Abstract
How stones are retained within the kidney while small in size is still not fully understood. In this paper we show two examples of how stones are retained during early growth: One is growth on Randall’s (interstitial) plaque, and the other is growth on mineral that has formed as a luminal plug in a terminal collecting duct. These two mechanisms of stone retention during early growth have distinctive morphologic features that can be seen by methods that show the microscopic structure of the stones. Stones growing on Randall’s plaque display an apatite region that is typically not large in size (less than 0.5 mm across) but which usually shows luminal spaces, which are signs of its origin in the connective tissue of the papilla. Stones growing on ductal plugs also show attachment to a piece of apatite, but the apatite regions are typically larger (often >1 mm long and >0.5 mm wide), and they are solid, without spaces running through them. We propose that knowing the mechanisms of stone retention during early stone formation could allow for better treatment of stone diseases.
Keywords: nephrolithiasis, imaging
Introduction
The great mystery about kidney stones is not that they form; precipitation of mineral from urine is to be expected, since urine is supersaturated for multiple minerals in most people [1], and many people will occasionally have crystalluria [2] reflecting this supersaturation of mineral. The mystery is that stones are retained in the kidney while small, so that they are able to grow to a size to cause symptoms.[3] Normal kidneys are able to excrete particles that are up to a few mm in size, so how are stones retained within the kidney when they are small?
The answer to this question is turning out to be complex. Specifically, even for stones composed of calcium oxalate (CaOx, the most common mineral in stones), there is not just one mechanism by which these stones are retained within the kidney during early growth. Moreover, there are some patients for whom the best-understood mechanisms of stone retention seem not to apply.
In this paper we show examples of two forms of CaOx stone growth—on Randall’s (interstitial) plaque or on ductal plugs. For both of these mechanisms the combination of endoscopic study of the renal papilla and the subsequent scrutiny of the microstructure of the stones has given a clear picture of how the stones are retained during their early growth. We will also show a case in which neither of these mechanisms seems to account for the retention of nascent CaOx stones.
The intent of this paper is to emphasize the concept that not all stones begin in the same way. It is likely that different mechanisms of stone initiation indicate different pathologies underlying stone formation, and thus also different paths for optimal treatment of the underlying disease. There is much to learn about these different mechanisms, but until we can separate stone formers by these underlying pathologies, it will be difficult to study treatment efficacy in appropriate ways.
Methods
Our group has used a standard protocol for the study of stone patients that involves high-resolution endoscopic imaging of as many papillae as can be accessed, combined with the collection of stones one-by-one for subsequent analysis.[4–11] Briefly, patients who are receiving minimally invasive treatment for renal stones (either percutaneous nephrolithotomy, PNL, or ureteroscopy, URS) are consented for study. (All procedures performed in studies involving human participants are in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.) During the procedure, each accessible calyx is mapped as to its location within the kidney (by fluoroscopy) and the papilla is examined with a digital endoscope. Stones that are present are identified as being either free or attached to tissue, and then removed by basket (the stones being kept in an unbroken state whenever possible).
Stones are photographed, allowed to dry, and then scanned using micro-computed tomographic (micro CT) imaging. Typically, a stone is scanned on a Skyscan 1172 micro CT system, at 60 kVp, 0.5 mm Al filter, for a final voxel size of 2–5 µm.[12,13] Following examination of the micro CT reconstructions, selected portions of stone are dissected out and analyzed using conventional Fourier-transform infrared spectroscopic analysis (FT-IR) for confirmation of mineral content.[12]
Results from the study of stones is then placed in context with the observations recorded during the surgery and additional study of videos from the endoscopy. This combination of microscopic study of the stone specimens and a careful recording of the site of origin of each stone allows inference of stone origins.
Results
Stones that grow on Randall’s plaque
A significant number of stone formers make stones on Randall's plaque, which is a calcification within the interstitial tissue of the renal papilla [14]. In this form of early stone retention and growth, the initial lesion is entirely interstitial, and the calcification is not exposed to the urine at all. It appears that the papillary epithelium that covers the Randall's plaque is somehow lost, and that with this event, mineral is able to deposit onto Randall's plaque from the urine. As long as the Randall's plaque remains a part of the papilla, the stone will remain attached, and thus can grow over time [3]. The Randall's plaque can be released from the papilla spontaneously, and the stone passed while small, but the stone still retains the signs of having grown on Randall's plaque [15].
How many stone formers make stones in this way? Recent work by Letavernier et al. [16] reports data on over 10,000 such stones, each of which showed morphologic signs of having formed on Randall's plaque. Specifically, each stone was identified as showing residue of Randall's plaque, typically as part of a concave region of the stone, suggesting that the stone had formed up against the surface of the papilla. Overall, out of over 30,000 CaOx stones analyzed over two decades by that group, 34% appeared to have formed on Randall's plaque. Thus it would seem that the formation of stones on Randall's plaque is very common, indeed.
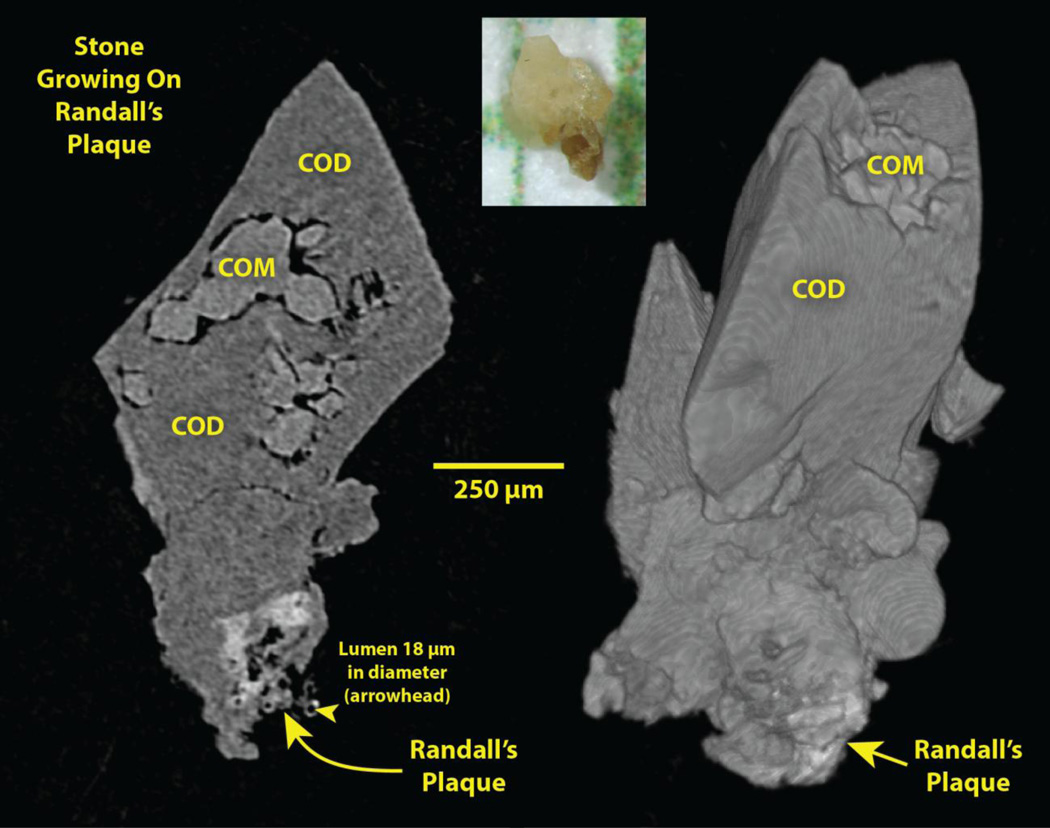
An example of stones from such a stone former is shown in Figure 1. Several features of the morphology of these stones points to their having been formed on Randall’s (interstitial) plaque: 1) The stone possesses a concave surface, as would happen if it formed against the curved surface of the renal papilla. 2) Within this concave surface of the stone is a region of apatite (whiter than calcium oxalate by micro CT [17]). 3) This region of apatite displays a characteristic diffuse x-ray density that diminishes as it extends away from the stone. 4) Most of these apatite regions show luminal spaces that can be followed through the three-dimensional image stack, consistent with the apatite region having come from the papillary interstitium, with the lumens of tubules and vessels persisting through the calcified tissue. 5) Finally, many of these apatite regions display at their distal periphery (farthest from the stone) what appear to be calcified tubules, similar to what was described by Cifuentes so many years ago [15]. These peripheral, calcified tubular shapes typically have an inner diameter of about 30 µm, suggesting that they may be calcified basement membrane of thin limbs of Henle’s loop, a location that appears to be the initial site of formation of Randall’s plaque [14].
Figure 1.
Stone growing on Randall’s plaque, plucked from the papilla of a patient during ureteroscopic removal of renal stones. Photo, top, on mm paper. Left: Micro CT slice of stone. Right: Surface rendering of micro CT image stack. COM: calcium oxalate monohydrate. COD: calcium oxalate dihydrate.
The sizes of apparent Randall’s plaque found attached to stones averages <500 µm wide (across the face of the stone) and ~300 µm deep (from the stone out to the tip of the apatite region). The vast majority of the these apatite regions showed cylindrical spaces curving through the mineral, spaces of appropriate diameter to be lumens of tubules or vessels. When patients have stones growing on Randall’s plaque, they usually show multiple stones such as these. In a recent series of 25 calcium oxalate stone formers with Randall’s plaque stones, with an average of 16 stones removed per patient, over half of the stones removed were apparently grown on Randall’s plaque. Indeed, some of the stones that were removed and did not show signs of plaque might have also grown on Randall’s plaque and the signs of the plaque lost (for example, by breakage during removal) but over half the stones were classed as having grown on plaque by the criteria described above.
We note that the morphology we describe here is not different from what has been used by others to identify stones that grew on Randall’s plaque. For example, Prien described such stones in great detail in the middle of the 20th century [18] and publically challenged the stone research community to seek to understand this kind of stone formation better [19]. Micro CT is allowing us to describe these stones with even more confidence, and we continue with the goal of understanding how this kind of stone forms.
Stones that grow on ductal plugs
A number of types of stone disease show plugging of medullary collecting ducts with mineral [20]. Such plugging of tubular lumens is not seen in the patient making stones solely by the mechanism of growth on Randall’s plaque [10], but it does appear in some calcium oxalate stone formers [21].
Because ductal plugs of mineral can extend from the collecting duct lumen out into the calyceal urine, they form a potential site for stone growth that would keep the stone anchored to the renal papilla while it is small. In our experience, ductal plugs growing nascent stones are composed of apatite, and are much larger than the size of residual Randall’s plaque, averaging ~500 µm in width and >1000 µm in length. These apatite plugs never show the luminal spaces characteristic of Randall’s plaque, and their size and shape is consistent with their having formed in the lumens of dilated collecting ducts.
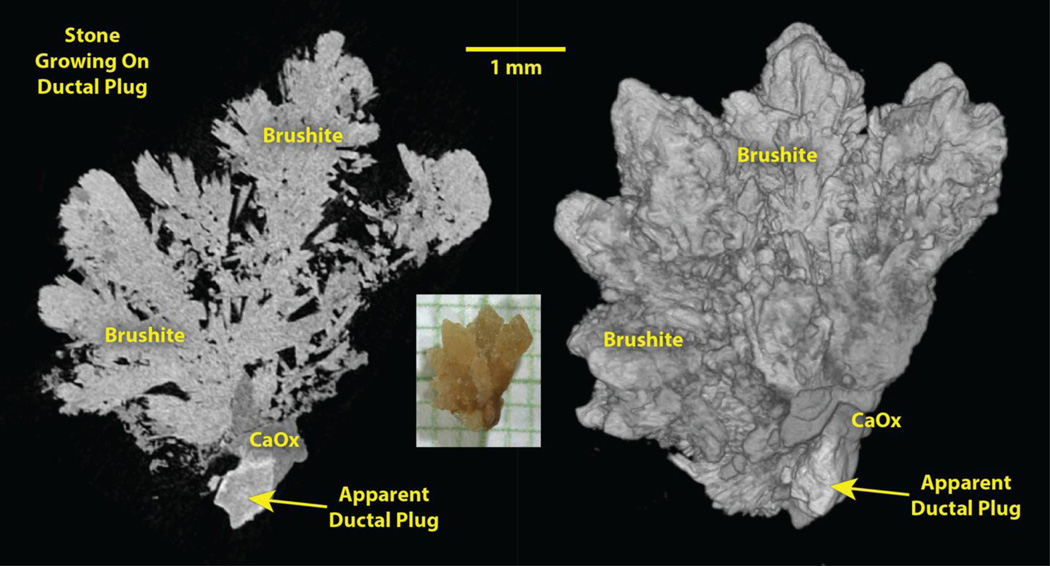
We have seen some examples of this kind of nascent stone growth, such as the one shown in Figure 2. This stone was plucked from the tip of a papilla in a brushite stone former, but the mineral found closest to the apparent plug was calcium oxalate. and the structure of the stone shows that it was growing on apatite, which presumably was a plug within a terminal collecting duct.
Figure 2.
Stone growing on apparent ductal plug, plucked from the papilla of a patient during ureteroscopic removal of renal stones. Photo, center, on mm paper. Left: Micro CT slice of stone. Right: Surface rendering of micro CT image stack. CaOx: calcium oxalate.
There may be several reasons why we have seen fewer of this type of nascent stone than we have seen stones growing on Randall’s plaque. First, it is our experience that stones growing on ductal plugs are much more easily dislodged from the papilla than stones growing on Randall’s plaque. Often the removal of a stone on Randall’s plaque requires a firm pull to rip the plaque out of the papillary connective tissue, while ductal plugs are more likely to slip out rather easily. This means that it is more likely for nascent stones on ductal plugs to be dislodged by irrigant flow or guide wires, and so these stones often will not be observed as being attached to the renal papilla when collected.
Second, stones growing on ductal plugs have been seen most often in patients making brushite stones, a kind that tend to grow rapidly [10,22], and aggressive apatite stone formers (such as those with hyperparathyroidism [7]). Thus in this type of active stone former there will tend to be large stones in the kidneys that will require fragmentation and removal before the papillae can be examined for nascent stones. This process of stone removal may also result in many nascent stones being dislodged from the papillae so that they never observed in their state of early growth.
Finally, we note that it is possible to infer the growth of a stone from Randall’s plaque by observing by micro CT an apatite region inside a calcium oxalate stone [23]. That is, it is possible for the evidence of past Randall’s plaque connection be observed inside a stone that has been detached form the papilla and grown to a larger size. However, in a stone composed largely of brushite or apatite, distinguishing internal apatite that once was a ductal plug is difficult to do [24], as it will not stand out as easily as does Randall’s plaque in a calcium oxalate stone.
Nevertheless, given the large number of kinds of stone formers with significant ductal plugging [20], it seems probable that the retention of nascent stones by their growing on ductal plugs is a likely mechanism by which early stones form. Documentation of such stone formation in majority CaOx stone formers has not yet been published, but such a mechanism of nascent CaOx stone formation is likely, as shown by the early stages of the stone in Figure 2.
Renal stones growing without an obvious microstructural mechanism for retention in the kidney
This paper would not be complete without recognizing that there are stone formers whose stones show no obvious signs of how they were retained within the kidney when small. An example of this is shown in Figure 3.
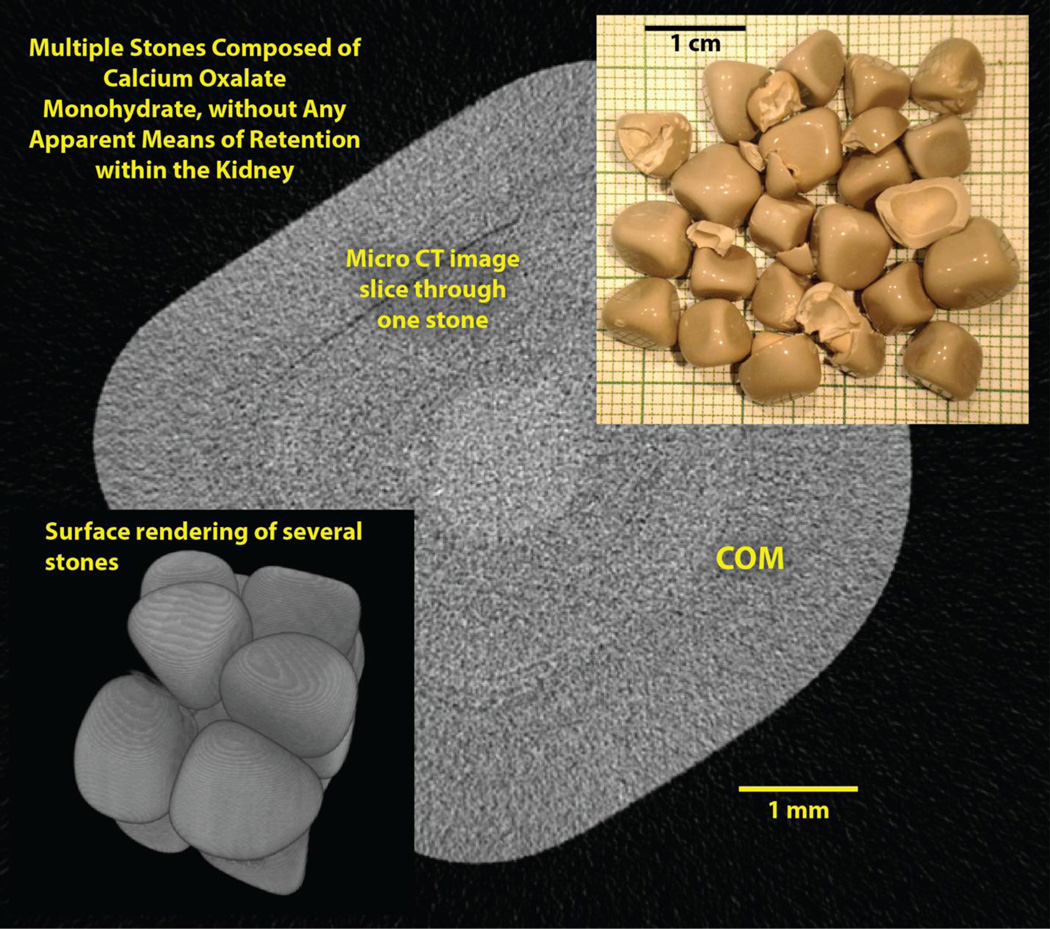
Figure 3.
Stones from a percutaneous procedure that show no sign of any known mechanism of retention during early growth. Background shows micro CT slice through one of the stones; the lighter region in the center of the stone was COM by FT-IR, so the stones appear to be pure COM.
The patient whose stones are shown in Figure 3 underwent percutaneous nephrolithotomy for multiple stones in the right renal pelvis. About 20 of the stones were removed intact, and each of them was remarkably pure by micro CT, and by FT-IR shown to be composed solely of calcium oxalate monohydrate (COM). All of the stone fragments removed had the same, pure appearance by micro CT as did the intact stones. Thus the evidence from the stones in this patient showed no signs of growth of nascent stones on either Randall’s plaque or ductal plug. These COM stones looked like marbles, but with flattened sides.
The papillae in this kidney were unremarkable. Most of them were quite normal, with very little Randall’s plaque. A few of the papillae showed what appeared to be dilated ducts, and what was described as a small amount of pitting, but there were none of the mineral inclusions that would indicate plugging of the collecting ducts.
How does one explain the growth of so many stones in a kidney, with no obvious mechanism of early stone retention? Daudon has reported that these smooth COM stones (called by him type Id) are rather rare, occurring in only 1% of cases, and are associated with hyperoxaluria, a long history of stone disease, and/or anatomical factors that restrict stone passage [25]. The patient whose stones are shown in Fig. 3 had repeated lithotripsy for a large stone 10 years prior to the recent procedure, but no history of passing stones. During the recent percutaneous procedure, we noted that the ureteral-pelvic junction (UPJ) was located unusually high on the renal pelvis, such that a wire could not be passed into the ureter during the process of obtaining access to the kidney. By non-contrast CT, the other kidney was completely free of stones. This patient had perfectly normal urine, with 24-hour collection (collected off-medication, 9 weeks after PNL) showing a volume of 3.0 L, pH 6.1, Ca 180 mg, oxalate 34 mg, citrate >1200 mg, and a supersaturation of CaOx of only 2.50.
Thus the most likely explanation for the formation of the stones shown in Figure 3 is an anatomical variation that prevented small stones from being expelled from the kidney, namely the ureter joining with the renal pelvis in a more cranial location than is typical. We make this conclusion because of the following factors: The patient had no systemic pathology to give rise to stones, as is evidenced by the absence of stones in the other kidney. The amount of Randall’s plaque in the stone-forming kidney was minimal, as is seen even in non-stone forming persons [4,21], and the stones did not show the microstructural characteristics seen in those that grew in Randall’s plaque. The stone-forming kidney showed no signs of mineral plugs within the collecting ducts, and the stones showed no signs of any apatite, which would be expected if they had grown, even at an early stage, on ductal plugs. Thus, with no other mechanisms to explain the stones, we are left with the possibility that the unusual anatomy of the UPJ allowed small particles of COM to collect in the lower part of the kidney, and that these grew over time into the substantial stones shown in Figure 3.
Discussion
The thrust of this paper is that it is possible to identify the mechanisms of early retention for some kinds of kidney stones. In the long run, we anticipate that classifying patients by the mechanisms of early stone retention will provide rational bases for distinctive treatment of these different, underlying mechanisms. However, in the meantime it is difficult to assign patients to different mechanistic groups using the concepts put forth in this paper. (Few patients will have their stones analyzed using micro CT, at least at the present time.)
A possible clinical correlate with stone retention mechanism is the visual appearance of the papillae, as viewed during endoscopy. It seems reasonable that a patient with a great deal of Randall’s plaque on endoscopy will also be a patient who makes stones by init iation of stones on that interstitial plaque. On the other hand, a patient whose papillae show a great deal of ductal plugging is more likely to initiate stones on those. Patients who have neither of these papillary characteristics presumably with have other pathologies that allow small stones to be retained in the kidney.
Efforts to quantify the endoscopic appearance of Randall’s plaque include the recent effort to develop a simple scoring system that could be used by the surgeon to identify kidneys with certain pathologies [26]. In this scoring system, papillae are identified as having Randall’s plaque, ductal plugging, pitting, or loss of papillary contour, each on a zero, one-plus, two-plus, kind of scale. The scoring scale was developed with the intent that it could be used by a surgeon to provide a qualitative indication of the type of papillary pathology in a patient. It is hoped that future studies will be able to show whether such a simple scoring system will be valuable for classifying patients into clinically rational groups.
As it stands at the present time, physicians must treat their patients for stones using only data concerning the type of stone formed, and the composition of 24-hour urine specimens. If, as seems to be the case, the mechanisms of stone initiation are diverse among CaOx stones, persons with that common stone type will be treated without any information about the mechanisms that are at work to form their stones. Thus it is possible that common treatments for CaOx stones may be inappropriate for some of those stone formers, but at the present time it is not possible to study patients by any such grouping. If the endoscopic appearance of renal papillae turns out to be able to separate CaOx stone formers into groups based on the apparent mechanisms of early stone retention, the possibility exists for improvement in therapies for this common type of kidney stone. Such is the direction of research that we see as important in the coming years.
In conclusion, study of the microstructure of kidney stones has shown that some CaOx stones initiate on Randall’s plaques, and their growth on this interstitial plaque holds them within the renal urinary space while they are small. Other stones seem to form on plugs of mineral extending from the mouth of collecting ducts, and we propose that this is another—and very different—mechanism for retention of stones in the kidney during early growth. Finally, other CaOx stones form without microstructural evidence of either of these two known mechanisms of retention in the kidney. We suggest urinary stasis within a collecting system with an anomalous anatomy is a reasonable explanation for some of these CaOx stones, but more study is required to elucidate the veracity of such alternative explanations. In the end, however, we think that treatment of patients will be improved by being able to identify the underlying causes for their kidney stones.
Acknowledgments
Funded by NIH P01 DK56788
Footnotes
Conflict of interest: none
References
- 1.Robertson WG, Peacock M, Nordin BE. Activity products in stone-forming and non-stone-forming urine. Clinical Science. 1968;34:579–594. [PubMed] [Google Scholar]
- 2.Daudon M, Jungers P. Clinical value of crystalluria and quantitative morphoconstitutional analysis of urinary calculi. Nephron Physiol. 2004;98(2):31–36. doi: 10.1159/000080261. [DOI] [PubMed] [Google Scholar]
- 3.Williams JC, Jr, McAteer JA. Retention and growth of urinary stones—Insights from imaging. J Nephrol. 2013;26(1):25–31. doi: 10.5301/jn.5000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111(5):607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evan AP, Lingeman JE, Coe FL, Shao YZ, Parks JH, Bledsoe SB, Worcester EM, Sommer AJ, Paterson RF, Kuo RL, Grynpas M. Apatite deposits in collecting duct lumens produce epithelial cell injury and interstitial inflammation in patients forming brushite renal stones. J Urol. 2004;171(4 suppl. S):299. (abstract) [Google Scholar]
- 6.Evan AP, Coe FL, Lingeman JE, Shao Y, Matlaga BR, Kim SC, Bledsoe SB, Sommer AJ, Grynpas M, Phillips CL, Worcester EM. Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int. 2006;69(12):2227–2235. doi: 10.1038/sj.ki.5000268. [DOI] [PubMed] [Google Scholar]
- 7.Evan AP, Lingeman JE, Coe FL, Miller NL, Bledsoe SB, Sommer AJ, Williams JC, Jr, Shao Y, Worcester EM. Histopathology and surgical anatomy of patients with primary hyperparathyroidism and calcium phosphate stones. Kidney Int. 2008;74:223–229. doi: 10.1038/ki.2008.161. [DOI] [PubMed] [Google Scholar]
- 8.Evan AP, Lingeman JE, Coe FL, Bledsoe SB, Sommer AJ, Williams JC, Jr, Krambeck AE, Worcester EM. Intra-tubular deposits, urine and stone composition are divergent in patients with ileostomy. Kidney Int. 2009;76:1081–1088. doi: 10.1038/ki.2009.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evan AP, Lingeman JE, Worcester EM, Bledsoe SB, Sommer AJ, Williams JC, Jr, Krambeck AE, Philips CL, Coe FL. Renal histopathology and crystal deposits in patients with small bowel resection and calcium oxalate stone disease. Kidney Int. 2010;78(3):310–317. doi: 10.1038/ki.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evan AP, Lingeman JE, Worcester EM, Sommer AJ, Phillips CL, Williams JC, Jr, Coe FL. Contrasting histopathology and crystal deposits in kidneys of idiopathic stone formers who produce hydroxyapatite, brushite, or calcium oxalate stones. Anat Rec. 2014;297(4):731–748. doi: 10.1002/ar.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evan AP, Worcester EM, Williams JC, Sommer AJ, Lingeman JE, Phillips CL, Coe FL. Biopsy Proven Medullary Sponge Kidney: Clinical Findings, Histopathology, and Role of Osteogenesis in Stone and Plaque Formation. Anat Rec. 2015;298(5):865–877. doi: 10.1002/ar.23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JC, Jr, McAteer JA, Evan AP, Lingeman JE. Micro-computed tomography for analysis of urinary calculi. Urol Res. 2010;38:477–484. doi: 10.1007/s00240-010-0326-x. [DOI] [PubMed] [Google Scholar]
- 13.Williams JC, Jr, Lingeman JE, Coe FL, Worcester EM, Evan AP. Micro-CT imaging of Randall’s plaques. Urolithiasis. 2015;43(suppl 1):13–17. doi: 10.1007/s00240-014-0702-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evan AP, Lingeman J, Coe FL, Worcester E. Randall’s plaque: Pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006;69:1313–1318. doi: 10.1038/sj.ki.5000238. [DOI] [PubMed] [Google Scholar]
- 15.Cifuentes Delatte L, Miñón-Cifuentes JL, Medina JA. Papillary stones: calcified renal tubules in Randall's plaques. J Urol. 1985;133(3):490–494. doi: 10.1016/s0022-5347(17)49039-2. doi: 3974005. [DOI] [PubMed] [Google Scholar]
- 16.Letavernier E, Vandermeersch S, Traxer O, Tligui M, Baud L, Ronco P, Haymann JP, Daudon M. Demographics and characterization of 10,282 Randall plaque-related kidney stones: a new epidemic? Medicine (Baltimore) 2015;94(10):e566. doi: 10.1097/MD.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarse CA, McAteer JA, Sommer AJ, Kim SC, Hatt EK, Lingeman JE, Evan AP, Williams JC., Jr Nondestructive analysis of urinary calculi using micro computed tomography. BMC Urology. 2004;4:15. doi: 10.1186/1471-2490-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prien EL. Studies in urolithiasis: II. Relationships between pathogenesis, structure and composition of calculi. J Urol. 1949;61(5):821–836. doi: 10.1016/S0022-5347(17)69150-X. [DOI] [PubMed] [Google Scholar]
- 19.Prien EL. The riddle of Randall's plaques. J Urol. 1975;114(4):500–507. doi: 10.1016/s0022-5347(17)67068-x. [DOI] [PubMed] [Google Scholar]
- 20.Coe FL, Evan AP, Lingeman JE, Worcester EM. Plaque and deposits in nine human stone diseases. Urol Res. 2010;38(4):239–247. doi: 10.1007/s00240-010-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linnes MP, Krambeck AE, Cornell L, Williams JC, Korinek M, Bergstralh EJ, Li X, Rule AD, McCollough CM, Vrtiska TJ, Lieske JC. Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int. 2013;84(4):818–825. doi: 10.1038/ki.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krambeck AE, Handa SE, Evan AP, Lingeman JE. Profile of the brushite stone former. J Urol. 2010;184(4):1367–1371. doi: 10.1016/j.juro.2010.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller NL, Williams JC, Evan AP, Bledsoe SB, Coe FL, Worcester EM, Munch LC, Handa SE, Lingeman JE. In idiopathic calcium oxalate stone-formers, unattached stones show evidence of having originated as attached stones on Randall's plaque. BJU International. 2010;105(2):242–245. doi: 10.1111/j.1464-410X.2009.08637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evan AP, Worcester EM, Coe FL, Williams J, Jr, Lingeman JE. Mechanisms of human kidney stone formation. Urolithiasis. 2015;43(Suppl 1):19–32. doi: 10.1007/s00240-014-0701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daudon M. Analyse et classification des calculs: contribution à l'etiologie de la maladie lithiasique. Revue medicale de la Suisse romande. 2004;124(8):445–453. [PubMed] [Google Scholar]
- 26.Borofsky MS, Paonessa JE, Evan AP, Williams JC, Jr, Coe FL, Worcester EM, Lingeman JE. A Proposed Grading System to Standardize the Description of Renal Papillary Appearance at the Time of Endoscopy in Patients with Nephrolithiasis. J Endourol. 2016;30(1):122–127. doi: 10.1089/end.2015.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]