergic Neurons. Eur J Neurosci. 2003;18:1979–1992. doi: 10.1046/j.1460-9568.2003.02898.x. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma Oscillations Correlate with Working Memory Load in Humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Hasselmo ME, Seamans JK. What Is the Functional Relevance of Prefrontal Cortex Entrainment to Hippocampal Theta Rhythms? Front Neurosci. 2011;5:24. doi: 10.3389/fnins.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. Nmda Receptor Hypofunction Produces Concomitant Firing Rate Potentiation and Burst Activity Reduction in the Prefrontal Cortex. Proc Natl Acad Sci U S A. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Katayama T, Okamoto M, Suzuki Y, Hoshino K, Kayama Y. Differences in Responsiveness of Mediodorsal Thalamic and Medial Prefrontal Cortical Neurons to Social Interaction and Systemically Administered Phencyclidine in Rats. Neuroscience. 2010;170:1153–1164. doi: 10.1016/j.neuroscience.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of Anxiety through Blockade of Anandamide Hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kiss T, Hoffmann WE, Hajos M. Delta Oscillation and Short-Term Plasticity in the Rat Medial Prefrontal Cortex: Modelling Nmda Hypofunction of Schizophrenia. Int J Neuropsychopharmacol. 2011;14:29–42. doi: 10.1017/S1461145710000271. [DOI] [PubMed] [Google Scholar]
- Kucewicz MT, Tricklebank MD, Bogacz R, Jones MW. Dysfunctional Prefrontal Cortical Network Activity and Interactions Following Cannabinoid Receptor Activation. J Neurosci. 2011;31:15560–15568. doi: 10.1523/JNEUROSCI.2970-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated Endogenous Cannabinoids in Schizophrenia. Neuroreport. 1999;10:1665–1669. doi: 10.1097/00001756-199906030-00008. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol Enhances Anandamide Signaling and Alleviates Psychotic Symptoms of Schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, Mcevoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of Antipsychotic Drugs in Patients with Chronic Schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Linszen DH, Dingemans PM, Lenior ME. Cannabis Abuse and the Course of Recent-Onset Schizophrenic Disorders. Arch Gen Psychiatry. 1994;51:273–279. doi: 10.1001/archpsyc.1994.03950040017002. [DOI] [PubMed] [Google Scholar]
- Madsen MV, Peacock L, Werge T, Andersen MB. Effects of the Cannabinoid Cb1 Receptor Agonist Cp55,940 and Antagonist Sr141716a on D-Amphetamine-Induced Behaviours in Cebus Monkeys. J Psychopharmacol. 2006;20:622–628. doi: 10.1177/0269881106063816. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Rovetti CC, Winston EN, Lowe JA., 3rd Effects of the Cannabinoid Cb1 Receptor Antagonist Sr141716a on the Behavior of Pigeons and Rats. Psychopharmacology (Berl) 1996;124:315–322. doi: 10.1007/BF02247436. [DOI] [PubMed] [Google Scholar]
- Martin RS, Secchi RL, Sung E, Lemaire M, Bonhaus DW, Hedley LR, et al. Effects of Cannabinoid Receptor Ligands on Psychosis-Relevant Behavior Models in the Rat. Psychopharmacology (Berl) 2003;165:128–135. doi: 10.1007/s00213-002-1240-x. [DOI] [PubMed] [Google Scholar]
- Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, et al. Neuroimaging in Cannabis Use: A Systematic Review of the Literature. Psychol Med. 2010;40:383–398. doi: 10.1017/S0033291709990729. [DOI] [PubMed] [Google Scholar]
- Mason O, Morgan CJ, Dhiman SK, Patel A, Parti N, Patel A, et al. Acute Cannabis Use Causes Increased Psychotomimetic Experiences in Individuals Prone to Psychosis. Psychol Med. 2009;39:951–956. doi: 10.1017/S0033291708004741. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Tant SR. Acute Changes in Cerebral Blood Flow Associated with Marijuana Smoking. Acta Psychiatr Scand. 1989;79:118–128. doi: 10.1111/j.1600-0447.1989.tb08579.x. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Chiu NY, Turkington TG, Degrado TR, Coleman RE. Regional Cerebral Blood Flow and Depersonalization after Tetrahydrocannabinol Administration. Acta Psychiatr Scand. 1999;100:67–75. doi: 10.1111/j.1600-0447.1999.tb10916.x. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Humphreys DF, Lowe JV, Wiethe KE. Regional Cerebral Blood Flow after Marijuana Smoking. J Cereb Blood Flow Metab. 1992;12:750–758. doi: 10.1038/jcbfm.1992.106. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Dekker N, Koeter MW, Quee PJ, Van Beveren NJ, Meijer CJ, et al. Cannabis and Cognitive Performance in Psychosis: A Cross-Sectional Study in Patients with Non-Affective Psychotic Illness and Their Unaffected Siblings. Psychol Med. 2012;42:705–716. doi: 10.1017/S0033291711001656. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Arvanitis L, Bauer D, Rein W, Meta-Trial Study G. Placebo-Controlled Evaluation of Four Novel Compounds for the Treatment of Schizophrenia and Schizoaffective Disorder. Am J Psychiatry. 2004;161:975–984. doi: 10.1176/appi.ajp.161.6.975. [DOI] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis Use and Risk of Psychotic or Affective Mental Health Outcomes: A Systematic Review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Morrison PD, Nottage J, Stone JM, Bhattacharyya S, Tunstall N, Brenneisen R, et al. Disruption of Frontal Theta Coherence by Delta9-Tetrahydrocannabinol Is Associated with Positive Psychotic Symptoms. Neuropsychopharmacology. 2011;36:827–836. doi: 10.1038/npp.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T. Phencyclidine Animal Models of Schizophrenia: Approaches from Abnormality of Glutamatergic Neurotransmission and Neurodevelopment. Neurochem Int. 2007;51:173–184. doi: 10.1016/j.neuint.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Negrete JC, Knapp WP, Douglas DE, Smith WB. Cannabis Affects the Severity of Schizophrenic Symptoms: Results of a Clinical Survey. Psychol Med. 1986;16:515–520. doi: 10.1017/s0033291700010278. [DOI] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, Mclean SL, et al. Animal Models of Cognitive Dysfunction and Negative Symptoms of Schizophrenia: Focus on Nmda Receptor Antagonism. Pharmacol Ther. 2010;128:419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- O'leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, et al. Effects of Smoking Marijuana on Brain Perfusion and Cognition. Neuropsychopharmacology. 2002;26:802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- O'leary DS, Block RI, Turner BM, Koeppel J, Magnotta VA, Ponto LB, et al. Marijuana Alters the Human Cerebellar Clock. Neuroreport. 2003;14:1145–1151. doi: 10.1097/00001756-200306110-00009. [DOI] [PubMed] [Google Scholar]
- Parolaro D, Realini N, Vigano D, Guidali C, Rubino T. The Endocannabinoid System and Psychiatric Disorders. Exp Neurol. 2010;224:3–14. doi: 10.1016/j.expneurol.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press Australia. , Google Scholar; Sydney, Australia: 1986. [Google Scholar]
- Pesaran B. Uncovering the Mysterious Origins of Local Field Potentials. Neuron. 2009;61:1–2. doi: 10.1016/j.neuron.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The Molecular Logic of Endocannabinoid Signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Yurgelun-Todd D. The Residual Cognitive Effects of Heavy Marijuana Use in College Students. JAMA. 1996;275:521–527. [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, Mcnaughton BL, Buzsaki G. Cannabinoids Reveal Importance of Spike Timing Coordination in Hippocampal Function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Roser P, Vollenweider FX, Kawohl W. Potential Antipsychotic Properties of Central Cannabinoid (Cb1) Receptor Antagonists. World J Biol Psychiatry. 2010;11:208–219. doi: 10.3109/15622970801908047. [DOI] [PubMed] [Google Scholar]
- Schubart CD, Sommer IE, Fusar-Poli P, De Witte L, Kahn RS, Boks MP. Cannabidiol as a Potential Treatment for Psychosis. Eur Neuropsychopharmacol. 2014;24:51–64. doi: 10.1016/j.euroneuro.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Seillier A, Giuffrida A. Inhibition of Fatty Acid Amide Hydrolase Modulates Anxiety-Like Behavior in Pcp-Treated Rats. Pharmacol Biochem Behav. 2011;98:583–586. doi: 10.1016/j.pbb.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Seillier A, Advani T, Cassano T, Hensler JG, Giuffrida A. Inhibition of Fatty-Acid Amide Hydrolase and Cb1 Receptor Antagonism Differentially Affect Behavioural Responses in Normal and Pcp-Treated Rats. Int J Neuropsychopharmacol. 2010;13:373–386. doi: 10.1017/S146114570999023X. [DOI] [PubMed] [Google Scholar]
- Seillier A, Dominguez Aguilar D, Giuffrida A. The Dual Faah/Magl Inhibitor Jzl195 Has Enhanced Effects on Endocannabinoid Transmission and Motor Behavior in Rats as Compared to Those of the Magl Inhibitor Jzl184. Pharmacol Biochem Behav. 2014;124:153–159. doi: 10.1016/j.pbb.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillier A, Martinez AA, Giuffrida A. Phencyclidine-Induced Social Withdrawal Results from Deficient Stimulation of Cannabinoid Cb(1) Receptors: Implications for Schizophrenia. Neuropsychopharmacology. 2013;38:1816–1824. doi: 10.1038/npp.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano MS, Fadda P, Frau R, Fattore L, Fratta W. Cannabinoid Self-Administration Attenuates Pcp-Induced Schizophrenia-Like Symptoms in Adult Rats. Eur Neuropsychopharmacol. 2010;20:25–36. doi: 10.1016/j.euroneuro.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Spano MS, Fattore L, Cadeddu F, Fratta W, Fadda P. Chronic Cannabinoid Exposure Reduces Phencyclidine-Induced Schizophrenia-Like Positive Symptoms in Adult Rats. Psychopharmacology (Berl) 2013;225:531–542. doi: 10.1007/s00213-012-2839-1. [DOI] [PubMed] [Google Scholar]
- Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, et al. Cannabinoid Activation of Ppar Alpha; a Novel Neuroprotective Mechanism. Br J Pharmacol. 2007;152:734–743. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Jodo E, Takeuchi S, Niwa S, Kayama Y. Acute Administration of Phencyclidine Induces Tonic Activation of Medial Prefrontal Cortex Neurons in Freely Moving Rats. Neuroscience. 2002;114:769–779. doi: 10.1016/s0306-4522(02)00298-1. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, et al. The Cb1 Receptor Antagonist Sr141716a Selectively Increases Monoaminergic Neurotransmission in the Medial Prefrontal Cortex: Implications for Therapeutic Actions. Br J Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano D, Guidali C, Petrosino S, Realini N, Rubino T, Di Marzo V, et al. Involvement of the Endocannabinoid System in Phencyclidine-Induced Cognitive Deficits Modelling Schizophrenia. Int J Neuropsychopharmacol. 2009;12:599–614. doi: 10.1017/S1461145708009371. [DOI] [PubMed] [Google Scholar]
- Young AM, Stubbendorff C, Valencia M, Gerdjikov TV. Disruption of Medial Prefrontal Synchrony in the Subchronic Phencyclidine Model of Schizophrenia in Rats. Neuroscience. 2015;287:157–163. doi: 10.1016/j.neuroscience.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, et al. The Impact of Cannabis Use on Cognitive Functioning in Patients with Schizophrenia: A Meta-Analysis of Existing Findings and New Data in a First-Episode Sample. Schizophr Bull. 2012;38:316–330. doi: 10.1093/schbul/sbq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JA, Hallak JE, Bhattacharyya S, Atakan Z, Martin-Santos R, et al. A Critical Review of the Antipsychotic Effects of Cannabidiol: 30 Years of a Translational Investigation. Curr Pharm Des. 2012;18:5131–5140. doi: 10.2174/138161212802884681. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Hallak JE, Dursun SM, Morais SL, Sanches RF, Musty RE, et al. Cannabidiol Monotherapy for Treatment-Resistant Schizophrenia. J Psychopharmacol. 2006;20:683–686. doi: 10.1177/0269881106060967. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid Receptors on Sensory Nerves Mediate the Vasodilator Action of Anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Normalized Single unit activity in the PFC. A) Z score plot depicting normalized firing rate of each individual neuron across time. Bright red colors represent increases while dark blue colors represent decreases in firing rate normalized to a cell’s own baseline firing rate. Recordings spaced 30, 60, 90, and 120 minutes after URB597 administration (x axis) were measured from multiple cells (y axis). URB597 has no net effect on neuronal activity (A), while THC decreases firing rate in saline rats (B). The opposite response is observed in PCP treated rats, i.e. an increased firing of a subset of neurons in response to URB597 (C) and THC (D).
Supplementary Figure 2. Normalized Single unit activity in the vHipp A) Z score plot depicting normalized firing rate of each individual neuron across time. Bright red colors represent increases while dark blue colors represent decreases in firing rate normalized to a cell’s own baseline firing rate. Recordings spaced 30, 60, 90, and 120 minutes after URB597 administration (x axis) were measured from multiple cells (y axis). URB597 has no net effect on neuronal activity (A), while THC appears to decrease firing rate in saline rats (B). The opposite response is observed in PCP treated rats, i.e. an increased firing of a subset of neurons in response to URB597 (C) and THC (D).