Abstract
Retinitis pigmentosa (RP) is an inherited photoreceptor-degenerative disease, and neuronal degeneration in RP is exacerbated by glial activation. Cassia seed (Jue-ming-zi) is a traditional herbal medicine commonly used to treat ocular diseases in Asia. In this report, we investigated the retina-protective effect of chrysophanol, an active component of Cassia seed, in an N-methyl-N-nitrosourea (MNU)-induced mouse model of RP. We determined that chrysophanol inhibited the functional and morphological features of MNU-induced retinal degeneration using scotopic electroretinography (ERG), optical coherence tomography (OCT), and immunohistochemistry analysis of R/G opsin and rhodopsin. Furthermore, TUNEL assays revealed that chrysophanol attenuated MNU-induced photoreceptor cell apoptosis and inhibited the expression of the apoptosis-associated proteins PARP, Bax, and caspase-3. In addition, chrysophanol ameliorated reactive gliosis, as demonstrated by a decrease in GFAP immunolabeling, and suppressed the activation of matrix metalloproteinase (MMP)-9-mediated gelatinolysis. In vitro studies indicated that chrysophanol inhibited lipopolysaccharide (LPS)-induced iNOS and COX-2 expression in the BV2 mouse microglia cell line and inhibited MMP-9 activation in primary microglia. Our results demonstrate that chrysophanol provided neuroprotective effects and inhibited glial activation, suggesting that chrysophanol might have therapeutic value for the treatment of human RP and other retinopathies.
Retinitis pigmentosa (RP) refers to a heterogeneous group of inherited retinal degenerative diseases that are characterized by progressive photoreceptor cell death. Genetic aberrations are directly implicated in the apoptosis of rod photoreceptors and the development of night blindness. Neurotrophic factor depletion and increases in oxidative stress following massive rod cell death can also induce apoptosis in cone photoreceptors, thereby promoting the loss of bright and color vision. Modes of glial activation, including microglia recruitment and reactive gliosis, are also involved in the pathogenesis of RP in humans and in mouse models1,2,3. A recent study demonstrated that microglia infiltrate the outer nuclear layer during early stages of retinal degeneration3. In addition to removing debris and apoptotic neurons, dysfunctional microglia execute neuronal cell death via a process of phagoptosis4,5. RP commonly results in vision loss, especially in patients who develop RP in adolescence or infancy. Therefore, visual acuity is disrupted during the early stages of RP, resulting in significantly higher healthcare costs and disability-adjusted life years for these patients6. Although RP affects millions of individuals worldwide, there are no therapies currently approved for the treatment of this disease.
Cassia seed (Jue-ming-zi, Cassia toro) is a traditional herbal medicine widely used in Asia to treat eye complications7,8. Previous studies have demonstrated that Cassia seed provides clinical benefits to patients with ophthalmic conditions, such as glaucoma and cataracts9,10. Chrysophanol, an active anthraquinone isolated from Cassia seed, exhibits various pharmacological effects, including anti-oxidative11, anti-hyperlipidemia12, and anti-inflammatory effects13,14. However, few studies have evaluated the protective effects of chrysophanol in the context of ocular diseases. In the present study, we investigated the retina-protective effects of chrysophanol in an N-methyl-N-nitrosourea (MNU)-induced mouse model of RP in vivo15. Similar to the mechanism of cell death in human RP, MNU selectively induces cell death in photoreceptor cells via apoptosis16. Therefore, the MNU-induced mouse model of RP is a reliable and appropriate approach to investigate the neuroprotective effects and mechanisms mediated by bioactive compounds, such as chrysophanol, in RP.
Chrysophanol prevented the functional and morphological features of MNU-induced photoreceptor apoptosis and retinal degeneration, and ameliorated glial activation. These findings suggest that the primary active component of Cassia seed, chrysophanol, has the potential to inhibit RP progression and prevent retinal injury. However, the mechanisms underlying this phenomenon, as well as the toxicity and long-term effects of chrysophanol, require further investigation.
Results
N-methyl-N-nitrosourea (MNU)-induced photoreceptor cell loss and retinal dysfunction in a dose-dependent manner
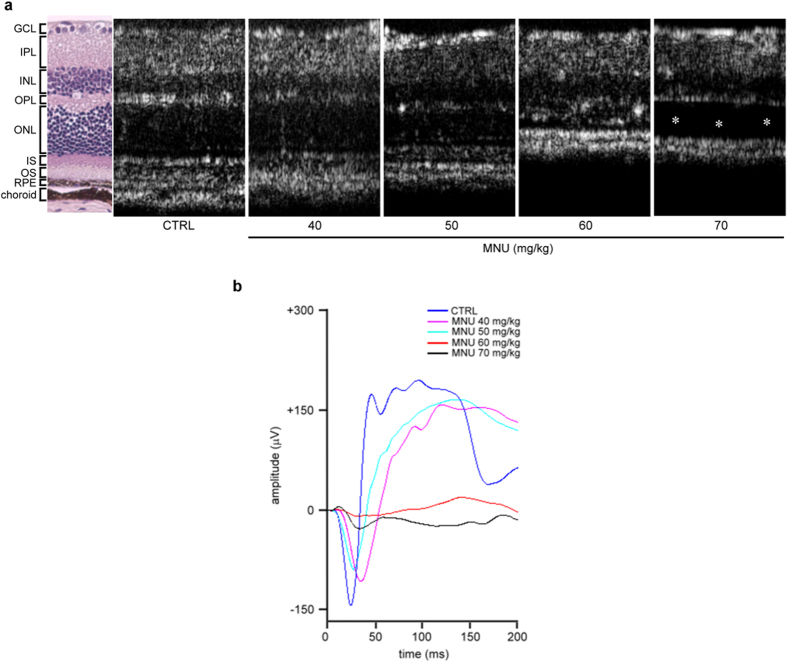
As shown in Fig. 1a, OCT images revealed that MNU-induced degeneration of the outer nuclear layer (ONL) in the retinas of C57BL/6 mice in a dose-dependent manner 7 days after it was administered. At day 7, photoreceptor degeneration resulting from photoreceptor cell loss was clearly observed in the MNU-treated group (60 mg/kg), and the total retinal thickness was significantly reduced. In addition, scotopic electroretinogram (ERG) responses of the a-wave and b-wave amplitude were strongly reduced in the MNU-treated group (60 mg/kg) compared to control mice (Fig. 1b). Consistently, delayed implicit time (time to peak) of a-wave and b-wave was found in MNU-treated group. These findings suggested that a significant loss in retinal function had occurred. Retinal detachment was observed in the 70 mg/kg MNU-treated group compared with the 60 mg/kg MNU-treated group. Therefore, MNU was used at a dose of 60 mg/kg in subsequent experiments.
Figure 1. Morphological and functional defects in mouse retina 7 days after treatment with various doses of MNU.
(a) OCT images revealed retinal ONL degeneration 7 days after the mice were treated with various doses of MNU. Retinal detachment (asterisk) was observed in the group treated with MNU (70 mg/kg). (b) Scotopic electroretinogram (ERG) responses of a- and b-waves elicited by light at an intensity of 19.1 cd · s/m2 were recorded at the same time point. CTRL: control; GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; OPL: outer plexiform layer; ONL: outer nuclear layer; IS: inner segment; OS: outer segment; RPE: retinal pigment epithelium.
Chrysophanol treatment restored retinal function of elctroretinogram (ERG) in MNU-exposed photoreceptors
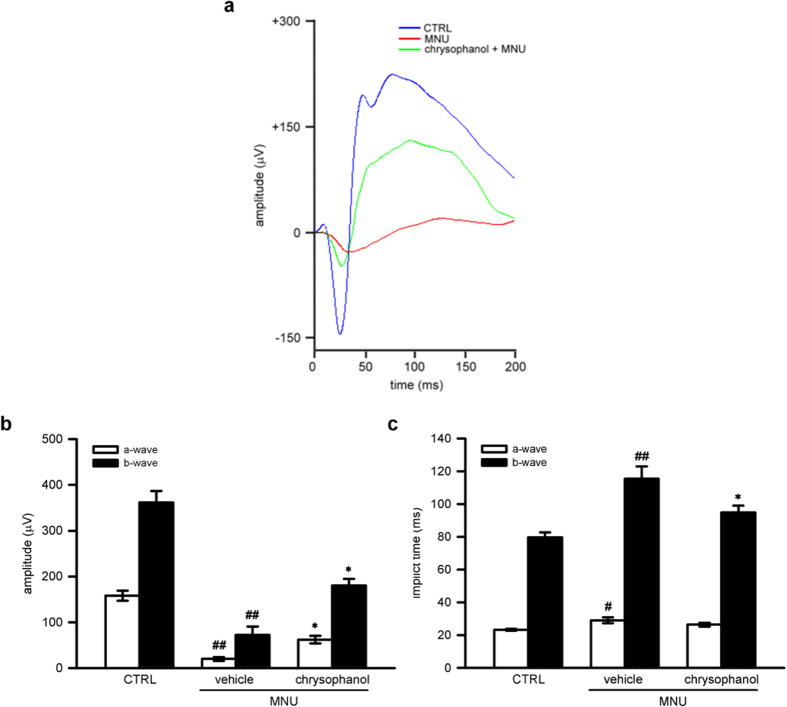
To assess whether chrysophanol treatment can restore photoreceptor cell function, we measured scotopic responses of electroretinogram (ERG) in MNU-exposed retinas. A standard scotopic ERG is composed of an a-wave and a b-wave17. The a-wave reflects the integrity of the combined rod and cone response, and the b-wave reflects the response of ON bipolar cells and the function of Muller cells18. On day 7, the a-wave amplitude and b-wave amplitude were strongly reduced in MNU-exposed retinas (60 mg/kg; a-wave, 20.0 ± 4.0 μV; b-wave, 72.3 ± 18.3 μV) compared with the control group (a-wave, 158.2 ± 11.2 μV; b-wave, 361.3 ± 25.5 μV). Chrysophanol (50 mg/kg) treatment rescued MNU-induced retinal dysfunction and increased ERG responses (a-wave, 62.0 ± 8.2 μV; b-wave, 180.1 ± 14.7 μV) (Fig. 2a,b). Moreover, delayed implicit time was found in MNU-exposed group (a-wave, 29.0 ± 1.8 milliseconds (ms); b-wave, 115.5 ± 7.5 ms) compared with the control group (a-wave, 23.3 ± 0.6 ms; b-wave, 79.7 ± 3.0 ms). Chrysophanol treatment protected against MNU-induced retinal dysfunction and improved ERG responses (a-wave, 26.4 ± 1.2 ms; b-wave, 94.9 ± 4.2 ms) (Fig. 2a,c).
Figure 2. Chrysophanol preserved MNU-injured photoreceptor function on day 7.
(a) Representative scotopic ERG responses from control mice (blue curve), and MNU-exposed mice treated with the vehicle control (red curve) or chrysophanol (green curve). (b,c) Quantification of the average amplitudes and implicit time (time to peak) from control mice (n = 7), and MNU-exposed mice treated with the vehicle control (n = 5) or chrysophanol (n = 11). The ERG a-waves were negative, and the a-wave amplitudes are presented as the absolute value. The data were presented as the mean ± SEM. CTRL: control. #p < 0.05 compared with the control group treated with vehicle, ##p < 0.001 compared with the control group treated with vehicle, *p < 0.01 compared with MNU-exposed group treated with vehicle.
Chrysophanol inhibited MNU-induced photoreceptor degeneration
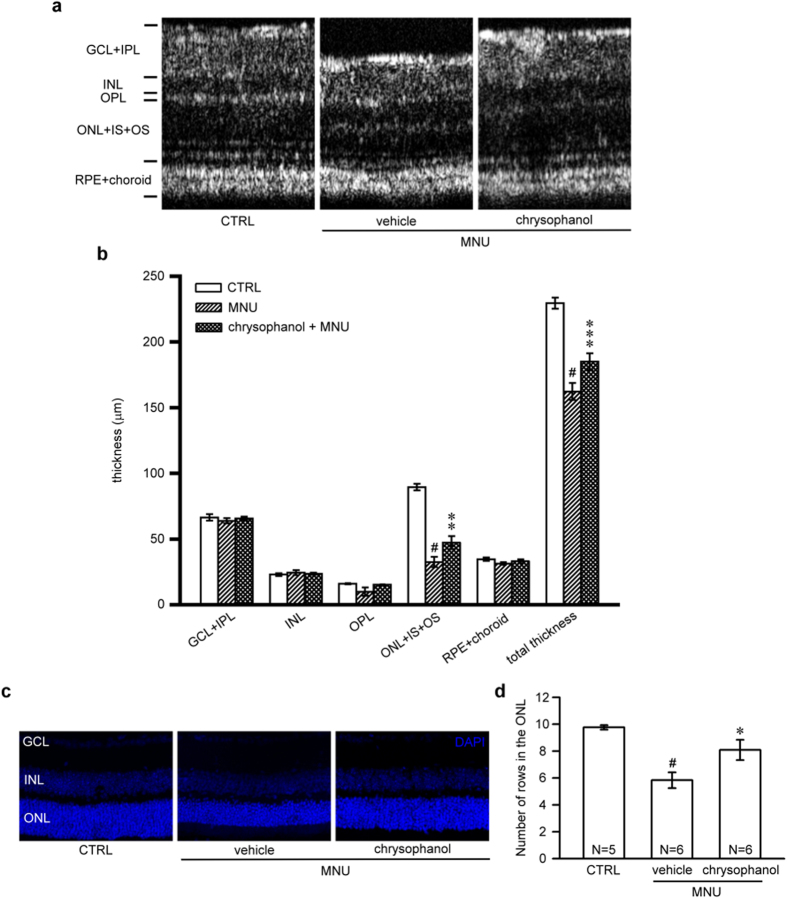
Representative OCT images of control retinas and MNU-exposed retinas treated with chrysophanol (50 mg/kg) or the vehicle control were shown in Fig. 3a. OCT images revealed that the cumulative thickness of the ONL, the inner segments (IS), and the outer segments (OS) layer (32.6 ± 3.9 μm) was significantly reduced 7 days after the administration of MNU compared with the control retinas (89.5 ± 2.5 μm). This effect was prevented by chrysophanol treatment (47.3 ± 5.0 μm). Consistent with these findings, the total thickness of MNU-exposed retinas (162.3 ± 6.5 μm) significantly decreased 7 days after the administration of MNU compared with the control group (229.5 ± 4.2 μm). This effect was significantly protected in retinas treated with chrysophanol (184.9 ± 5.4 μm) (Fig. 3a,b). DAPI staining demonstrated that the normal morphology of control retinas was characterized by three layers: the ganglion cell layer (GCL), the inner nuclear layer (INL), and the ONL (Fig. 3c). Compared with the control group, the number of rows of photoreceptor nuclei in the ONL was significantly reduced in MNU-exposed retinas (5.8 ± 0.6) compared to control group (9.8 ± 0.2), and this reduction was markedly prevented by chrysophanol treatment (8.1 ± 0.8; Fig. 3c,d). DAPI staining of photoreceptor nuclei demonstrated similar results acquired from OCT.
Figure 3. Chrysophanol attenuated MNU-induced photoreceptor degeneration on day 7.
(a) OCT was used to evaluate changes in retinal thickness in control mice (n = 7) and in mice treated with the vehicle control (n = 6) or chrysophanol (n = 8) 7 days after the administration of MNU (60 mg/kg). (b) Quantification of the thickness of the separated layer using InSight XL software. (c) The arrangement of nuclei in the GCL, INL and ONL in DAPI-counterstained retina sections. (d) Quantification of the number of photoreceptor nuclei rows in the ONL. The data are presented as the mean ± SEM. CTRL: control; N: number of animals in each group; GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; OPL: outer plexiform layer; ONL: outer nuclear layer; IS: inner segment; OS: outer segment; RPE: retinal pigmented epithelium. #p < 0.001 compared with the control group treated with vehicle, *p < 0.05 compared with the MNU-exposed group treated with vehicle, **p < 0.01 compared with the MNU-exposed group treated with vehicle, ***p < 0.001 compared with the MNU-treated group treated with vehicle.
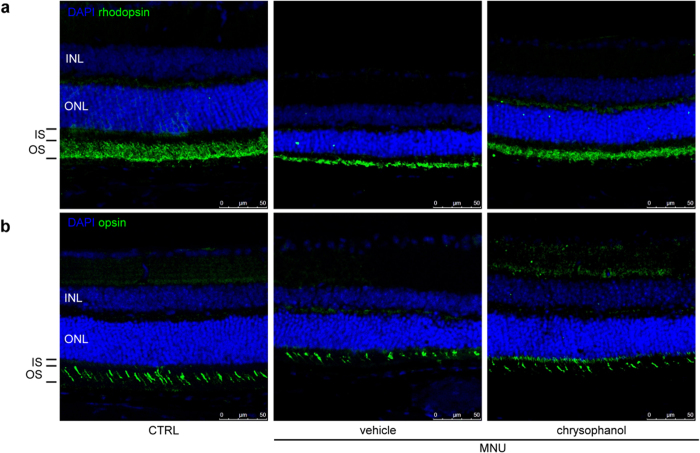
To further evaluate the protective effect of chrysophanol on photoreceptor cell loss, we stained vertical retinal sections with antibodies against rhodopsin and red/green opsin to selectively label rod and cone photoreceptors, respectively19. The rod OS in MNU-treated retinas exhibited an abnormal, truncated pattern compared with control retinas (Fig. 4a), and chrysophanol treatment rescued the defects in OS organization and length. In addition to the protection of rods, we observed that chrysophanol inhibited MNU-induced damage to cone photoreceptors (Fig. 4b). Opsin immunostaining revealed that the amount and morphology of the MNU-exposed cone OS was disrupted compared with the well-arranged pattern observed in the control group, and this defect was rescued by chrysophanol treatment. Therefore, chrysophanol protected both rod and cone cells from MNU-induced morphological changes.
Figure 4. Chrysophanol rescued MNU-induced cone and rod degeneration on day 7.
(a) The rod photoreceptors (labeled by rhodopsin in green) primarily localized to the OS. (b) The cone photoreceptors (labeled by red/green opsin in green) primarily localized to the OS. CTRL: control; INL: inner nuclear layer; ONL: outer nuclear layer; IS: inner segment; OS: outer segment.
Chrysophanol inhibited MNU-induced photoreceptor cell apoptosis and the expression of apoptosis-associated proteins
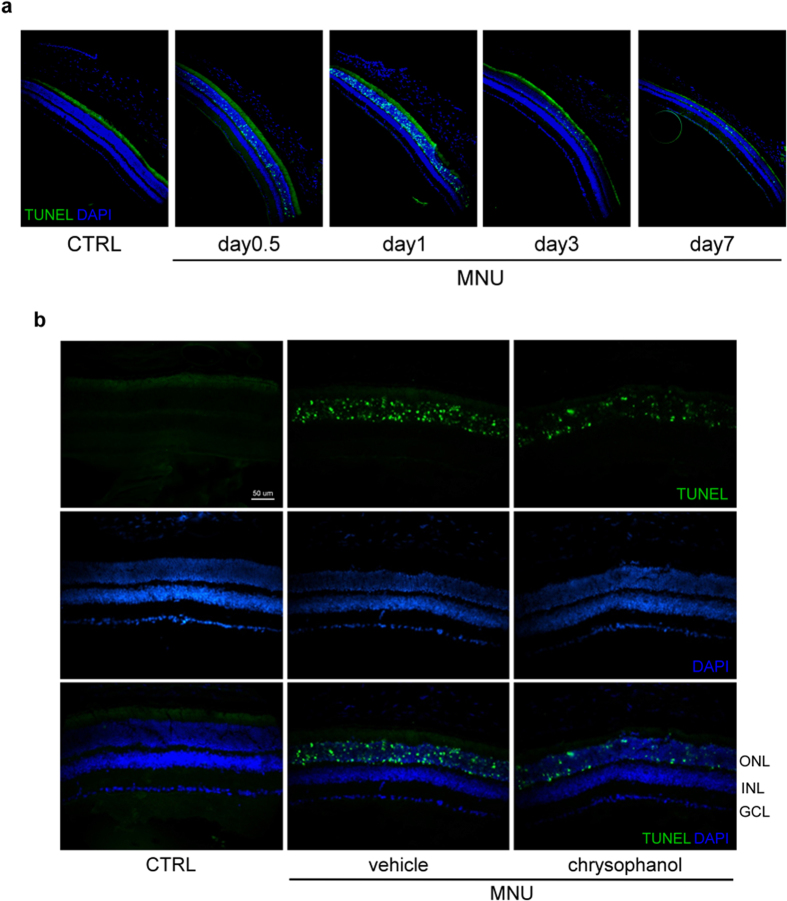
The signals of TUNEL were found in the ONL after 12 hours exposure of MNU (60 mg/kg). Peak levels of apoptosis were observed on day 1, and the number of apoptotic cells gradually decreased on days 3 and 7 (Fig. 5a). Chrysophanol treatment significantly reduced the number of TUNEL-labeled cells in the ONL (Fig. 5b) 1 day after MNU-exposure. These results suggested that chrysophanol treatment blocked early photoreceptor cell apoptosis in MNU-exposed retinas.
Figure 5. Chrysophanol significantly reduced MNU-induced photoreceptor cell death on day 1.
(a) The time course of retinal cell death after MNU (60 mg/kg) administration as determined by TUNEL-labeling (green dot). The number of apoptotic cells peaked 1 day after the administration of MNU. (b) TUNEL-stained sections revealing dead photoreceptors in DAPI-counterstained retinal sections. CTRL: control; GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer.
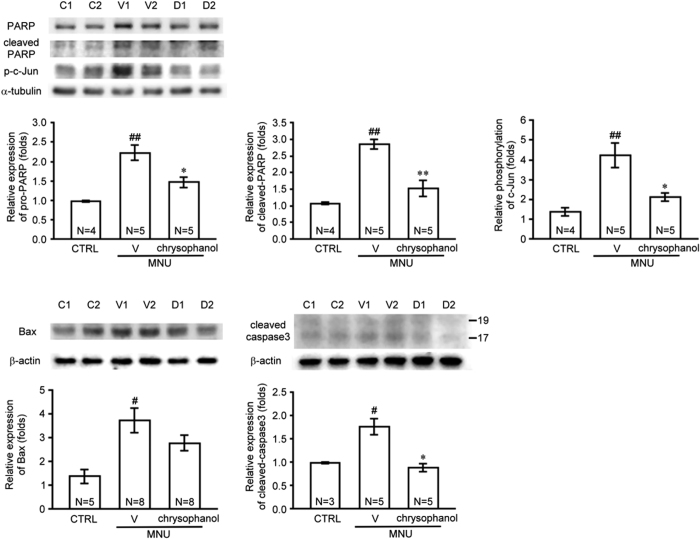
To identify the mechanism underlying the anti-apoptotic effect of chrysophanol, we examined the expression of PARP, caspase-3, Bax, and phosphorylated c-Jun in MNU-exposed retinas (Fig. 6). Pro-PARP and cleaved-PARP levels increased 2.3- and 2.7-fold, respectively, in MNU-exposed retinas compared with control retinas. And pro-PARP and cleaved-PARP levels significantly decreased 1.5- and 1.9-fold in the chrysophanol-treated MNU-exposed group compared with the MNU-exposed group, respectively. In addition, Bax levels were 2.7-fold higher in MNU-exposed retinas compared with control retinas, whereas Bax levels decreased 1.3-fold in the chrysophanol-treated MNU-exposed retinas compared with MNU-exposed retinas. Compared with control retinas, caspase-3 levels increased 1.8-fold in MNU-exposed retinas, and chrysophanol significantly reduced caspase-3 levels by 1.9-fold compared with the MNU-exposed retinas. In addition, phosphorylated c-Jun levels increased 3.1-fold in MNU-exposed retinas compared with control retinas and decreased 1.9-fold in the chrysophanol-treated group compared with the MNU-exposed group. Together, these results revealed that the anti-apoptotic effect of chrysophanol on photoreceptor cells exposed to MNU was mediated by the down-regulation of PARP, Bax, caspase-3, and phosphorylated c-Jun levels.
Figure 6. Chrysophanol inhibited MNU-induced expression of apoptosis-related proteins.
PARP, Bax, caspase-3, and p-c-Jun protein levels in retinal homogenates were evaluated using western blot. All the Western blotting experiments were performed under the same conditions. After transferring the blots onto nitrocellulose membranes, we immediately cropped the targeted blots according to referenced indicating markers (Supplementary Fig. S1), and then targeted proteins were immunoblotted with its specific monoclonal antibody. The quantification values were presented as the mean ± SEM of at least 3 retinas from each group. Representative bands from 2 independent experiments for each group: the control (C1, C2), vehicle-treated (V1, V2), and chrysophanol-treated (D1, D2) groups. C, CTRL: control; V: vehicle; D: chrysophanol-treated; N: number of animals in each group. #p < 0.01 compared with the control groups treated with vehicle, ##p < 0.001 compared with the control groups treated with vehicle, *p < 0.01 compared with the MNU-treated group treated with vehicle, **p < 0.001 compared with the MNU-treated group treated with vehicle.
Chrysophanol inhibited glial fibrillary acidic protein (GFAP) expression and MMP-9 activation in MNU-exposed retinas
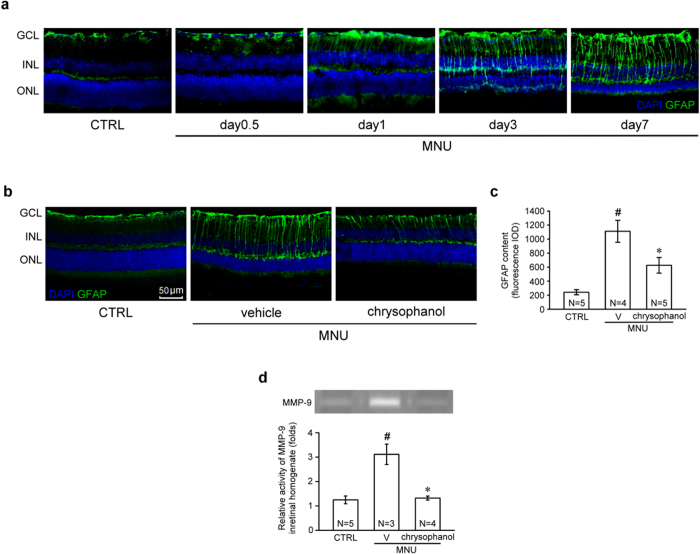
In the resting phase, GFAP localization is primarily restricted to the nerve fiber layer (NFL) and the GCL. Immunofluorescence assays demonstrated that GFAP was progressively up-regulated in a time-dependent manner in mice exposed to MNU (60 mg/kg) (Fig. 7a). These GFAP-positive processes were twisted and extended from GCL to INL. GFAP protein levels strongly increased and extended in MNU-treated group on day 7, and this effect was significantly inhibited by chrysophanol (Fig. 7b,c). The expression and activation of matrix metalloproteinase-9 (MMP-9) has previously been observed in retinopathies, and this effect is directly associated with an increase in GFAP expression20. Therefore, we evaluated MMP-9 activation in our MNU-induced mouse model of RP. The results of gelatin-zymography analysis demonstrated that MMP-9 expression and activation was significantly enhanced in MNU-exposed retinas (3.1 ± 0.4-fold) compared with control retinas (1.2 ± 0.2-fold; Fig. 7d). Chrysophanol (50 mg/kg) significantly inhibited MNU-induced gelatinolysis (1.3 ± 0.1-fold). These results suggested that MMP-9 activity is positively correlated with the pathological cascade of MNU-induced retinal injury. Furthermore, chrysophanol effectively inhibited MMP-9 activation in vivo.
Figure 7. Chrysophanol inhibited MNU-induced reactive gliosis and retinal MMP-9 activation on day 7.
(a) The time course of GFAP localization (green) after MNU (60 mg/kg) administration. (b) GFAP content in DAPI-counterstained retinal sections. (c) MMP-9 gelatinolytic activity in retinal homogenates was detected using zymography. All the zymographic experiments were performed under the same conditions. After gelatin staining, we immediately cropped the gelatinolytic regions in the gels (Supplementary Fig. S2). The data are presented as the mean ± SEM. CTRL: control; V: vehicle; N: number of animals in each group; GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer; IOD: integrated optical density. #p < 0.001 compared with the control group treated with vehicle, *p < 0.001 compared with the MNU-exposed group treated with vehicle.
Chrysophanol concentration-dependently inhibited lipopolysaccharide (LPS)-induced expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in BV-2 cells and inhibited MMP-9 gelatinolysis in primary microglia
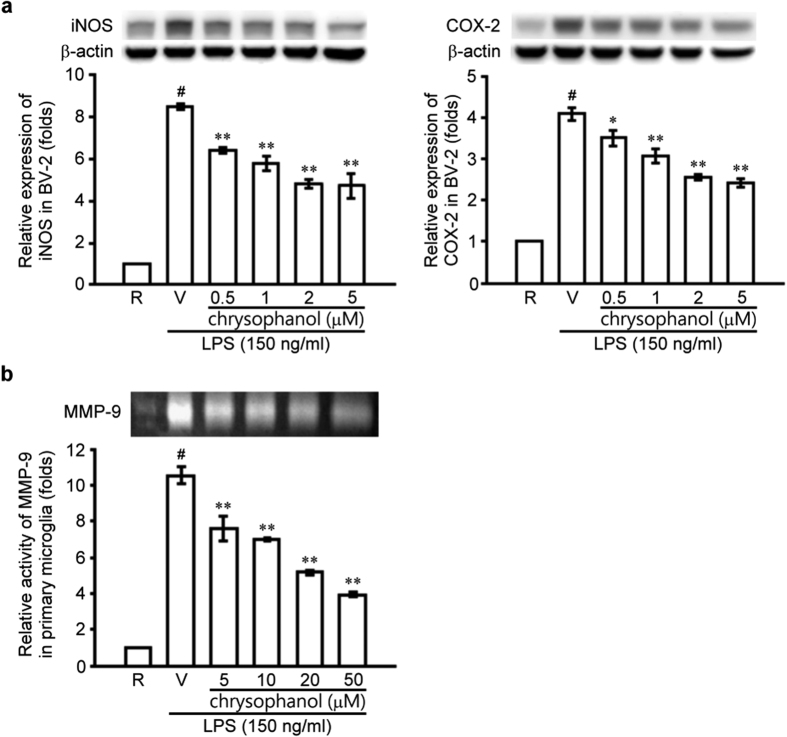
Microglial activation plays a key role in the host inflammatory response and contributes to the pathogenesis of retinal degeneration21,22,23. Accordingly, photoreceptor proteins released from RP retinas could activate microglia via Toll-like receptor 4 (TLR4) signaling3,24,25. Therefore, we used lipopolysaccharide (LPS), a TLR4 agonist, as an activator to stimulate the microglia cell line BV2. LPS (150 ng/mL) significantly increased iNOS and COX-2 protein levels in BV2 microglial cells (Fig. 8a), and chrysophanol (0.5–5 μM) significantly inhibited LPS-induced iNOS and COX-2 expression in a concentration-dependent manner. We also examined MMP-9 gelatinolytic activity in LPS-stimulated primary microglia (Fig. 8b) and found that MMP-9 activation was significantly induced and activated after LPS stimulation (up to 10.5 ± 0.5-fold) compared with the control group. In addition, chrysophanol significantly and concentration-dependently inhibited MMP-9 activation in primary microglia (Fig. 8b).
Figure 8. Chrysophanol inhibited iNOS and COX-2 expression and MMP-9 activation in activated microglia.
(a) Microglial BV2 cells were pretreated with the vehicle (DMSO) or chrysophanol (0.5, 1, 2, and 5 μM) and stimulated with LPS (150 ng/ml) for 24 hours. iNOS and COX-2 expression in cell lysates was evaluated using western blot. All the Western blotting experiments were performed under the same conditions. After transferring the blots onto nitrocellulose membranes, we immediately cropped the targeted blots according to referenced indicating markers (Supplementary Fig. S3), and then targeted proteins were immunoblotted with its specific monoclonal antibody. (b) Primary microglia cells were pretreated with the vehicle (DMSO) or chrysophanol (5, 10, 20, and 50 μM) and stimulated with LPS (150 ng/mL) for 24 hours. MMP-9 activation in cell supernatants was evaluated using zymography. All the zymographic experiments were performed under the same conditions. After gelatin staining, we immediately cropped the gelatinolytic regions in the gels (Supplementary Fig. S3). The data are presented as the mean ± SEM of at least 3 experiments in each group. R: resting; V: vehicle. #p < 0.001 compared with the control group treated with vehicle, *p < 0.01 compared with the LPS-exposed group treated with vehicle, **p < 0.001 compared with the LPS-exposed group treated with vehicle.
Discussion
Among the retinal degenerative diseases, RP is hereditarily characterized by progressive rod and cone photoreceptor cell death. In fact, photoreceptor apoptosis is a commonly observed mode of cell death in ocular diseases such as RP and age-related macular disease (AMD)26. Therefore, blocking retinal apoptosis might delay the progression of RP or AMD27. MNU is a potent retinal toxin that promotes retinal degeneration28. Specifically, MNU induces the degeneration of the ONL, a region abundant in photoreceptor cells, and this effect resembles the pathogenesis of end-stage human RP15. The present study was conducted to investigate the potential anti-apoptotic and retinal-protective effects of chrysophanol in an MNU-induced mouse model of RP. Based on the previous studies that evaluated the therapeutic profiles of Cassia seed and chrysophanol in ophthalmic and neuroprotective studies9,29, chrysophanol was used at a dose of 50 mg/kg in this study. We demonstrated that chrysophanol treatment attenuated photoreceptor cell degeneration and improved scotopic ERG responses in MNU-exposed retinas. Notably, chrysophanol effectively restored the integrity and structure of both rod and cone photoreceptors 7 days after MNU-induced retinal injury. Moreover, we found that the protective effect of chrysophanol in MNU-exposed retinas was mediated by the inhibition of photoreceptor cell apoptosis, proliferative gliosis, microglial activation, and MMP-9 activation.
A recent study reported that apoptosis-associated DNA fragmentation was observed in rat retinas during 7 days after MNU exposure30. Consistent with these findings, we observed an increase in TUNEL-labeled apoptotic cells in the ONL of mouse retinas 1 day after MNU exposure, and chrysophanol significantly abrogated this effect. An increase in the expression of apoptotic effectors demonstrated that the DNA adduct formation caused by MNU induced poly ADP-ribose polymerase (PARP) activation, further inducing cell death by depleting cellular energy reserves31. PARP has been shown to regulate activator protein-1 (AP-1)32, a dimeric transcription factor primarily composed of c-Jun and c-Fos that plays an important role in the regulation of cell proliferation and apoptosis33. Previous studies demonstrated that the induction of c-Jun and the phosphorylation of c-Jun at its N-terminal are associated with neuronal apoptosis34,35. In the apoptosis cascade, Bax is up-regulated 24 hours after the administration of MNU, and cysteine aspartate-specific protease-3 (caspase-3) coordinates and executes the apoptotic pathway36,37. A previous study indicated that inhibiting both PARP activity and the JNK/AP-1 signaling pathway blocks MNU-induced photoreceptor cell apoptosis30. Consistent with these findings, we found that chrysophanol attenuated the MNU-induced increase in PARP, cleaved PARP, phosphorylated c-Jun, Bax and cleaved caspase-3 levels 1 day after the administration of MNU. In summary, our findings suggested that the anti-apoptotic effects of chrysophanol were mediated by the inhibition of PARP activation, c-Jun phosphorylation, and apoptotic effectors.
Nearly all retinal diseases are associated with Müller cell and astrocyte gliosis38,39. The various consequences of proliferative gliosis, including the blood-retinal barrier breakdown, antioxidant deficiency, monocyte recruitment, and aggravated neuronal cell death19,40. In the present study, we evaluated the inhibitory effects of chrysophanol on glial cell activation. Glial fibrillary acidic protein (GFAP), a protein that localizes to the end-feet and processes of astrocytes and Müller cells, is a biomarker of gliosis in retinal degeneration38,41. In the present study, chrysophanol significantly attenuated GFAP overexpression in MNU-exposed retinas.
An increase in matrix metalloproteinase-9 (MMP-9) activity and colocalization of MMP-9 and GFAP have been observed in other animal models of retinopathy, such as the optic nerve ligation-induced mouse model of retinopathy20. Furthermore, several studies have demonstrated that increased MMP-9 activity plays a critical role in neuronal cell death in retinopathies42,43,44. Therefore, we investigated MMP-9 activity in MNU-exposed retinas. As expected, MMP-9 expression and activity significantly increased in MNU-exposed retinas, and this phenomenon was inhibited by chrysophanol. Although the inhibitory effect of chrysophanol on MMP-9 activity was directly associated with an inhibition of photoreceptor cell apoptosis, a more detailed characterization of this correlation requires additional experiments.
Microglia activation is associated with photoreceptor degeneration and subsequent primary rod photoreceptor cell death in RP in humans and in mouse models1,3,24. A previous study found that microglia migrate to the outer retina and phagocytose rod cell debris1. Photoreceptor proteins released from RP retinas could activate microglia via Toll-like receptor 4 (TLR4) signaling, and TLR4-mediated microglial phagoptosis might enhance neuronal death by promoting the phagocytosis of injured but living photoreceptors3,24,25. Therefore, we used lipopolysaccharide (LPS), a TLR4 agonist, as an activator to stimulate the microglia cell line BV2. In addition, an increase in the expression of the proinflammatory effectors inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), as well as other factors expressed in activated microglia, enhanced photoreceptor apoptosis21,23,45,46. A previous clinical study reported that the aqueous humor and levels of serum nitric oxide (NO) species are enhanced in RP patients compared with healthy controls, further suggesting that NO exacerbates oxidative damage to cone cells in RP47. In this study, we found that chrysophanol significantly inhibited LPS-induced iNOS and COX-2 expression in a concentration-dependent manner.
A previous study indicated that down-regulating MMP-9 activity could prevent the apoptotic cell death of retinal ganglion cells48. Therefore, we investigated the inhibitory effect of chrysophanol on MMP-9 activation in microglia. Consistent with previous findings, we observed that chrysophanol inhibited MMP-9 activation in LPS-activated primary microglia in a concentration-dependent manner. Taken together, these findings indicated that chrysophanol effectively inhibited microglia activation by down-regulating inflammatory factors and MMP-9 activity and that these effects potentially inhibited disease progression.
In conclusion, we demonstrated that chrysophanol prevented the functional and morphological features of MNU-induced retinal degeneration. The retina-protective effects provided by chrysophanol might be mediated by the inhibition of apoptosis, proliferative gliosis, microglia activation, and MMP-9 induction. We observed that chrysophanol provides neuroprotective effects on retinas, suggesting that chrysophanol might have therapeutic value for the treatment of RP and retinal injury. Further studies investigating the precise mechanisms underlying the protective and long-term effects associated with chrysophanol are warranted.
Methods
Animals and treatment
Male C57BL/6 mice (25–30 g body weight) were obtained from BioLASCO Taiwan Co., Ltd (Taipei, Taiwan) and maintained in a 12 hour light/12 hour dark cycle at 25 ± 1 °C and 39–45% relative humidity. Water and food were available ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee of Taipei Medical University (LAC-2016-0159), and conducted in accordance with guidelines of ARVO statement for the use of animals in ophthalmic and vision research.
To characterize MNU-induced retinal degeneration, C57BL/6 mice were intraperitoneally injected with various doses of MNU (40, 50, 60, and 70 mg/kg; Chem Service, Westchester, PA, USA) and evaluated at day 1 or day 7 after the injection. The eyes were rapidly enucleated and processed as described below.
Chrysophanol (50 mg/kg; Sigma, St. Louis, MO) or the vehicle control (0.1% carboxymethyl cellulose) was orally administered using a feeding needle. For the 7 day experiments, chrysophanol was delivered daily for 3 days starting the day before MNU was administered. For the 1 day experiments, a single dose of chrysophanol was delivered prior to the administration of MNU.
Scotopic electroretinography (ERG) analysis
Scotopic ERGs were used to evaluate the cone and rod photoreceptor responses to light stimulias previously described49. The electroretinography system was composed of a MP-36 4-channel amplifier and acquisition system (Biopac Systems, Inc., Pershore, UK) connected to a PS33-PLUS photic stimulator (Grass Technologies, Warwick, RI USA). Recordings were obtained using 10 msec flash stimuli with an intensity of 16 (19.1 cd · s/m2). ERG a-wave amplitudes were measured from baseline to the negative peak, and the b-wave amplitude was measured from the trough of the a-wave to the peak of the positive wave. The temporal properties of the ERG response were defined by the time-to-peak (implicit time) of the a- and b-wave, and are measured from stimulus onset to the peak of the a- and b-wave. OCT imaging was performed immediately after the ERG recording as described below.
Spectral-Domain Optical Coherence Tomography (SD-OCT) imaging
SD-OCT was used to non-invasively acquire cross-sectional tomographic images of the retina. This approach allowed for the monitoring of retinal morphology in live experimental animals. The Micron III intraocular imaging system (Phoenix Research Labs, Pleasanton, CA), composed of an OCT engine and a scanning lens, was used as previously described49. The resulting OCT image was imported into the InSight XL software (Phoenix Research Laboratories) to measure the total retinal thickness as well as the thickness of the different retinal layers.
Tissue homogenization
All of the animals were euthanized with an injection of a ketamine and xylazine mixture, and the eye balls were immediately enucleated. After removing the extraocular tissue, each eye ball was transferred into a 2 mL Precellys homogenization tube containing five 2.8-mm ceramic beads (Bertin Technologies, Rockville, Maryland) and immersed in 200 μl of homogenization buffer supplemented with 1× protease inhibitor (Roche, Mannheim, Germany). The samples were homogenized using a tissue homogenizer (Minilys®; Bertin Technologies, Rockville, Maryland) at 5,000 rpm for 30 seconds. The homogenized sample was centrifuged at 5,000 g for 5 min at 4 °C, and the resulting supernatants were incubated at 4 °C for 30 min. The samples were subsequently centrifuged at 12,000 g for 20 min at 4 °C and stored at −80 °C prior to additional experiments.
Immunofluorescence and TUNEL assays
To obtain retinal sections, the mouse eyes were resected immediately following transcardial perfusion with 4% paraformaldehyde (PFA) and fixed in Davidson’s fluid at 4 °C overnight. Then, the eyes were dehydrated with 30% sucrose, frozen-embedded in OCT, and serially sectioned at a thickness of 13 μm using a cryostat (Cryotome-E; Thermo Shandon, Cheshire, UK). The sections were pre-treated with citrate-EDTA buffer (pH 6.2) for epitope retrieval and subsequently incubated in blocking solution comprised of 10% normal goat serum and 0.5% Triton X-100 in 0.1 M phosphate buffer (pH 7.4) for 1 hour. Then, the sections were incubated with anti-GFAP (1:100; ProSci Inc., Poway, CA), anti-rhodopsin (1:50; Abcam, Burlingame, CA), and anti-red/green opsin antibody (1:100; Chemicon, Temecula, CA) at 4 °C overnight. The sections were rinsed and subsequently incubated with the fluorescence-conjugated secondary antibody Dylight 488 (1:200; GeneTex, Irvine, CA) for 2 hours at room temperature. Then, the sections were rinsed and mounted on cover slips using HIGHDEF® IHC fluoromount (Enzo Life Sciences, Farmingdale, NY) with 2 μM 4,6-Diamidino-2-phenylindole (DAPI; AAT Bioquest, Inc., Sunnyvale, CA). DAPI-stained retinal sections were used to calculate the number of rows of photoreceptor nuclei in the outer nuclear layer. Three sections nearing the central retinal area were counted and averaged in each group, and 3 fields were counted per section. Apoptosis was evaluated using the terminal deoxynucleotidyl transferase dUTP nick and labeling (TUNEL) assay as previously described49 with some modifications. The results of the TUNEL assays are presented as immunofluorescence images.
The retinal sections were evaluated using an Eclipse 80i fluorescent microscope (Nikon Instruments, Melville, NY) and a Leica TCS SP5 confocal microscope imaging system (Leica Microsystems, Wetzlar, Germany) equipped with argon and diode lasers. ImageJ software was used to quantify the number of photoreceptor nuclei rows in the ONL and the fluorescence content of GFAP.
Cell culture
The mouse BV-2 microglia cell line was cultured in DMEM supplemented with penicillin (90 units/ml), streptomycin (90 μg/mL), l-glutamine (3.65 mM), HEPES (18 mM), NaHCO3 (23.57 mM), and 10% heat-inactivated fetal bovine serum (FBS) at 37 °C in a humidified atmosphere (95% O2, 5% CO2). The cell culture conditions and cell treatments have been previously described50.
Isolation of primary microglia
The protocol used to isolate primary microglia was conducted as previously described with some modifications51. After the rats were perfused, the brain was removed and the meninges were resected. The fresh brain tissue was transferred into a dish with 5 mL of 0.25% trypsin (Gibco, Burlington, Canada) supplemented with 10 μg/mL deoxyribonuclease I (Roche, Indianapolis, IN) and minced roughly by using scissors. After an incubation period, the brain tissues were further minced with 10 mL of DMEM (Gibco, Grand Island, NY) supplemented with 10% FBS, and centrifuged at 1,000 rpm for 10 min. The tissue pellet was resuspended in 10% DMEM supplemented with FBS and incubated for the indicated period of time. Microglia were harvested from flasks of mixed glial cell cultures shaken for 2 hours, and the cells were collected and prepared for further experiments.
Western blot analysis
Western blot was used to analyze PARP, caspase-3, Bax and p-c-Jun levels in retinal homogenates, and iNOS and COX-2 levels in cell lysates as previously described52. The quantitative densitometry analysis was conducted using BIO-PROFIL Bio-1D light analytical software (Vilber Lourmat, Marue La Vallee, France). Protein levels are presented as the relative intensity compared with the control. The ratio of the optical density of the protein product to the internal control (α-tubulin or β-actin) was obtained and was expressed as a ratio or percentage of the control value in the Figures.
Gelatin zymography analysis
MMP-9 expression and the activation of retinal homogenates and conditioned medium from cultured primary microglia were evaluated using gelatin zymography as previously described53. Clear zones (bands) in a blue background represent MMP-9 degradatory activity. The image was acquired using a IP-008-SP Photo-print digital imaging system (Vilber Lourmat, Marue La Vallee, France), and BIO-PROFIL Bio-1D light analytical software (Vilber Lourmat, Marue La Vallee, France) was used for the densitometric quantitation of the gelatinolytic zones.
Statistical analyses
The experimental results were expressed as the mean ± SEM from the indicated number of experiments. The results were analyzed using one-way analysis of variance (ANOVA) with the Sigma Stat v3.5 software. The Student-Newman-Keuls test was used to evaluate statistically significant differences between groups. A p-value < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Lin, F.-L. et al. The natural retinoprotectant chrysophanol attenuated photoreceptor cell apoptosis in an N-methyl-N-nitrosourea-induced mouse model of retinal degeneration. Sci. Rep. 7, 41086; doi: 10.1038/srep41086 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Science Council of Taiwan (NSC 102-2628-B-038-009-MY3 and NSC 102-2320-B-038-018-MY3).
Footnotes
Author Contributions F.L.L., Y.W.C., and G.H. designed the experiments. L.L.F., C.H.L., J.D.H., J.L.Y., and H.M.C. carried out the experiments. F.L.L. and C.H.L. analyzed the results and prepared the figures. F.L.L., G.C.Y.C., Y.W.C., and G.H. wrote the manuscript. All of the authors reviewed the manuscript.
References
- Gupta N., Brown K. E. & Milam A. H. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res 76, 463–471 (2003). [DOI] [PubMed] [Google Scholar]
- Zeiss C. J. & Johnson E. A. Proliferation of microglia, but not photoreceptors, in the outer nuclear layer of the rd-1 mouse. Invest Ophthalmol Vis Sci 45, 971–976 (2004). [DOI] [PubMed] [Google Scholar]
- Zhao L. et al. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med 7, 1179–1197 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C. & Neher J. J. Microglial phagocytosis of live neurons. Nat Rev Neurosci 15, 209–216 (2014). [DOI] [PubMed] [Google Scholar]
- Brown G. C. & Neher J. J. Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem Sci 37, 325–332 (2012). [DOI] [PubMed] [Google Scholar]
- Zheng A., Li Y. & Tsang S. H. Personalized therapeutic strategies for patients with retinitis pigmentosa. Expert Opin Biol Ther 15, 391–402 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. C. In The Pharmacology of Chinese Herbs 2nd edn (ed. Huang K. C. ) 123 (CRC Press, 1998). [Google Scholar]
- Li Danny C., Yi Feng. Z. W. In Handbook of Traditional Chinese Medicine Vol. 2, (ed. S. T. Stevenson Xutian, Chun-Su Yuan ) 513 (World Scientific, 2014). [Google Scholar]
- Horng C. T. et al. The activity of lowering intraocular pressure of cassiae seed extract in a DBA/2J mouse glaucoma model. J Ocul Pharmacol Ther 29, 48–54 (2013). [DOI] [PubMed] [Google Scholar]
- Sreelakshmi V. & Abraham A. Cassia tora leaves modulates selenite cataract by enhancing antioxidant status and preventing cytoskeletal protein loss in lenses of Sprague Dawley rat pups. J Ethnopharmacol 178, 137–143 (2016). [DOI] [PubMed] [Google Scholar]
- Choi J. S., Lee H. J. & Kang S. S. Alaternin, cassiaside and rubrofusarin gentiobioside, radical scavenging principles from the seeds of Cassia tora on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical. Arch Pharm Res 17, 462–466 (1994). [DOI] [PubMed] [Google Scholar]
- Luo X. et al. Experiment study of total anthraquinone in cassiae semen on lipid peroxidation and PPAR-gamma expression in liver tissues of rats with alcoholic fatty liver. Zhongguo Zhong Yao Za Zhi 36, 1654–1659 (2011). [PubMed] [Google Scholar]
- Zhang N. et al. Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice. Mediators Inflamm 2014, 370530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rim H. K. et al. SoSoSo or its active ingredient chrysophanol regulates production of inflammatory cytokines & adipokine in both macrophages & adipocytes. Indian J Med Res 137, 142–150 (2013). [PMC free article] [PubMed] [Google Scholar]
- Tsubura A., Yoshizawa K., Kuwata M. & Uehara N. Animal models for retinitis pigmentosa induced by MNU; disease progression, mechanisms and therapeutic trials. Histol Histopathol 25, 933–944 (2010). [DOI] [PubMed] [Google Scholar]
- van Soest S., Westerveld A., de Jong P. T., Bleeker-Wagemakers E. M. & Bergen A. A. Retinitis pigmentosa: defined from a molecular point of view. Surv Ophthalmol 43, 321–334 (1999). [DOI] [PubMed] [Google Scholar]
- Marmor M. F. et al. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol 118, 69–77 (2009). [DOI] [PubMed] [Google Scholar]
- Miller R. F. & Dowling J. E. Intracellular responses of the Muller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol 33, 323–341 (1970). [DOI] [PubMed] [Google Scholar]
- Cuenca N. et al. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res 43, 17–75 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang X., Cheng M. & Chintala S. K. Optic nerve ligation leads to astrocyte-associated matrix metalloproteinase-9 induction in the mouse retina. Neurosci Lett 356, 140–144 (2004). [DOI] [PubMed] [Google Scholar]
- Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol 81, 1345–1351 (2007). [DOI] [PubMed] [Google Scholar]
- Karlstetter M. et al. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res 45, 30–57 (2015). [DOI] [PubMed] [Google Scholar]
- Yoshida N. et al. Laboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology 120, e5–12 (2013). [DOI] [PubMed] [Google Scholar]
- Kohno H. et al. Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J Biol Chem 288, 15326–15341 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher J. J. et al. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol 186, 4973–4983 (2011). [DOI] [PubMed] [Google Scholar]
- Ding X., Patel M. & Chan C. C. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res 28, 1–18 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. et al. Retinal structure and function preservation by polysaccharides of wolfberry in a mouse model of retinal degeneration. Sci Rep 4, 7601 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Uemura Y., Nakao I. & Tsubura A. Induction of mammary carcinomas by the direct application of crystalline N-methyl-N-nitrosourea onto rat mammary gland. Cancer Lett 92, 105–111 (1995). [DOI] [PubMed] [Google Scholar]
- Zhang K. et al. P2X7 as a new target for chrysophanol to treat lipopolysaccharide-induced depression in mice. Neurosci Lett 613, 60–65 (2016). [DOI] [PubMed] [Google Scholar]
- Uehara N., Miki K., Tsukamoto R., Matsuoka Y. & Tsubura A. Nicotinamide blocks N-methyl-N-nitrosourea-induced photoreceptor cell apoptosis in rats through poly (ADP-ribose) polymerase activity and Jun N-terminal kinase/activator protein-1 pathway inhibition. Exp Eye Res 82, 488–495 (2006). [DOI] [PubMed] [Google Scholar]
- Berger N. A. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res 101, 4–15 (1985). [PubMed] [Google Scholar]
- Zingarelli B. et al. Activator protein-1 signalling pathway and apoptosis are modulated by poly(ADP-ribose) polymerase-1 in experimental colitis. Immunology 113, 509–517 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Liu Z. & Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 9, 240–246 (1997). [DOI] [PubMed] [Google Scholar]
- Oshitari T., Yamamoto S. & Roy S. Increased expression of c-Fos, c-Jun and c-Jun N-terminal kinase associated with neuronal cell death in retinas of diabetic patients. Curr Eye Res 39, 527–531 (2014). [DOI] [PubMed] [Google Scholar]
- Xia Z., Dickens M., Raingeaud J., Davis R. J. & Greenberg M. E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270, 1326–1331 (1995). [DOI] [PubMed] [Google Scholar]
- Yoshizawa K. et al. Mechanisms of photoreceptor cell apoptosis induced by N-methyl-N-nitrosourea in Sprague-Dawley rats. Lab Invest 79, 1359–1367 (1999). [PubMed] [Google Scholar]
- Yang L. et al. Microarray expression analysis of the early N-methy-N-nitrosourea-induced retinal degeneration in rat. Neurosci Lett 418, 38–43 (2007). [DOI] [PubMed] [Google Scholar]
- Nakazawa T. et al. Attenuated glial reactions and photoreceptor degeneration after retinal detachment in mice deficient in glial fibrillary acidic protein and vimentin. Invest Ophthalmol Vis Sci 48, 2760–2768 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A. et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res 25, 397–424 (2006). [DOI] [PubMed] [Google Scholar]
- Penn J. S. et al. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 27, 331–371 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan J. P. & Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin Drug Saf 4, 433–442 (2005). [DOI] [PubMed] [Google Scholar]
- Ahuja S. et al. rd1 mouse retina shows imbalance in cellular distribution and levels of TIMP-1/MMP-9, TIMP-2/MMP-2 and sulfated glycosaminoglycans. Ophthalmic Res 38, 125–136 (2006). [DOI] [PubMed] [Google Scholar]
- De Groef L., Van Hove I., Dekeyster E., Stalmans I. & Moons L. MMPs in the neuroretina and optic nerve: modulators of glaucoma pathogenesis and repair? Invest Ophthalmol Vis Sci 55, 1953–1964 (2014). [DOI] [PubMed] [Google Scholar]
- Yong V. W., Power C., Forsyth P. & Edwards D. R. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci 2, 502–511 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H. Y. et al. Identification of sequential events and factors associated with microglial activation, migration, and cytotoxicity in retinal degeneration in rd mice. Invest Ophthalmol Vis Sci 46, 2992–2999 (2005). [DOI] [PubMed] [Google Scholar]
- Song F. et al. Schizandrin A Inhibits Microglia-Mediated Neuroninflammation through Inhibiting TRAF6-NF-kappaB and Jak2-Stat3 Signaling Pathways. PLoS One 11, e0149991 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Fernandez de la Camara C. et al. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLoS One 8, e74223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh B. S. & Chintala S. K. Inhibition of reactive gliosis attenuates excitotoxicity-mediated death of retinal ganglion cells. PLoS One 6, e18305 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. H. et al. Long-term Fluorometholone Topical Use Induces Ganglion Cell Damage in Rats Analyzed With Optical Coherence Tomography. Toxicol Sci 147, 317–325 (2015). [DOI] [PubMed] [Google Scholar]
- Wang C. H. et al. Carbamazepine attenuates inducible nitric oxide synthase expression through Akt inhibition in activated microglial cells. Pharm Biol 52, 1451–1459 (2014). [DOI] [PubMed] [Google Scholar]
- Hsiao G. et al. A potent antioxidant, lycopene, affords neuroprotection against microglia activation and focal cerebral ischemia in rats. In Vivo 18, 351–356 (2004). [PubMed] [Google Scholar]
- Chung C. L. et al. Dynasore, a dynamin inhibitor, induces PAI-1 expression in MeT-5A human pleural mesothelial cells. Am J Respir Cell Mol Biol 40, 692–700 (2009). [DOI] [PubMed] [Google Scholar]
- Chou Y. C. et al. Nuclear-targeted inhibition of NF-kappaB on MMP-9 production by N-2-(4-bromophenyl) ethyl caffeamide in human monocytic cells. Chem Biol Interact 184, 403–412 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.