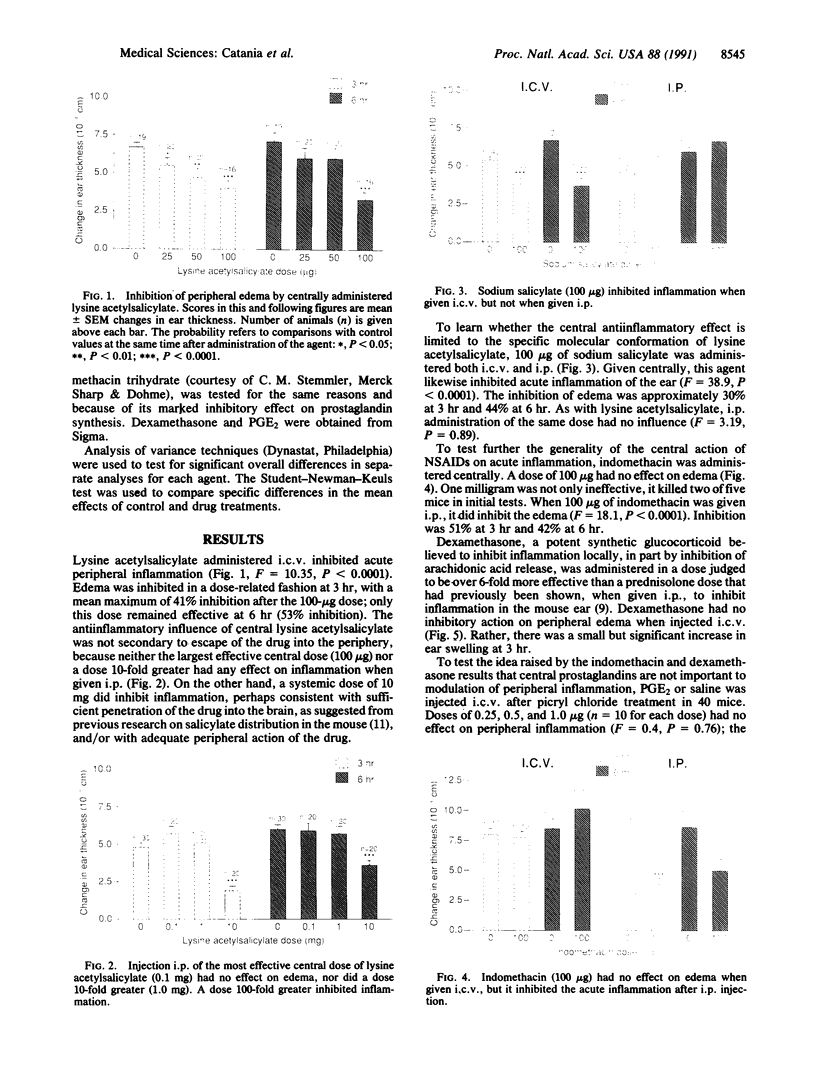
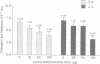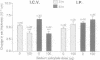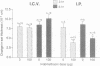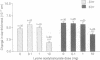Abstract
Understanding of the antiinflammatory actions of nonsteroidal drugs is incomplete, but these actions are believed to occur in the periphery, without any contribution from the central nervous system. Recent research on the antipyretic antiinflammatory neuropeptide alpha-melanocyte-stimulating hormone indicates that it can act centrally to inhibit peripheral inflammation; this raises the possibility that other agents, such as nonsteroidal antiinflammatory drugs, may have similar activity. In the present research both lysine acetylsalicylate and sodium salicylate inhibited edema, induced in the mouse ear by topical application of picryl chloride, when injected into the lateral cerebral ventricle. This inhibitory activity on a measure of acute inflammation was not due to escape of the drugs into the periphery, because systemic injection of doses that were effective centrally did not affect inflammation. In contrast, central administration of a dose of indomethacin that was antiinflammatory when given intraperitoneally did not inhibit peripheral inflammation. Thus indomethacin apparently lacks the central antiinflammatory action of the salicylates. This observation, plus our inability to demonstrate either an antiinflammatory effect of intracerebroventricular dexamethasone, a prostaglandin inhibitor, or a pro-inflammatory influence of prostaglandin E2, suggests that prostaglandins are not important to central modulation of inflammation. The results indicate that, in addition to having central influences on fever and pain, salicylates can act within the brain to inhibit acute inflammation in the periphery.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besson J. M., Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987 Jan;67(1):67–186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- Braga P. C. Ketoprofen: i.c.v. injection and electrophysiological aspects of antinociceptive effect. Eur J Pharmacol. 1990 Aug 10;184(2-3):273–280. doi: 10.1016/0014-2999(90)90619-h. [DOI] [PubMed] [Google Scholar]
- Chahl L. A., Ladd R. J. Local oedema and general excitation of cutaneous sensory receptors produced by electrical stimulation of the saphenous nerve in the rat. Pain. 1976 Mar;2(1):25–34. doi: 10.1016/0304-3959(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Chen A. C., Chapman C. R. Aspirin analgesia evaluated by event-related potentials in man: possible central action in brain. Exp Brain Res. 1980;39(4):359–364. doi: 10.1007/BF00239300. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Basbaum A. I., Levine J. D. Neural control of vascular permeability: interactions between primary afferents, mast cells, and sympathetic efferents. J Neurophysiol. 1989 Jul;62(1):48–58. doi: 10.1152/jn.1989.62.1.48. [DOI] [PubMed] [Google Scholar]
- Denko C. W., Petricevic M. Sympathetic or reflex footpad swelling due to crystal-induced inflammation in the opposite foot. Inflammation. 1978 Mar;3(1):81–86. doi: 10.1007/BF00917323. [DOI] [PubMed] [Google Scholar]
- Domenjoz R. Synthetic anti-inflammatory drugs: concepts of their mode of action. Adv Pharmacol. 1966;4:143–217. doi: 10.1016/s1054-3589(08)60099-x. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Lorenzetti B. B., Corrêa F. M. Central and peripheral antialgesic action of aspirin-like drugs. Eur J Pharmacol. 1978 Dec 15;53(1):39–48. doi: 10.1016/0014-2999(78)90265-0. [DOI] [PubMed] [Google Scholar]
- HALEY T. J., MCCORMICK W. G. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother. 1957 Mar;12(1):12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltz M. E., Lipton J. M. Antiinflammatory activity of a COOH-terminal fragment of the neuropeptide alpha-MSH. FASEB J. 1989 Sep;3(11):2282–2284. [PubMed] [Google Scholar]
- Jurna I., Brune K. Central effect of the non-steroid anti-inflammatory agents, indomethacin, ibuprofen, and diclofenac, determined in C fibre-evoked activity in single neurones of the rat thalamus. Pain. 1990 Apr;41(1):71–80. doi: 10.1016/0304-3959(90)91111-U. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Gamse R. Substance P in peripheral sensory processes. Ciba Found Symp. 1982;(91):35–54. doi: 10.1002/9780470720738.ch4. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Dardick S. J., Basbaum A. I., Scipio E. Reflex neurogenic inflammation. I. Contribution of the peripheral nervous system to spatially remote inflammatory responses that follow injury. J Neurosci. 1985 May;5(5):1380–1386. doi: 10.1523/JNEUROSCI.05-05-01380.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Goetzl E. J., Basbaum A. I. Contribution of the nervous system to the pathophysiology of rheumatoid arthritis and other polyarthritides. Rheum Dis Clin North Am. 1987 Aug;13(2):369–383. [PubMed] [Google Scholar]
- Lim R. K. Pain mechanisms. Anesthesiology. 1967 Jan-Feb;28(1):106–110. [PubMed] [Google Scholar]
- Lipton J. M., Macaluso A., Hiltz M. E., Catania A. Central administration of the peptide alpha-MSH inhibits inflammation in the skin. Peptides. 1991 Jul-Aug;12(4):795–798. doi: 10.1016/0196-9781(91)90135-c. [DOI] [PubMed] [Google Scholar]
- Lipton J. M. Modulation of host defense by the neuropeptide alpha-MSH. Yale J Biol Med. 1990 Mar-Apr;63(2):173–182. [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Shyu K. W., Lin M. T. Hypothalamic monoaminergic mechanisms of aspirin-induced analgesia in monkeys. J Neural Transm. 1985;62(3-4):285–293. doi: 10.1007/BF01252242. [DOI] [PubMed] [Google Scholar]
- Sturman J. A., Dawkins P. D., McArthur N., Smith M. J. The distribution of salicylate in mouse tissues after intraperitoneal injection. J Pharm Pharmacol. 1968 Jan;20(1):58–63. doi: 10.1111/j.2042-7158.1968.tb09619.x. [DOI] [PubMed] [Google Scholar]
- Vane J. The evolution of non-steroidal anti-inflammatory drugs and their mechanisms of action. Drugs. 1987;33 (Suppl 1):18–27. doi: 10.2165/00003495-198700331-00005. [DOI] [PubMed] [Google Scholar]