Significance
The RAF-ERK kinase pathway drives cell proliferation and cancer growth. ERK kinase activity is terminated by dual-specificity MAPK phosphatases (MKP/DUSPs), which are often assumed to be tumor suppressors. We demonstrate that the MKP DUSP5 terminates nuclear ERK signaling but, surprisingly, promotes ERK activation in the cytoplasm by relieving feedback inhibition of upstream kinases. Cancer-causing RAF kinase mutations, which occur in ∼8% of tumors and are refractory to feedback inhibition, reprogram DUSP5 to become a cell-wide attenuator of ERK signaling that prevents cellular senescence and promotes oncogenic transformation. Our results establish that interactions between feedback loops in the ERK cascade control localized signal promotion or suppression, which in turn govern cell proliferation and transformation.
Keywords: ERK, MAPK, MKP, DUSP, signaling
Abstract
Deregulated extracellular signal-regulated kinase (ERK) signaling drives cancer growth. Normally, ERK activity is self-limiting by the rapid inactivation of upstream kinases and delayed induction of dual-specificity MAP kinase phosphatases (MKPs/DUSPs). However, interactions between these feedback mechanisms are unclear. Here we show that, although the MKP DUSP5 both inactivates and anchors ERK in the nucleus, it paradoxically increases and prolongs cytoplasmic ERK activity. The latter effect is caused, at least in part, by the relief of ERK-mediated RAF inhibition. The importance of this spatiotemporal interaction between these distinct feedback mechanisms is illustrated by the fact that expression of oncogenic BRAFV600E, a feedback-insensitive mutant RAF kinase, reprograms DUSP5 into a cell-wide ERK inhibitor that facilitates cell proliferation and transformation. In contrast, DUSP5 deletion causes BRAFV600E-induced ERK hyperactivation and cellular senescence. Thus, feedback interactions within the ERK pathway can regulate cell proliferation and transformation, and suggest oncogene-specific roles for DUSP5 in controlling ERK signaling and cell fate.
Activation of ERK (extracellular signal-regulated kinase), the prototypic MAPK (mitogen-activated protein kinase), regulates a range of cell fate decisions, including differentiation, proliferation, and death (1, 2). Most stimuli engage ERK signaling by activating RAS GTPases, which promote the dimerization and activation of RAF kinases. RAF then phosphorylates and activates MEK (MAPK/ERK kinase), which in turn phosphorylates both threonine and tyrosine residues within a signature T-E-Y motif to activate ERK (2, 3). Under physiological conditions, ERK activity is self-limiting by the rapid phosphorylation and inhibition of upstream components [such as RAF kinases (4–6), RAS exchange factors (7), and receptor tyrosine kinases (RTKs) (8, 9)] but also by delayed induction of dual-specificity MAP kinase phosphatases (MKPs/DUSPs), which dephosphorylate and inactivate ERK (10, 11). Whether these temporally distinct negative feedback loops have cooperative or distinct roles in regulating ERK signaling is currently unclear.
Oncogenic mutations in RAS (RASmut) or a V600E substitution in BRAF frequently drive tumor progression through constitutive downstream MEK-ERK activation (12). ERK-inhibitory proteins, such as the MKP/DUSPs, are therefore often assumed to be tumor suppressors, but both decreased and increased MKP/DUSP expression is linked to tumor progression in different contexts (13). These observations may, at least in part, be explained by evidence showing that high-intensity ERK activation in some cell and tumor types engages tumor suppressive mechanisms, such as senescence (14–18). In such circumstances, the attenuation of ERK signaling by MKP/DUSPs may actually promote tumor progression (16, 17).
DUSP5, a nuclear-localized member of the MKP/DUSP family, is induced in response to ERK activation (19, 20) and specifically dephosphorylates and anchors ERK in the nucleus (21). We recently established that Dusp5 knockout (KO) mice are sensitized to HRASQ61L-driven DMBA/TPA (7,12-dimethylbenz[a]anthracene/12-O-tetra-decanoylphorbol-13-acetate)-initiated skin carcinogenesis, due to increased nuclear ERK activity and ERK-dependent SerpinB2 expression (22). Furthermore, the down-regulation of DUSP5 in gastric and prostate cancers is also associated with a poor prognosis (23, 24), suggesting that DUSP5 can function as a tumor suppressor. In contrast, DUSP5 expression is commonly retained or even enhanced in BRAFV600E-driven colorectal, melanoma, and thyroid cancer cell lines and tumors (25–27), which suggests that DUSP5 may either promote or limit the oncogenic potential of ERK signaling, depending on cellular context.
Here, we report that in addition to its role in the nuclear inactivation of ERK, DUSP5 increases RAF, MEK, and ERK activity in the cytoplasm. This latter effect is caused by the relief of upstream kinase inhibition, and depends on both the turnover rate of DUSP5 and its ability to sequester inactive ERK in the nucleus. Expression of the BRAFV600E oncoprotein, which is insensitive to feedback inhibition, alters the function of DUSP5 to become a cell-wide inhibitor of ERK, which in turn enables cells to avoid ERK hyperactivation and senescence. These data may explain why DUSP5 may be associated with tumor suppression or promotion in different tumor types.
Results
DUSP5 Controls both Nuclear and Cytoplasmic ERK Activity.
We have previously demonstrated that DUSP5 causes both nuclear accumulation and dephosphorylation of ERK in MEFs when stimulated with the phorbol ester TPA (22). These localized effects were revealed using confocal and high-content microscopy (HCM) (22). We therefore used similar methodology to compare the effects of DUSP5 on ERK responses to serum and growth factors.
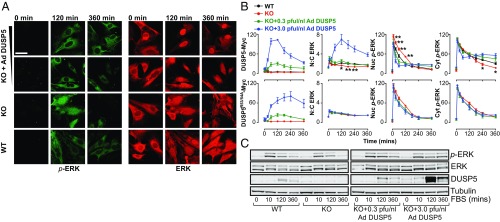
FBS stimulation of serum-starved primary WT MEFs rapidly elicited high levels of dually (pT-E-pY) phosphorylated ERK (p-ERK) in the nucleus and cytoplasm (from 5 to 60 min), followed by nuclear dephosphorylation of ERK and a prolonged phase (120–360 min) of lower-intensity cytoplasmic p-ERK signaling (∼30–50% of peak values; Fig. 1 A and B and Fig. S1 A and B). This observation is consistent with studies in several cell types showing that prolonged growth factor or serum stimulus typically causes transient nuclear, but sustained cytoplasmic, ERK activation (28, 29). Maximal increases in ERK nuclear accumulation [reflected by the nuclear:cytoplasmic (N:C) ratio of total ERK] occurred concurrently with the onset of nuclear ERK dephosphorylation (Fig. 1 A and B and Fig. S1B) and DUSP5 expression (Fig. 1C), suggesting that DUSP5 may be responsible for the nuclear accumulation and dephosphorylation of ERK (21, 22). In agreement with this hypothesis, Dusp5 deletion both reduced the N:C ERK ratio and elevated nuclear p-ERK levels (Fig. 1 A and B and Fig. S1A). Surprisingly, Dusp5 deletion also reduced the amplitude of the prolonged (240–360 min) cytoplasmic p-ERK response, suggesting that in addition to its nuclear inactivation of ERK, DUSP5 may also promote cytoplasmic ERK activation (Fig. 1 A and B).
Fig. 1.
DUSP5 propagates cytoplasmic ERK signaling. Serum-starved primary MEFs from WT or Dusp5 KO mice were infected with either 0.3–3.0 pfu/nL Ad ERK-responsive EGR1 promoter-driven DUSP5-Myc (DUSP5) or a KIM mutant of DUSP5-Myc (DUSP5R53/54A) and stimulated with 20% (vol/vol) FBS for times indicated. (A) Representative confocal images of p-ERK and ERK are shown. (Scale bar: 60 μm.) (B) HCM was used to quantify >104 cells per condition per experiment for levels of Myc tag, nuclear (Nuc), or cytoplasmic (Cyt) p-ERK intensity, or N:C of total ERK fluorescence. Data are normalized mean arbitrary fluorescence unit (AFU) values ± SEM, n = 4–8, *P < 0.05, **P < 0.01 comparing WT vs. KO using two-way repeated-measures ANOVA and Bonferroni posttest. Note: KO data are identical in Upper and Lower plots. (C) Western blots of whole-cell lysates are shown for total ERK, p-ERK, β-tubulin, and DUSP5. A representative blot is shown from n = 3 similar experiments; blot quantification is shown in Fig. S1C.
Fig. S1.
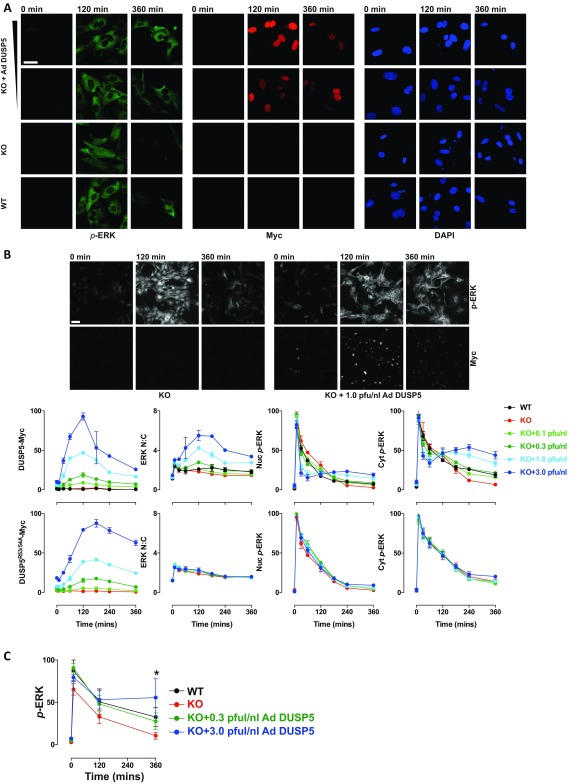
DUSP5 increases cytoplasmic ERK responses. Primary WT or Dusp5 KO MEFs were infected with either empty Ad or 0.1–3.0 pfu/nL Ad ERK-dependent EGR1 promoter-driven DUSP5-Myc or a KIM mutant (DUSP5R53/54A-Myc) before stimulation with 20% (vol/vol) FBS as indicated. (A) Representative images are shown for p-ERK, Myc-tag, and DAPI staining from confocal microscopy experiments comparing rescue using 0.3 and 3 pfu/nL Ad DUSP5. (Scale bar: 60 μm.) (B) Representative images and quantification in plots are shown for a single HCM experiment comparing the full range of DUSP5 or DUSP5R53/54A rescue conditions from 0.1–3.0 pfu/nL Ad concentrations. Note: KO data are identical in Upper and Lower plots. Data are shown as normalized population averages of AFU ± SD, n = 2–4. (Scale bar: 100 μm.) (C) Primary WT or Dusp5 KO MEFs were infected with either empty Ad or 0.3–3.0 pfu/nL Ad DUSP5-Myc before 20% (vol/vol) FBS stimulus and Western blotting as described in Fig. 1C. Data shown are normalized p-ERK levels measured in whole-cell lysates ± SEM, n = 3, *P < 0.05 comparing KO vs. KO + 3.0 pfu/nL DUSP5 using two-way repeated-measures ANOVA and Bonferroni posttest.
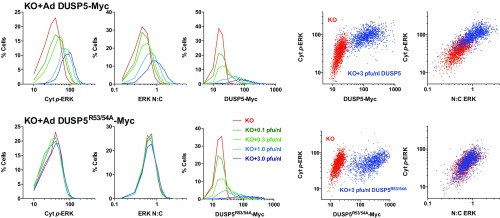
Previous reports show that constitutive overexpression of DUSP5 causes the constitutive nuclear accumulation and dephosphorylation of ERK (21). We therefore hypothesized that the propagation of cytoplasmic p-ERK levels (Fig. 1 A and B) may arise because DUSP5 is induced by ERK activity (19, 20). To test this prediction, we rescued DUSP5 expression in Dusp5 KO MEFs using adenovirus (Ad) vectors containing an ERK-responsive 1.2-kb EGR1 immediate early gene promoter to drive DUSP5-Myc expression (22). Low-titer (0.3 pfu/nL) Ad DUSP5-Myc restored transient DUSP5 expression (Fig. 1C), as well as corresponding changes in ERK N:C ratio, ERK nuclear dephosphorylation and the cytoplasmic rebound in p-ERK (Fig. 1 A and B and Fig. S1). The addition of higher titer Ad DUSP5-Myc (up to 3 pfu/nL) caused supraphysiological DUSP5 expression (Fig. 1C) and exaggerated all endpoints of ERK regulation, including the propagation of cytoplasmic ERK activity (Fig. 1 A and B and Fig. S1B). These effects of Dusp5 KO and rescue on cytoplasmic p-ERK were also reflected in Western blots of whole-cell lysates, most likely because the cytoplasm occupies a much greater volume of MEFs than the nucleus (compare Fig. 1 B and C and Fig. S1C). Negative control experiments using Ad to express an R53/54A kinase interaction motif (KIM) mutant of DUSP5-Myc that is unable to bind to ERK and driven by the EGR1 promoter (21, 22) failed to influence ERK responses at any titer, indicating the necessity of DUSP5 association with ERK to influence ERK signaling (Fig. 1B and Fig. S1B). Plotting of single-cell data revealed that increased cytoplasmic p-ERK levels after 240-min FBS stimulus correlated with increases in both DUSP5-Myc (but not DUSP5R53/54A-Myc) expression levels and ERK N:C ratio, indicating that the effects of DUSP5 are cell autonomous (Fig. S2).
Fig. S2.
DUSP5 regulation of ERK is dose dependent, cell autonomous, and reliant upon the kinase interaction motif. Dusp5 KO MEFs were infected with empty Ad, Ad EGR1 promoter-driven DUSP5-Myc, or a KIM mutant (DUSP5R53/54A-Myc) using indicated pfu/nL titers and stimulated for 240 min with 20% (vol/vol) FBS before HCM. Single-cell data from a representative of n = 4 experiments is shown. (Left) Frequency histograms of Cyt p-ERK, ERK N:C ratio, and whole-cell Myc levels. (Right) Scatterplots of either ERK N:C or Myc intensity vs. Cyt p-ERK intensity are shown comparing cells infected with 3 pfu/nL empty Ad (red), DUSP5-Myc (blue, Upper), or Ad DUSP5R53/54A-Myc (blue, Lower). All values are in raw AFU per cell.
Our previous studies, using TPA stimulation of MEFs without serum starvation, showed that endogenous DUSP5 suppressed nuclear ERK activity, but did not alter cytoplasmic p-ERK responses (22). We repeated these experiments, but included Ad DUSP5-Myc or Ad DUSP5R53/54A-Myc to rescue expression from sub- to supraphysiological levels in Dusp5 KO MEFs. DUSP5-Myc, but not DUSP5R53/54A-Myc, caused dose-dependent nuclear dephosphorylation and anchoring of ERK in response to TPA, but cytoplasmic p-ERK responses were only increased at supraphysiological levels of DUSP5 (Fig. S3A). DUSP5-Myc overexpression also increased cytoplasmic p-ERK responses to NGF stimulation in rat PC12 cells (Fig. S3B). These data collectively indicate a novel function of DUSP5—the maintenance or propagation of cytoplasmic p-ERK signaling—that is cell autonomous, dose dependent, and reliant upon association with ERK via the KIM domain.
Fig. S3.
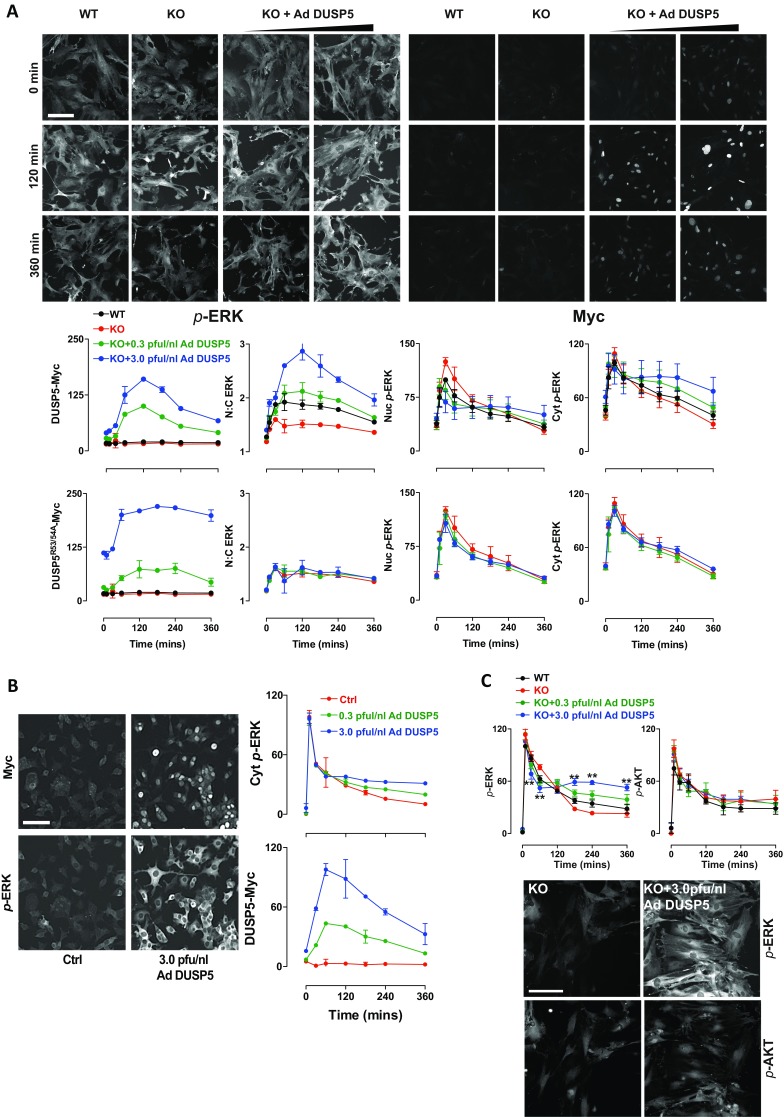
DUSP5 regulates cytoplasmic ERK signaling in response to phorbol ester and NGF stimuli, and does not influence serum-induced AKT signaling. (A) Primary WT and Dusp5 KO MEFs were infected with empty Ad, Ad EGR1 promoter-driven DUSP5-Myc, or DUSP5R53/54A-Myc using indicated pfu/nL titers before stimulation with 10 ng/mL TPA and HCM. Three fields per well per fluorophore were acquired from cells. (Upper) Representative images of ∼10% of a single field. (Scale bar: 150 μm.) (Lower) Graphs represent population averaged normalized AFU for whole-cell Myc, Nuc, and Cyt p-ERK intensity, or N:C ratio of total ERK intensity derived from a representative experiment ± SD, n = 2–4. (B) PC12 cells were infected with empty Ad (Ctrl) or indicated titers of Ad DUSP5-Myc before serum starvation and stimulation with 3 nM NGF and analysis using HCM. (Left) Representative images of cells are shown from HCM experiments following 360-min NGF treatment. (Scale bar: 50 μm.) (Right) Graphs show normalized population mean AFU values for whole-cell Myc levels and Cyt p-ERK intensity derived from a representative experiment ± SD, n = 2. (C) Primary WT and Dusp5 KO MEFs were infected with empty Ad, Ad EGR1 promoter-driven DUSP5-Myc, or DUSP5R53/54A-Myc using indicated pfu/nL titers before stimulation with 20% (vol/vol) FBS for up to 360 min and were costained for p-ERK and p-Ser473 AKT before HCM. Representative images are shown for 360 min FBS stimulus; plots show normalized population means of whole-cell p-ERK and p-AKT levels in AFU ± SEM, n = 4–5. (Scale bar: 50 μm.) **P < 0.01 using two-way repeated-measures ANOVA and Bonferroni posttest, comparing KO and KO + 3 pfu/nL Ad DUSP5-Myc conditions.
DUSP5 Propagates ERK Signaling by Increasing RAF and MEK Activation.
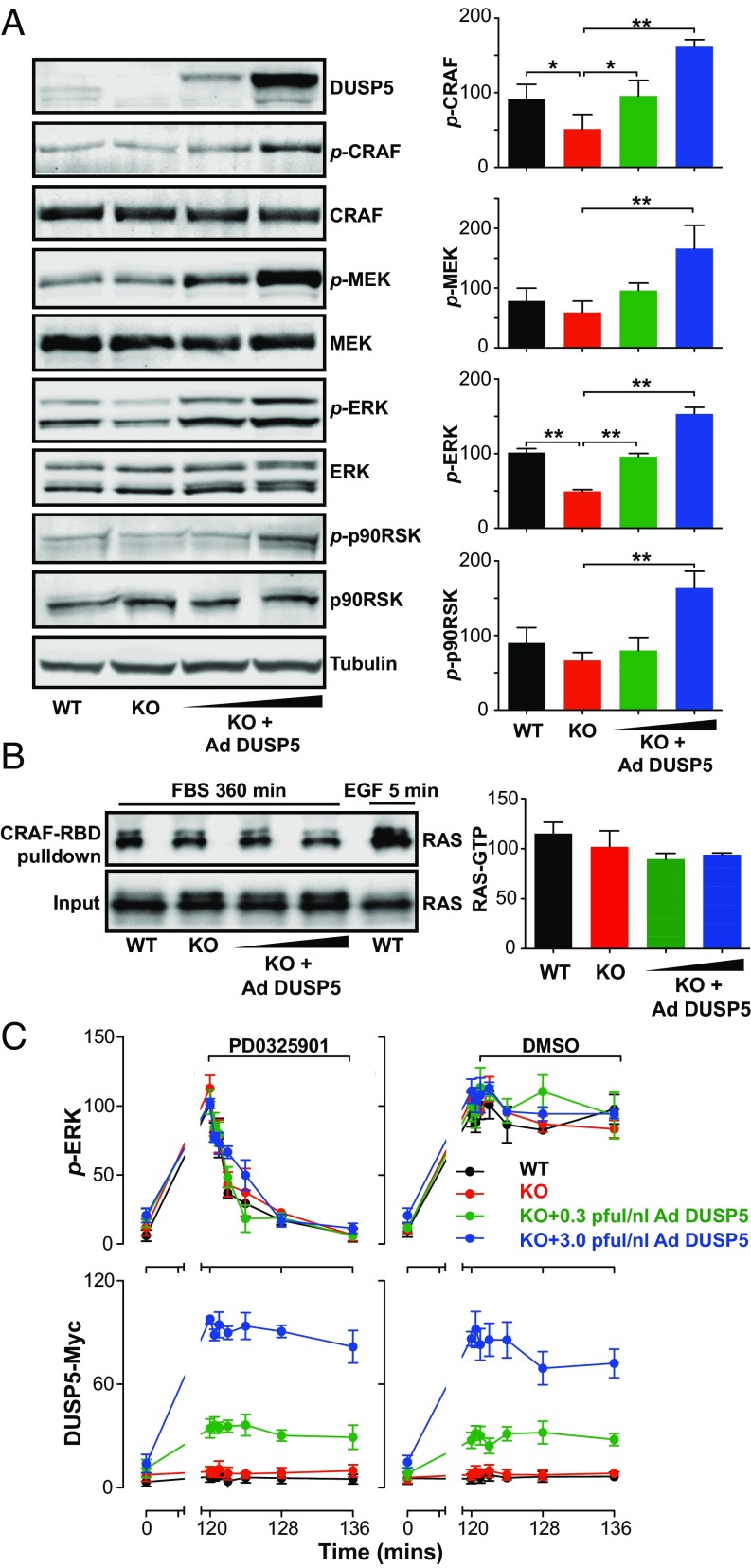
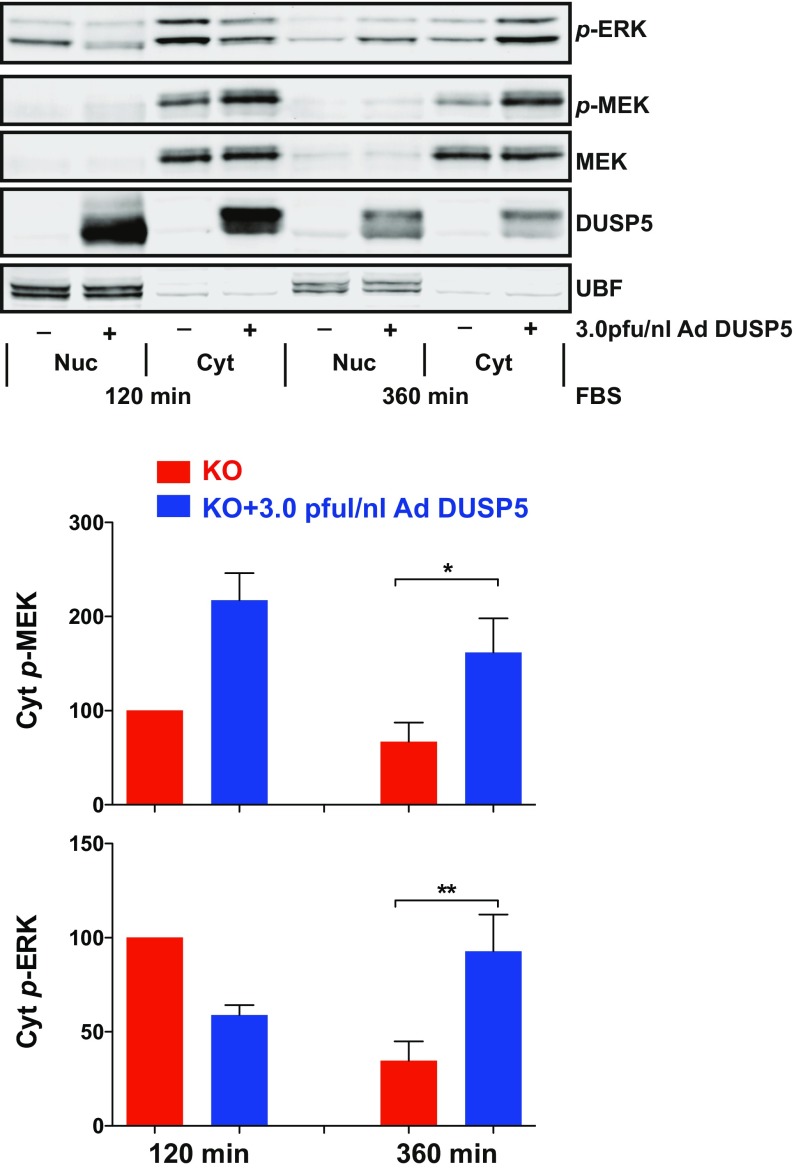
To determine how DUSP5 may cause increased ERK signaling, we examined the effects of Dusp5 deletion and rescue on the core ERK pathway components—CRAF, MEK and ERK, as well as the ERK substrate p90 ribosomal S6 kinase (p90RSK)—after 360-min FBS stimulus. Although only p-ERK levels showed a very obvious and substantial reduction following Dusp5 deletion, p-CRAF levels were also significantly reduced, and Ad DUSP5-Myc rescue caused clear, dose-dependent increases in the levels of all four phosphokinases measured (Fig. 2A). It is likely that the more readily detectable differences in p-ERK levels reflects the greater relative abundance of ERK, and because of signal amplification through the RAF-MEK-ERK cascade (30). Comparisons of MEK and ERK responses to FBS in fractionated lysates showed that DUSP5 significantly increased cytoplasmic p-MEK levels without influencing its localization or expression, whereas p-ERK responses echoed those observed in our microscopy assays (compare Fig. S4 and Fig. 1B). Strikingly, DUSP5 did not influence FBS-induced RAS activation, even when expressed at supraphysiological levels (Fig. 2B). In contrast, elevated RAS-GTP was readily detected in positive control samples stimulated with EGF for 5 min (Fig. 2B). Whilst it is possible that there could be highly localized or subtle changes in RAS activation that we cannot measure in whole-cell lysates, the data suggest that DUSP5 increases ERK phosphorylation by promoting pathway activity downstream of RAS.
Fig. 2.
DUSP5 propagates ERK signaling by increasing RAF and MEK activity. WT and Dusp5 KO primary MEFs were infected with 0.3–3.0 pfu/nL Ad EGR1 promoter-driven DUSP5-Myc (Ad DUSP5) as indicated. (A) MEFs were stimulated for 360 min with 20% (vol/vol) FBS. Western blots of whole-cell lysates were probed for p-sites on ERK (TEY activation loop), MEK (Ser217/221), CRAF (Ser338), and p90RSK (Thr-359/Ser363) as well as total kinase levels and β-tubulin. Normalized blot quantification is shown ± SEM, n = 4. *P < 0.05, **P < 0.01 comparing KO vs. all columns using one-way ANOVA and Dunnett's posttest. (B) MEFs were stimulated either with 20% (vol/vol) FBS for 360 min or 50 ng/mL EGF for 5 min before lysis and pull-down of GTP-RAS using the RAS-binding domain (RBD) of CRAF. Input levels of total RAS in whole-cell lysates and levels of GTP-RAS in the pull-down were measured by Western blotting. Normalized mean blot quantification of GTP-RAS is shown ± SEM, n = 3. (C) MEFs were stimulated with 20% (vol/vol) FBS for 120 min before addition of either DMSO vehicle or 5 μM PD0325901 MEK inhibitor for times indicated. Levels of Myc-tag and whole-cell levels of p-ERK were measured using HCM. Data shown are normalized population AFU values, n = 3 ± SEM.
Fig. S4.
DUSP5 increases cytoplasmic MEK and ERK phosphorylation in fractionated lysates. Primary Dusp5 knockout (KO) MEFs were infected with 3 pfu/nL empty Ad or Ad EGR1 promoter-driven DUSP5-Myc before stimulation with 20% (vol/vol) FBS for 120 or 360 min, separation into nuclear and cytoplasmic fractions, and Western blotting. Representative blots are shown above quantified normalized band intensities of Cyt p-ERK and p-MEK in the plots. *P < 0.05, **P < 0.01 from n = 4 experiments using one-way ANOVA and Dunnett's posttest comparing indicated columns at 360-min stimulus.
To investigate the possibility that DUSP5 could also promote ERK activity by attenuating other ERK phosphatases, we compared rates of ERK dephosphorylation after 120-min FBS stimulus and addition of a specific MEK inhibitor (MEKi), PD0325901, under conditions of Dusp5 deletion and rescue. A complete loss of p-ERK was observed within 8 min following MEKi addition, irrespective of DUSP5 expression level (Fig. 2C). We were also unable to detect differences in p-ERK half-life under these conditions, which is likely due to the very high constitutive rates of ERK dephosphorylation observed even in the absence of DUSP5 expression. These results exclude the possibility that DUSP5 could promote p-ERK signaling over several hours by attenuating the activity of other ERK phosphatases, and indicate that continuous MEK activity is required for the propagation of ERK signaling by DUSP5. These data are also supported by our previous studies showing that Dusp5 KO does not change either serum or TPA-induced expression of other ERK-regulatory MKP/DUSPs in MEFs (DUSP4, 6, 7, and 9) (22).
Knockdown of DUSP5 in some cancer cell lines has been reported to influence AKT phosphorylation (31), suggesting DUSP5 may influence additional RAS-effector pathways. We therefore compared FBS-induced activation of the ERK and PI3K-AKT pathways by measuring p-AKT and p-ERK levels in Dusp5 KO and rescue experiments. Although p-ERK levels were again increased by DUSP5-Myc expression, p-AKT levels were unchanged in the same cells, indicating that in MEFs the effects of DUSP5 are unlikely to be mediated by changes in the activity of this alternative RAS effector pathway (Fig. S3C). These results are also consistent with our previous studies showing that Dusp5 deletion does not influence JNK or p38 MAPK responses to FBS or TPA stimuli (22). Thus, DUSP5 is most likely able to increase the cytoplasmic activity of RAF, MEK, and ERK without invoking pathway cross-talk.
DUSP5 Nuclear Localization Controls Cytoplasmic ERK Phosphorylation.
Our experiments revealing that DUSP5 can prolong ERK signaling raise the question of whether other MKP/DUSPs might also exhibit this behavior. DUSP6 (also known as MKP-3/Pyst1) is analogous to DUSP5 in its ERK-dependent promoter regulation (32), ERK substrate specificity (33), sequestration of ERK (34), and rate of turnover (20, 35), but is localized in the cytoplasm due to an N-terminal nuclear export sequence (33, 34), whereas a nuclear localization sequence (NLS) close to the KIM causes DUSP5 to accumulate in the nucleus (21).
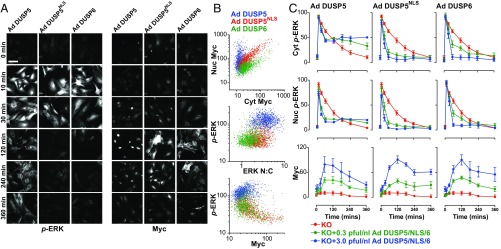
To test whether DUSP5 effects on cytoplasmic p-ERK were dependent on its nuclear localization, we used the EGR1 promoter in identical Ad backbone vectors to enable a direct comparison of DUSP5-Myc with DUSP6-Myc or an NLS mutant of DUSP5-Myc (DUSP5NLS), which impedes nuclear import but maintains catalytic and anchoring functions (21). FBS stimulation of Dusp5 KO cells infected with Ad DUSP5-Myc, DUSP5NLS-Myc or DUSP6-Myc induced comparable levels of expression but with different localization; DUSP5-Myc was almost exclusively nuclear, DUSP6-Myc was chiefly cytoplasmic, and DUSP5NLS-Myc was evenly distributed (Fig. 3 A and B), which is consistent with previous reports using these ORFs driven by constitutive promoters (21, 34). As expected, DUSP5-Myc expression led to elevated ERK N:C ratio, but maintained high levels of p-ERK per cell (Fig. 3B). Consistent with their ability to inactivate and sequester ERK in their respective cellular locations, DUSP5NLS-Myc and DUSP6-Myc reduced p-ERK and also the ERK N:C ratio (Fig. 3B). To determine if the differential effects of these DUSP-Myc transgenes could be due to altered expression kinetics, we compared time courses of p-ERK responses to FBS at varied Ad titers. Each of the DUSP-Myc proteins showed comparable magnitude and kinetics of induction, but only DUSP5-Myc was able to propagate cytoplasmic ERK activity. In contrast, both DUSP5NLS-Myc and DUSP6-Myc potently and dose-dependently decreased p-ERK throughout the cell (Fig. 3 A and C). These data suggest that DUSP5-mediated increases in cytoplasmic p-ERK are dependent upon its nuclear localization.
Fig. 3.
The DUSP5 nuclear localization signal is required for propagation of cytoplasmic p-ERK. Primary Dusp5 KO MEFs were infected with 0.3–3 pfu/nL Ad EGR1 promoter-driven DUSP5-Myc (Ad DUSP5), a nuclear localization sequence mutant of DUSP5-Myc or DUSP6-Myc (Ad DUSP5NLS and Ad DUSP6, respectively), and stimulated with 20% (vol/vol) FBS. (A) Representative HCM images show cells infected with 3 pfu/nL Ad DUSP-Myc before stimulation and staining for both p-ERK and Myc. Less than 1% imaged area per condition per experiment is shown. (Scale bar: 100 μm.) (B) Scatterplots show single-cell comparisons of nuclear vs. cytoplasmic levels of Myc tag (Top), ERK N:C ratio vs. whole-cell p-ERK (Middle) or whole-cell Myc-tag vs. whole-cell p-ERK levels (Bottom) from a single representative experiment of n = 4 comparing cells infected with 3 pfu/nL Ad DUSP and stimulated for 180 min with FBS. All nonratio values are shown in raw AFU per cell. (C) Plots show average Nuc p-ERK, Cyt p-ERK, and whole-cell Myc levels in cells stimulated with FBS. Data shown are mean, normalized AFU levels ± SEM, n = 3–5. Note: KO data are identical in each row and are shown in each plot to clarify the effects of Ad DUSP-Myc expression in the Dusp5-deleted cells.
DUSP5 Propagates ERK Signaling by Relieving Upstream Kinase Inhibition.
A major mechanism by which ERK self-limits its activity is through direct phosphorylation and inhibition of upstream components, such as RAS exchange factors (7), RTKs (8, 9), and RAF kinases, which prevent RAS-mediated RAF dimerization and activation (4–6). This rapid, negative feedback confers homeostatic control, such that pharmacological inhibition or a reduction in total ERK concentration causes strong compensatory increases in upstream kinase activity, which acts to maintain near constant p-ERK output levels (30, 36, 37). We therefore hypothesized that the nuclear sequestration and inactivation of ERK by DUSP5 may cause similar compensatory increases in upstream kinase activity, and that this may explain why cytoplasmic p-ERK levels are maintained or even prolonged in the presence of DUSP5.
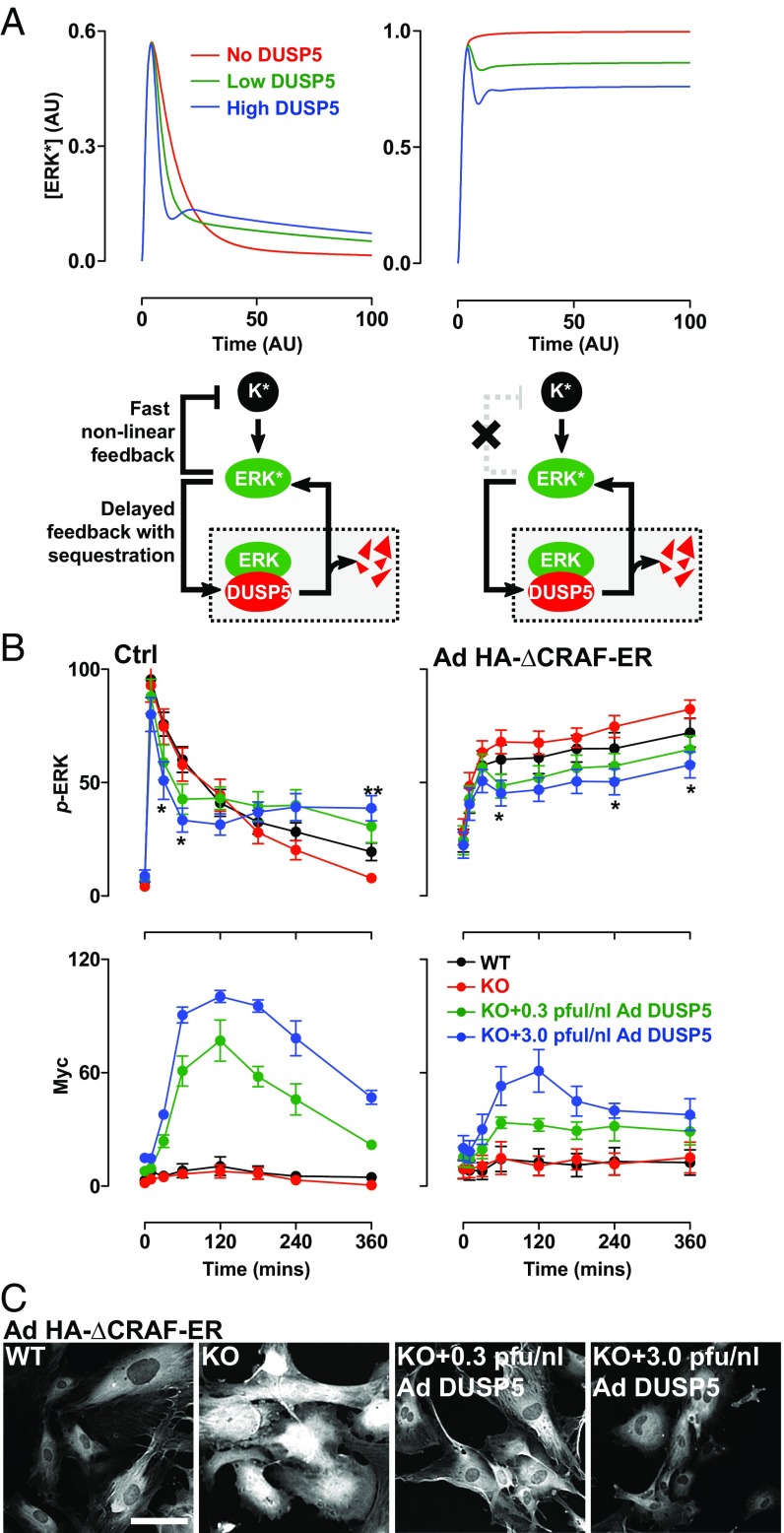
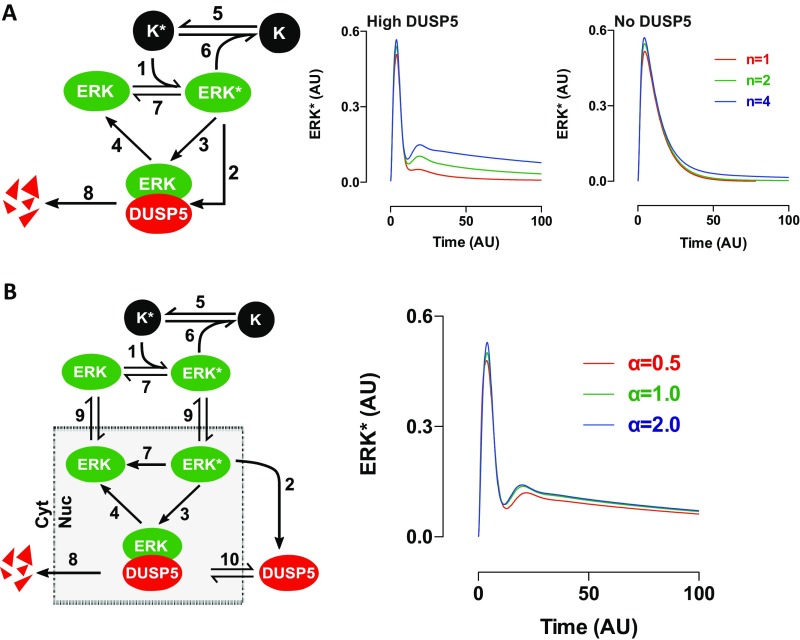
To assess whether our hypothesis was tenable, we devised a simplified mathematical model to explore the nature of interactivity among DUSP5, ERK, and upstream kinases that could cause DUSP5 to increase ERK signaling (Fig. 4A). In this network, K (representing all kinases upstream of ERK) is converted upon stimulus to an activated form, K*, which catalyzes the conversion of ERK to an active form, ERK*. To replicate typical receptor signaling, we modeled the conversion of K to K* as being rapid, followed by slower exponential conversion back to K. To represent rapid feedback inhibition of upstream kinases, ERK* was able to accelerate the deactivation of K* in our model (Fig. 4A, Fig. S5A, and Table S1; SI Materials and Methods). Previous studies indicate that ERK-mediated inhibition of RAF is chiefly responsible for homeostatic control of the ERK cascade, and is highly nonlinear in nature (30, 36, 37). We therefore modeled the relationship between ERK* and K* as highly cooperative by using a Hill function with a Hill coefficient of 4 (Table S1; SI Materials and Methods). Because ERK activation induces DUSP5 expression (19, 20), we modeled DUSP5 synthesis as dependent upon ERK*, but with a delay to replicate the time lag incurred by transcription and translation. Because DUSP5 differentially controls nuclear and cytoplasmic ERK activity (Fig. 1), we initially included compartmentalization and ERK traffic in our model (Table S2; SI Materials and Methods). However, biological rates of ERK nuclear shuttling are fast enough relative to the time scale of p-ERK propagation that their inclusion in the model did not influence in silico predictions of ERK* responses (Fig. S5B). Therefore, in our model we assumed for simplicity that all ERK associated with DUSP5 is inactive and that the release of inactive ERK occurs through DUSP5 degradation (Fig. 4A and SI Materials and Methods).
Fig. 4.
DUSP5 propagates ERK signaling by relieving upstream kinase inhibition. (A) Conceptual model network structures comprising ERK activation by an upstream kinase (K), feedback inhibition and sequestration of ERK by DUSP5, and nonlinear feedback inhibition of K by ERK. K* and ERK* denote activated forms of K and ERK, respectively. The plots show model predictions of ERK* concentration vs. time in arbitrary units (AU) under conditions where there is no, low, or high levels of DUSP5 synthesis and in which negative feedback between ERK and K is intact (Left) or completely disabled (Right). (B) WT and Dusp5 KO MEFs were infected with either empty Ad, or Ad expressing HA-ΔCRAF-ER, alongside either 0.3 or 3 pfu/nL of Ad EGR1 promoter-driven DUSP5-Myc. Cells were stimulated with either 20% (vol/vol) FBS (Left) or 0.1 µM 4HT (Right) before immunostaining for p-ERK and Myc followed by HCM analysis. Normalized population mean AFU values for whole-cell p-ERK and Myc intensity are shown ± SEM, n = 4–7, *P < 0.05, **P < 0.01 comparing KO vs. KO + 3.0 pfu/nL Ad DUSP5-Myc using two-way repeated-measures ANOVA and Bonferroni posttest. (C) Representative HCM images of p-ERK staining are shown from experiments described in B, comparing Ad HA-ΔCRAF-ER–infected MEFs after 360-min stimulus with 0.1 µM 4HT. (Scale bar: 100 μm.)
Fig. S5.
The nonlinearity of upstream negative feedback, but not ERK nuclear shuttling, determines the regulatory effects of DUSP5 in silico. (A) A network topology scheme (Left) represents a minimal interaction network comprising K (an upstream activator of ERK), ERK, and DUSP5. K* and ERK* represent activated versions of K and ERK, respectively. A full description of equations and a table describing reactions numbered as presented in the network scheme is included in SI Materials and Methods. The only reaction that is not modeled using mass action kinetics is the negative feedback between ERK* and K*, which is modeled using a Hill function (reaction no. 6 in Table S1). (Right) Plots show ERK* output kinetics under conditions in which different values of Hill coefficient (which alter cooperativity described in the Hill function) were used to model the feedback between ERK* and K* (n = 1, 2, or 4) in the presence of high levels of DUSP5 synthesis (High DUSP5) or no DUSP5 synthesis (No DUSP5). The plots only replicate our biological data, where high levels of DUSP5 cause a rebound in ERK signaling (Figs. 1 and 4), when the level of cooperativity between ERK* and K* is also high (Hill coefficient, n = 4). (B) An expanded network topology scheme is shown (Left), incorporating nuclear shuttling of ERK as well as cytoplasmic synthesis and nuclear import of DUSP5. Plots of ERK* output from model simulations in the compartmentalized model are shown (Right), in which DUSP5 synthesis levels were varied, and ERK nuclear shuttling rates (α in reaction no. 9, Table S2) were set at 0.5, 1.0, or 2.0 events per arbitrary unit of time. These estimates of shuttling rate (relative to the time course of DUSP5 effects) are consistent with published studies showing that rates of active and inactive ERK nuclear shuttling are very rapid (57). The rate of ERK nuclear shuttling in our model failed to appreciably influence the effects of DUSP5 on ERK* signal propagation even when varied to these extents. Because nuclear shuttling is unlikely to influence predictions on these much longer timescales, we therefore decided to use the simplified, reduced model in Fig. S5A in all further predictive simulations.
Table S1.
Reactions and typical parameter values used in a reduced model comprising DUSP5, ERK, and an upstream kinase (K) without cellular compartmentalization
| No. | Reaction | Rate | Value |
| 1 | ERK activation | ||
| 2 | DUSP synthesis | ||
| 3 | Sequestration | ||
| 4 | Release | ||
| 5 | Upstream input | ||
| 6 | Upstream negative feedback | ||
| 7 | ERK deactivation | ||
| 8 | DUSP degradation | ||
Table S2.
Reactions and typical parameter values used in an expanded model comprising DUSP5, ERK, and an upstream kinase, K, in which ERK and DUSP5 can shuttle to and from a nuclear compartment
| No. | Reaction | Rate | Value |
| 1 | ERK activation | ||
| 2 | DUSP synthesis | ||
| 3 | Sequestration | ||
| 4 | Release | ||
| 5 | Upstream input | ||
| 6 | Upstream negative feedback |
|
|
| 7 | ERK deactivation | ||
| 8 | DUSP degradation | ||
| 9 | ERK shuttling | ||
| (import) | |||
| (export) | |||
| 10 | DUSP shuttling | (import) | |
| (export) |
When K, ERK, and DUSP5 enzymes were linked this way in silico, increasing DUSP5 levels proportionally increased the ERK* response (Fig. 4A), which qualitatively replicated the kinetics of FBS-induced p-ERK responses observed in cells, using Dusp5 KO and rescue experiments (Fig. 4B). Reducing the cooperativity of feedback between ERK* and K* in our mathematical model reduced the ability of DUSP5 to propagate ERK* (Fig. S5A), whereas complete disruption of feedback between ERK* and K* reversed DUSP5 function, such that increasing DUSP5 levels proportionally reduced ERK* (Fig. 4A, compare Left and Right). These mathematical simulations suggest that the effects of DUSP5 are reliant upon the relief of upstream feedback mechanisms. To test this prediction, we generated Ad expressing an HA-tagged fusion protein of the conserved region 3 (CR3) kinase domain of CRAF (residues 305–648), and the estrogen receptor ligand-binding domain (HA-ΔCRAF-ER). The CR3 region of CRAF lacks five (of six) inhibitory ERK phosphorylation sites (4) and is therefore insensitive to feedback inhibition (30). HA-ΔCRAF-ER also lacks an N-terminal autoinhibitory domain, and the fusion to ER renders it conditionally activatable by the addition of 4-hydroxytamoxifen (4HT) (38). Accordingly, stimulation of MEFs expressing HA-ΔCRAF-ER with 4HT caused rapid, sustained increases in p-ERK (Fig. 4 B and C). Under these conditions, DUSP5 dose-dependently reduced p-ERK levels in both the nucleus and cytoplasm, mimicking the predictions generated in our mathematical model (Fig. 4A). These data indicate that key aspects of DUSP5 function, and specifically its ability to promote ERK activity, are dependent upon the relief of upstream negative feedback within the ERK cascade.
DUSP5 Degradation Controls the Amplitude and Duration of ERK Activity.
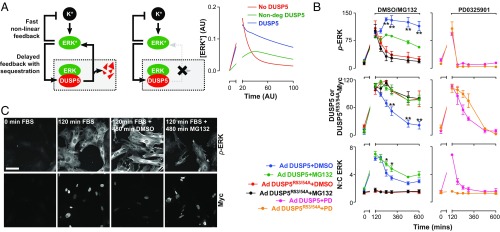
Published estimates of DUSP5 half-life are in the order of ∼45 min (20), but how this rapid turnover influences ERK regulation is unclear. We used our mathematical model to predict the effects of altering DUSP5 half-life, and found that abrogating DUSP5 degradation reduced the amplitude of sustained ERK* signaling in silico (Fig. 5A). To test this prediction, we reconstituted Dusp5 KO MEFs with supraphysiological levels of either DUSP5-Myc or DUSP5R53/54A-Myc, and used a proteasome inhibitor (MG132) to block DUSP5 degradation 120 min after FBS stimulation. MG132 blocked the degradation of DUSP5-Myc and caused a corresponding increase in ERK nuclear accumulation (ERK N:C ratio; Fig. 5B), but reduced p-ERK signal propagation by up to 50% (Fig. 5 B and C). In contrast, DUSP5R53/54A-Myc did not influence p-ERK responses or ERK N:C ratio, replicating the “No DUSP5” conditions in our mathematical model (Fig. 5B). Importantly, MG132 addition to cells expressing DUSP5R53/54A-Myc did not influence p-ERK signaling, indicating that its effects in cells expressing DUSP5-Myc arise specifically because the interaction between ERK and DUSP5 is prolonged (Fig. 5B). Controls, using MEKi addition after 120 min of FBS stimulus, reduced p-ERK and ERK N:C to basal levels within minutes, indicating that continuous MEK activity is required for DUSP5 to propagate ERK signaling and cause continuous ERK nuclear accumulation (Fig. 5B). Taken together, these data support a model where DUSP5 first sequesters dephosphorylated ERK in the nucleus, causing a relief of upstream kinase inhibition, and where the subsequent degradation of DUSP5 releases ERK from the nucleus, causing a rebound in p-ERK levels.
Fig. 5.
DUSP5 degradation controls the amplitude and duration of ERK signaling. (A) The cartoons show a “normal” network structure (Left) and one in which DUSP5 degradation is abrogated (Right). The plot compares model simulations for conditions where DUSP5 is synthesized and degraded (DUSP5), where DUSP5 is absent (No DUSP5) or synthesized but not degraded (Non-deg DUSP5). Comparisons of activated ERK (ERK*) concentrations vs. time are shown in AUs. Note: the break in the x axis is to enable direct comparison with the wet-laboratory data shown in B, in which only prolonged phases (>120 min) of FBS stimulus were measured. (B) Dusp5 KO MEFs were infected with 3 pfu/nL of Ad expressing EGR1 promoter-driven DUSP5-Myc or DUSP5R53/54A-Myc before 20% FBS stimulus for 120 min and treatment with either DMSO vehicle, 10 μM MG132 proteasome inhibitor, or 5 μM PD0325901 MEK inhibitor (PD). Plots show HCM readouts for whole-cell p-ERK, ERK N:C ratio and whole-cell Myc intensity and are shown as normalized population mean AFU ± SEM, n = 3. *P < 0.05, **P < 0.01 using two-way ANOVA and Bonferroni posttest comparing DMSO and MG132-treated cells. (C) Representative images of KO+Ad DUSP5 cells are shown from HCM experiments described in B. (Scale bar: 100 μm.)
DUSP5 Facilitates BRAFV600E-Driven Cell Proliferation and Transformation.
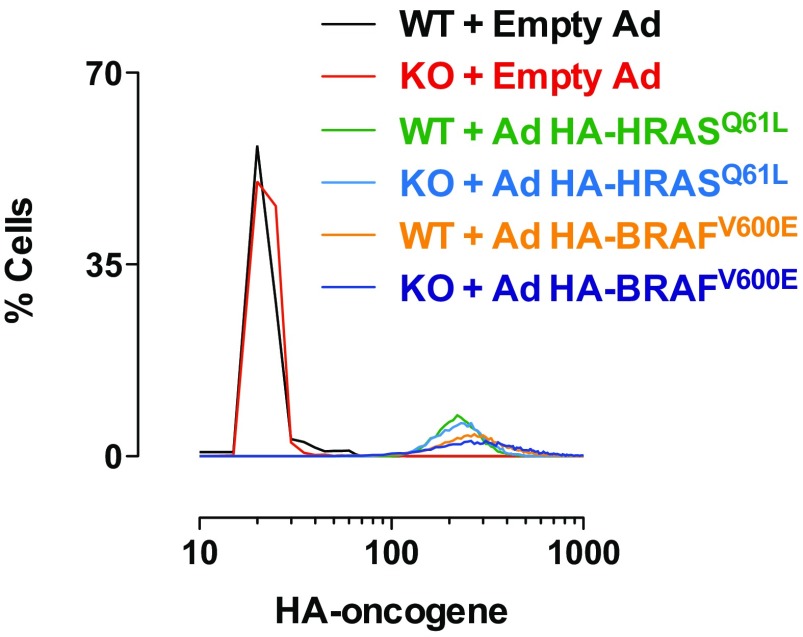
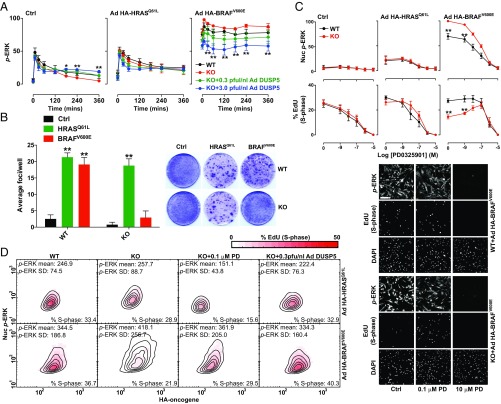
The oncogenic V600E substitution in BRAF causes constitutive kinase activation, irrespective of RAS activation or RAF dimerization status (5). As such, BRAFV600E is refractory to feedback inhibition by ERK (26, 30, 36, 37). In contrast, RASmut oncoproteins constitutively activate the ERK pathway, but leave ERK-RAF negative feedback intact. We therefore hypothesized that DUSP5 would cause more profound effects on p-ERK signaling in the presence of BRAFV600E than RASmut. To test this, we expressed comparable levels of HA-BRAFV600E or HA-HRASQ61L in WT and Dusp5 KO MEFs (Fig. S6) before FBS stimulation, and rescued DUSP5-Myc expression to normal or supraphysiological levels. In control cells lacking oncogene expression, DUSP5 again propagated FBS-induced p-ERK levels. HA-HRASQ61L expression marginally increased basal and FBS-induced p-ERK levels, but neither Dusp5 KO nor rescue significantly influenced responses (Fig. 6A). In contrast, HA-BRAFV600E expression caused high p-ERK signaling irrespective of FBS stimulus, and Dusp5 deletion further elevated p-ERK levels by ∼30%, which was dose-dependently inhibited by Ad DUSP5-Myc rescue at all time points (Fig. 6A). These data suggest that the loss of negative feedback caused by either ΔCRAF-ER or BRAFV600E expression is sufficient to transform the regulatory function of DUSP5, from a cytoplasmic propagator to cell-wide inhibitor of ERK (compare Figs. 4B and 6A).
Fig. S6.
Single-cell expression ranges of adenovirus-delivered HA-tagged oncogenes in MEFs. Primary WT and Dusp5 KO MEFs were infected with 3 pfu/nL empty Ad, Ad HA-HRASQ61L, or Ad HA-BRAFV600E for 24 h. Cells were stained for HA-tag before HCM. Four fields per well per fluorophore were acquired from cells. Frequency histograms of whole-cell HA-oncogene stain intensity using raw AFU values per cell from a representative experiment from n = 4 are shown.
Fig. 6.
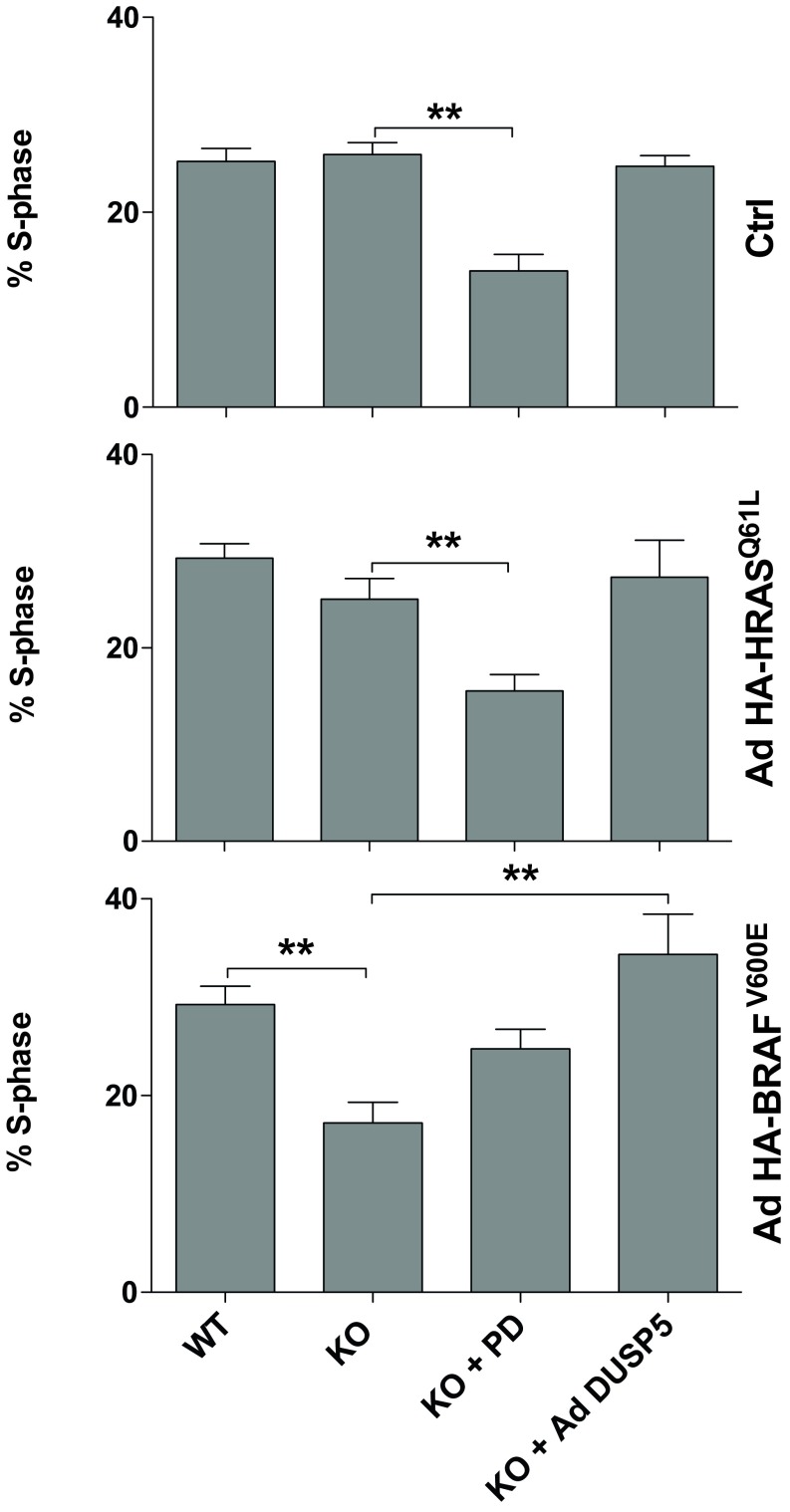
BRAFV600E expression combined with Dusp5 deletion causes ERK hyperactivation and proliferative arrest. (A) Primary WT and Dusp5 KO MEFs were infected with empty Ad, Ad HA-HRASQ61L, or Ad HA-BRAFV600E alongside 0.3–3 pfu/nL of Ad expressing EGR1 promoter-driven DUSP5-Myc before stimulation with 20% (vol/vol) FBS. Plots show time courses comparing whole-cell p-ERK levels using HCM. Normalized population mean AFU values are shown ± SEM, n = 4. *P < 0.05, **P < 0.01 comparing KO vs. KO + 3.0 pfu/nL Ad DUSP5-Myc using two-way repeated-measures ANOVA and Bonferroni posttest. (B) Immortalized WT and Dusp5 KO MEFs were infected with retrovirus control, HRASQ61L, or BRAFV600E vectors for 2 wk before assessment of transformed foci using crystal violet staining. Representative plate scans are shown and plots show average focus formation per well ± SEM, n = 4. **P < 0.01 comparing control and oncogene-expressing cells using two-way repeated-measures ANOVA and Bonferroni posttest. (C) Primary WT and Dusp5 KO MEFs were infected with either empty Ad, Ad HA-HRASQ61L, or Ad HA-BRAFV600E and treated with increasing doses of PD0325901 MEK inhibitor for 24 h before a 2-h fluorescent EdU pulse label, immunostaining for p-ERK, and analysis using HCM. Plots show Nuc p-ERK levels and the percentage of EdU-positive cells ± SEM, n = 3. **P < 0.01 using two-way repeated-measures ANOVA and Bonferroni posttest comparing WT and KO. Representative images are shown beneath. (Scale bar: 200 μm.) (D) WT and Dusp5 KO primary MEFs were infected with Ad HA-BRAFV600E (Lower), Ad HA-HRASQ61L (Upper), and 0.3 pfu/nL Ad DUSP5-Myc and treated with 0.1 µM PD0325901 (PD) as indicated for 24 h before 2-h fluorescent EdU pulse-labeling of S-phase cells and counterstaining for p-ERK and HA-tag. The plots show HA intensity vs. Nuc p-ERK in single cells; contour lines show equal cell density; and the heat map reflects the percentage of S-phase–positive cells within HA and p-ERK bins. Data plotted are in raw AFU and population statistics, including mean p-ERK levels, p-ERK SD, and overall percentage of S-phase–positive cells are shown within individual plots.
In the absence of other genetic mutations, RASmut or BRAFV600E expression can cause oncogene-induced cell-cycle arrest and senescence, which is mediated by hyperactivation of ERK and consequently limits cell transformation (16, 39–41). We have previously shown that Dusp5 KO does not influence HRASQ61L-mediated MEF proliferation or transformation (22), but reasoned that the more pronounced effects of Dusp5 KO on BRAFV600E signaling might be sufficient to mediate such changes. To test this, we performed colony formation assays in WT and Dusp5 KO MEFs immortalized by targeted Cdkn2a knockdown (22) and expressing either HRASQ61L or BRAFV600E. Both oncogenes induced comparable transformation rates in WT MEFs, but Dusp5 KO selectively abolished BRAFV600E-driven transformation (Fig. 6B). To establish whether these effects were accompanied by a similar influence on proliferation, we compared rates of S-phase entry 24 h after addition of HA-HRASQ61L or HA-BRAFV600E. As nuclear ERK activity is required for proliferation (42), and because the effects of Dusp5 KO on BRAFV600E signaling were most apparent in the nucleus, we specifically compared nuclear p-ERK with rates of S-phase entry. Dusp5 KO caused a 25% increase in HA-BRAFV600E-induced nuclear p-ERK and a corresponding 50% drop in S-phase positive cells (Fig. 6C). Partial blockade of p-ERK signaling in HA-BRAFV600E + Dusp5 KO cells using sub-IC50 (0.1 µM) MEKi completely restored rates of S-phase entry, whereas more complete p-ERK inhibition using higher MEKi concentrations (>1 μM) caused a complete cessation of proliferation (Fig. 6C). In either control populations without oncogene or HA-HRASQ61L-expressing populations, Dusp5 KO had no significant effect on nuclear p-ERK responses, S-phase entry or MEKi efficacy (Fig. 6C). These data are consistent with studies indicating that only a narrow range of ERK activity can promote cell cycle progression (14, 41), and suggest that Dusp5 KO + BRAFV600E increases ERK activity beyond thresholds that are compatible with cell proliferation.
To test whether the effects of Dusp5 deletion seen on proliferation rate reflected a particular subpopulation of cells, we compared oncogene expression vs. nuclear p-ERK and S-phase entry in single cells. We found that Dusp5 deletion selectively increased nuclear p-ERK and reduced S-phase entry across a 10-fold difference in HA-BRAFV600E (but not HA-HRASQ61L) expression, and also increased the cell-cell heterogeneity of the response (reflected by the increased spread of p-ERK histogram contours and SD values; Fig. 6D). Addition of Ad DUSP5-Myc or 0.1 µM MEKi reversed the effects of Dusp5 KO + HA-BRAFV600E on nuclear p-ERK levels, heterogeneity and S-phase response (Fig. 6 C and D and Fig. S7). Our data therefore support a model where proliferation of cells is robust to disruption of a single feedback loop by either BRAFV600E expression or Dusp5 deletion. However, when combined, these increase both the intensity and heterogeneity of the p-ERK response to such an extent that proliferation is inhibited.
Fig. S7.
DUSP5 facilitates BRAFV600E-driven cell proliferation. WT and Dusp5 KO MEFs were infected with 3 pfu/nL of empty Ad, Ad HA-HRASQ61L, or Ad HA-BRAFV600E and either treated with 100 nM PD0325901 MEK inhibitor (PD) or coinfected using 0.3 pfu/nL Ad DUSP5-Myc for 24 h before 2-h pulse labeling with fluorescent EdU for assessment of proliferative index using HCM. Plots show the percentage of EdU-positive cells (% S-phase) in each condition ± SEM, n = 3–5. **P < 0.01 using one-way ANOVA and Dunnett's posttest comparing all columns to KO.
DUSP5 Deletion Accelerates BRAFV600E-Driven Senescence.
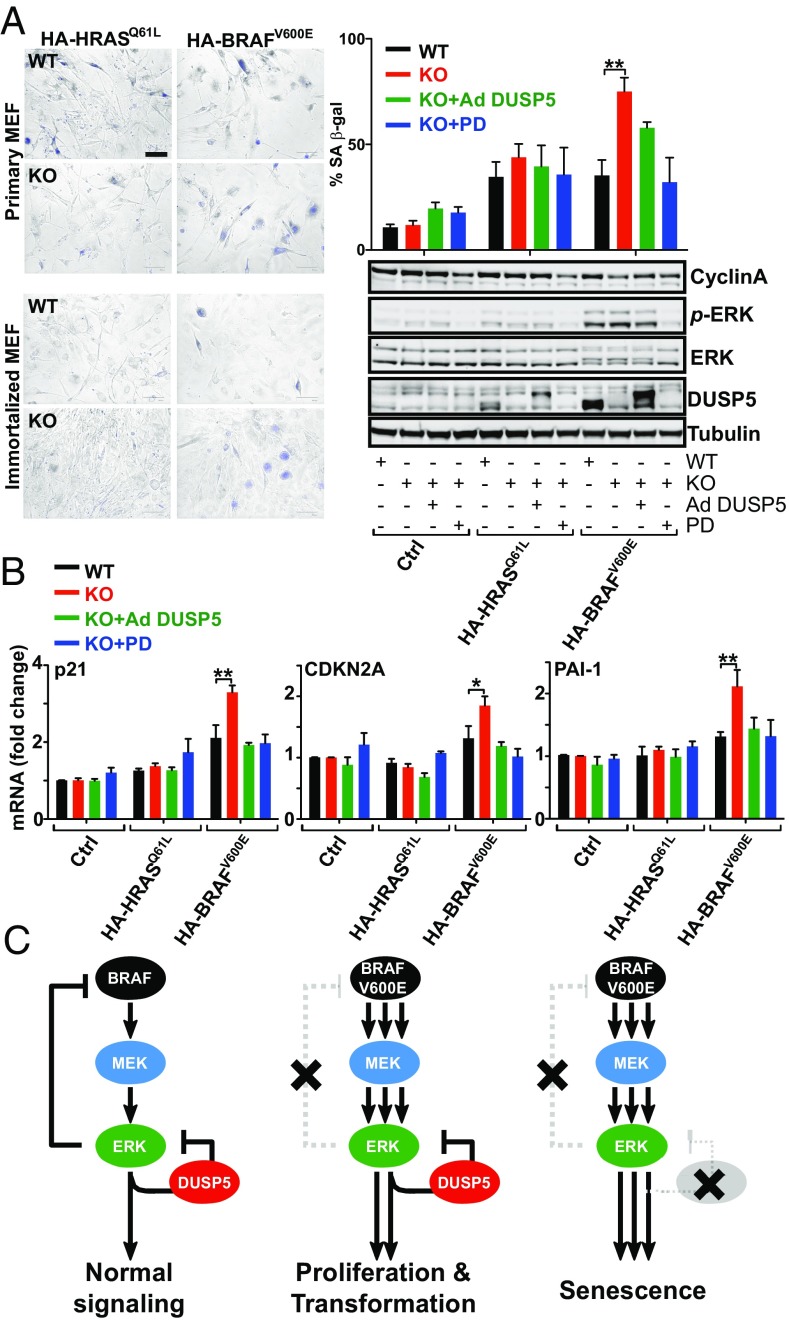
Finally, we investigated whether DUSP5 was able to influence HRASQ61L or BRAFV600E oncogene-induced senescence. As expected, expression of HA-BRAFV600E or HA-HRASQ61L in primary and immortalized MEFs increased the proportion of senescence-associated β-galactosidase-positive cells (SA β-gal+) within 72 h by ∼3.5-fold (Fig. 7A). However, Dusp5 deletion doubled the HA-BRAFV600E-induced proportion of SA β-gal+ cells, which was reversed by 0.3 pfu/nL Ad DUSP5-Myc rescue or the addition of 0.1 µM MEKi (Fig. 7A), suggesting that the onset of senescence is accelerated by Dusp5 deletion. We therefore sought to establish if molecular changes associated with senescence (39) were altered at earlier time points. Western blotting of whole-cell lysates showed that HA-BRAFV600E expression for just 24 h in primary Dusp5 KO MEFs caused a small, but not statistically significant, decrease in Cyclin A expression (Fig. 7A) and markedly elevated levels of Cdkn1a (p21CIP1), Cdkn2a (p16INK4A) and Serpine1 (PAI-1) mRNA (Fig. 7B). All of these changes were entirely restored to levels seen in WT cells by Ad DUSP5-Myc rescue or 0.1 µM MEKi, indicating that the elevation of these senescence markers is both DUSP5- and MEK-dependent. No such changes were seen when combining Dusp5 KO with HA-HRASQ61L expression or in the absence of oncogene expression. These data collectively suggest that in this MEF model of incipient tumorigenesis, Dusp5 deletion accelerates BRAFV600E-induced cell cycle arrest and senescence by causing ERK hyperactivation.
Fig. 7.
Dusp5 deletion accelerates BRAFV600E-driven senescence. (A) WT and Dusp5 KO primary and immortalized MEFs were infected with empty Ad, Ad HA-HRASQ61L, Ad HA-BRAFV600E, and Ad EGR1 promoter-driven DUSP5-Myc (Ad DUSP5) and treated with 0.1 µM PD0325901 (PD) MEK inhibitor as indicated. Representative images show SA β-gal staining of MEFs after 72 h. (Scale bar: 125 μm.) The quantification of SA β-gal staining is shown for primary MEFs after 72 h ± SEM, n = 3. **P < 0.01 using two-way repeated-measures ANOVA and Bonferroni posttest. Western blots compare indicated protein levels in primary MEFs 24 h after oncogene expression. (B) The plots show quantitative PCR comparisons of indicated mRNA levels from primary MEFs treated as in A for 24 h. *P < 0.05, **P < 0.01 using two-way repeated-measures ANOVA and Bonferroni posttest, ±SEM, n = 3. (C) Model depicting cellular consequences of combined BRAFV600Eexpression and DUSP5 deletion.
SI Materials and Methods
Mathematical Modeling.
Dynamic models were derived using ordinary differential equations. All parameters were chosen to mimic our experimental data within biologically reasonable boundaries, and the models were implemented using Mathematica (Wolfram). The following equations were used to model the interplay between reactants and products outlined in Table S1 and in the network diagram in Fig. S5A for the reduced model lacking nuclear compartmentalization, and were used to generate plots shown in Figs. 4A and 5A. Note that the numbering of the reactions represented in the network diagram in Fig. S5A correspond to the numbering of the reactions outlined in Table S1.
The equations below describe relationships between reactants and products outlined in Table S2 and in the network diagram in Fig. S5B for an expanded model, including nuclear and cytoplasmic compartmentalization. Again, the numbering of the reactions represented in the network diagram in Fig. S5B correspond to the numbering of the reactions outlined in the corresponding Table S2.
Chemicals, Antibodies, and Inhibitors.
The polyclonal anti-DUSP5 antibody was raised in sheep using full-length recombinant DUSP5 protein as previously described (20). Anti–total ERK1/2 (4695, rabbit mAb, clone 137F5), p-MEK1/2 (9154, rabbit mAb, clone 41G9), total MEK1/2 (4694, mouse mAb, clone L38C12), p-Akt (Ser-473) (3787, rabbit mAb, clone 736E11), p-p90RSK (Thr-359/Ser363) (9344, rabbit pAb), total p90RSK (9355, rabbit mAb, clone 32D7), p-CRAF (Ser338) (9427, rabbit mAb, clone 56A6), total CRAF (9422, rabbit pAb), Myc tag (2278, rabbit mAb clone 71D10), and HA tag (3724 rabbit mAb, clone C29F4) were all obtained from Cell Signaling Technologies. Anti–β-tubulin (T8328, mouse mAb, clone AA2) and anti p-ERK1/2 (M9692, mouse mAb, clone MAPK-YT) were purchased from Sigma. Anti-Cyclin A (sc-596, rabbit pAb C-19) was obtained from Santa Cruz Biotechnology. For the Li-Cor Odyssey, Alexa Fluor 680 donkey IgG from Life Technologies and anti-rabbit IgG DyLight 680 conjugate and anti-mouse IgG DyLight 800 conjugate from Cell Signaling Technology were used. For high-content or confocal microscopy imaging, primary antibodies were visualized using Alexa Fluor 488-conjugated, highly cross-adsorbed goat anti-mouse or Alexa Fluor 546-conjugated, highly cross-adsorbed goat anti-rabbit secondary antibodies (Invitrogen). PD0325901 MEK inhibitor and MG132 proteasome inhibitor were purchased from Selleck Chemicals. NGF growth factor and TPA phorbol ester were purchased from PeproTech and Sigma, respectively.
Molecular Biology.
EGR1 promoter fragments (54), DUSP5-Myc, DUSP5R53/54A-Myc, DUSP5NLS-Myc, and DUSP6-Myc ORFs (21, 33) were subcloned into pAd5 MCS K-N pA Ad shuttle vectors (Gene Transfer Vector Core, University of Iowa). HA-tagged HRASQ61L (22), BRAFV600E (a kind gift from Catrin Pritchard, University of Leicester, Leicester, United Kingdom) and ∆CRAF-ER (a kind gift from Simon Cook, Babraham Institute, Babraham, United Kingdom) were subcloned into pAd5 CMV K-N pA Ad shuttle vectors. Ad vectors were generated and purified as previously described (22, 55).
Cell Fractionation and Confocal Microscopy.
Nuclear and cytoplasmic fractions of cell lysates were isolated from primary MEFs using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) according to the manufacturer’s instructions. For confocal microscopy, MEFs were plated on glass coverslips, treated, and immunostained as indicated in the figure legends and imaged using a Zeiss LSM510META laser-scanning confocal microscope (Zeiss).
Western Blotting.
Whole-cell lysates were prepared and analyzed using SDS/PAGE using Novex 4–12% gradient gels (Invitrogen) as described previously (56), before quantification using primary antibodies and fluorescently tagged secondary antibodies on a Li-Cor Odyssey infrared imager using Image Studio software (Li-Cor Biosciences).
Senescence-Associated β-Gal Assay.
Cells were assessed for senescence-associated β-gal staining using a kit obtained from Cell Signaling Technology (9860) according to manufacturer’s instructions. Briefly, MEFs were plated onto 96-well or 6-well plates and treated as indicated in figure legends, before fixation and β-gal staining at pH 6, 37 °C for 16 h. Cells were stored under 70% (vol/vol) glycerol and counterstained using 300 nM DAPI. ImageJ freeware was used to assess the total number of cells using the DAPI stain and “particle finder” macro, and β-gal–positive cells were scored manually as being senescent. One image per well was scored for triplicate samples within each independent experiment.
RAS-GTP Pulldowns.
The 1.5 × 106 MEFs were seeded onto 15-cm plates. Following stimulation, cells were washed twice in PBS and lysed in lysis buffer (containing Halt protease and phosphatase inhibitors from Invitrogen) and detection using an Active RAS Detection Kit (Cell Signaling Technology, 8821) according to the manufacturer’s instructions. Briefly, 600 µg of total protein was incubated with 80 µg of CRAF-RBD-GST beads for 1 h at 4 °C. Beads were then washed three times before Ras-GTP was disassociated from the CRAF-RBD-GST beads with the addition of 2× SDS sample buffer at 95 °C for 5 min. Immunoblots were then performed as described.
Focus Formation Assays.
Phoenix cells were transfected with either pBABE-puro empty vector or pBABE-puro HRASQ61L or BRAFV600E using Fugene6 (Promega) in OptiMEM (Invitrogen). Media were changed after 6 h to OptiMEM with 10% (vol/vol) FBS, and again the following morning to DMEM with 10% (vol/vol) FBS. Cells were then incubated for 48 h at 32 °C to allow virus production before infection of immortalized WT and DUSP5 knockout MEFs. Cells were then reseeded onto 10-cm dishes (in triplicate) in DMEM containing 5% (vol/vol) FBS. The medium was changed every 2–3 d until cells reached confluence and transformed foci appeared. Cells were fixed in ice-cold 100% methanol and stained with 0.5% crystal violet diluted in 25% methanol. Foci ≥2 mm were counted on each plate, and the mean values calculated.
TaqMan Reverse Transcription-qPCR Analysis.
RNA was isolated from cells using an RNeasy MiniPrep Kit (Qiagen) according to the manufacturer’s instructions. TaqMan reverse-transcription-qPCR was carried out as described previously (32) using prevalidated probes (Applied Biosystems), and levels were normalized to a β-actin internal control.
Data Handling and Statistical Analysis.
All plots and statistical analyses of data were performed using either GraphPad Prism or MATLAB. Statistical tests used are indicated in the figure legends. Differences achieving P < 0.05 were accepted as statistically significant. Relative fluorescence intensity data from HCM experiments were normalized by expressing all data as relative to control (usually WT) samples within each independent experiment before data pooling for each data set and statistical analysis. Western blotting densitometry quantification values were corrected for loading using a housekeeping control and normalized by expressing all data as relative to either control WT samples or to the mean band intensity for all samples in the blot for each replicate experiment before statistical analysis.
All data used to create the figures in this paper are openly available from the University of Bath data archive (https://doi.org/10.15125/BATH-00317).
Discussion
We demonstrate that DUSP5, a phosphatase that is induced by ERK activity and specifically inactivates and anchors ERK in the nucleus (19–22), can also increase cytoplasmic ERK activation (Fig. 1). These positive effects of DUSP5 are dependent upon the relief of upstream kinase inhibition (Figs. 2 and 4), the nuclear localization of DUSP5 (Fig. 3) and rapid DUSP5 degradation (Fig. 5). Expression of the BRAFV600E oncogene, which is present in ∼8% of all human tumors (43) and is refractory to ERK feedback inhibition (5, 26), reprograms the regulatory role of DUSP5 to facilitate cell proliferation and transformation (Figs. 6 and 7). Critically, these data show that members of the MKP/DUSP family can exert substantial effects through ‘feedback relief' as a consequence of their interaction with target MAPKs. As most MKP/DUSPs have distinct MAPK substrate affinities, half-lives and promoter regulation (10, 11), our data have important consequences for understanding how the MKP/DUSPs influence MAPK signaling in different contexts, including malignant progression.
Although our data show that DUSP5 can propagate ERK signaling in response to sustained stimuli, such as serum, TPA or NGF (Fig. 1 and Fig. S3), it is likely that these effects are stimulus-specific. For example, it is difficult to envisage a mechanism where DUSP5 could possibly extend ERK signaling induced by transient stimuli that are desensitized before significant levels of DUSP5 have been expressed (<60 min). We used a conceptual three-component mathematical model in our study, which mimicked the propagation of ERK signaling by DUSP5 (Figs. 4 and 5 and Fig. S5A). A key predicted requirement for this signal propagation in our mathematical model is the highly nonlinear inhibition of upstream kinases by ERK (Fig. 4A). This prediction was tested in cells using expression of either a conditionally activatable ΔCRAF-ER fusion or the BRAFV600E oncogene, both of which disable ERK-mediated feedback inhibition of RAF (26, 30) and reversed the function of DUSP5, from propagator to inhibitor of ERK activity (Figs. 4 and 6). Our data therefore suggest that the regulatory effects of DUSP5 are profoundly dependent upon the relief of feedback between ERK and upstream kinases. Thus, signals that cause ERK activation via MEK-activating kinases other than RAF [such as Mos, MEKK1 (MAP3K1), and COT/Tpl2 (MAP3K8), which are not typically feedback inhibited by ERK (12, 44)] may also reverse the function of DUSP5 in a similar manner to ΔCRAF-ER or BRAFV600E (Figs. 4 and 6).
The notion that DUSP5 (and likely other MKP/DUSPs) should mediate effects through ‘feedback relief’ is perhaps to be expected in light of the number of studies demonstrating that pharmacological inhibitors of the ERK pathway cause similar effects (25, 30, 36, 37, 45, 46). Our results indicate that the nuclear sequestration of inactive ERK by DUSP5 evokes compensatory increases in upstream RAF and MEK activation through feedback relief (Fig. 2A and Fig. S4), and that the release of ERK following DUSP5 degradation causes a rebound in ERK activity (Fig. 5). Strikingly, we found that mutation of the nuclear localization signal (NLS) of DUSP5, which impedes nuclear import (21), causes potent inhibition of ERK throughout the cell. Similar data were seen following expression of DUSP6 (a cytoplasmic paralog of DUSP5 (11)), indicating that the nuclear localization of DUSP5 is a requirement for its propagation of cytoplasmic ERK activity (Fig. 3). These data are somewhat surprising, given that the inclusion of cellular compartmentalization in our mathematical model made no difference to our in silico simulations of ERK signal propagation by DUSP5 (compare Fig. 4A and Fig. S5 A and B). Our mathematical model is therefore predictive of DUSP5 behavior when it is confined to the nucleus, but cannot at present account for why cytoplasmic MKP/DUSPs may, in contrast, attenuate ERK activity so potently. Nevertheless, our experimental data clearly indicate that DUSP5 and DUSP6 play distinct roles in regulating ERK that are likely attributable to their subcellular distribution. Resolving the molecular basis for these differences will be the subject of future investigation.
Our data indicate that, despite the fact that DUSP5 can regulate both nuclear and cytoplasmic ERK signaling (22) (Fig. 1), its deletion does not obviously influence normal cell proliferation in primary or immortalized MEFs (Fig. 6). Furthermore, mice entirely lacking DUSP5 develop normally and display no obvious adult phenotype (22), suggesting that the physiological role of DUSP5 may only be fully apparent in the event of stress or pathological challenge. In support of this, our previous data demonstrate that DUSP5 suppresses HRASQ61L-driven tumors in a DMBA/TPA chemical carcinogenesis model (22). Our current study also supports this view by showing that DUSP5 facilitates BRAFV600E-driven cell proliferation and transformation by preventing ERK hyperactivation and senescence in MEFs (Figs. 6 and 7). These data may explain why DUSP5 is typically retained or even elevated in cells derived from advanced BRAFV600E tumors (25, 26). Our results also support a growing body of evidence demonstrating that both normal and tumor cells are under strong selection pressure to maintain ERK activity within a narrow threshold range, such that both ERK inhibition and hyperactivation may cause tumor suppression (14, 15, 18, 41). It is therefore possible that the inhibition or loss of DUSP5 may have beneficial effects in BRAFV600E tumors by causing ERK hyperactivation, as has been demonstrated for DUSP6, whose inhibition or deletion causes senescence in BCR-ABL-driven adult lymphoblastic leukemia (17).
The data in our present study indicates that Dusp5 deletion can alter MEK inhibitor efficacy (Fig. 6C). Future work will therefore focus on the possible interactions between DUSP5 and chemical inhibitors of ERK pathway components to better inform personalized therapy. Although it is logical to test compounds already in clinical use, it will be important to also examine emerging ERK-targeted compounds that are reported to act by inhibiting ERK dimerization (47) or nuclear translocation (48), as well as explore the possibility that DUSP5-specific inhibitors may be developed. It is likely that the most effective extension of our current work will be to determine the effects of Dusp5 loss in human cancer cell lines with different oncogenic backgrounds combined with the use of murine models that allow tissue-specific conditional expression of BRAFV600E and Dusp5 deletion to further define the in vivo significance of our results. Finally, DUSP5 has recently been shown to regulate ERK-dependent gene expression and survival in eosinophils, and to modulate responses to helminth infection in mice (49). The latter results indicate that the relevance of our work may extend beyond the regulation of oncogenic signaling to other diseases in which abnormal ERK activity has been implicated. These include inflammatory disease, cardiac hypertrophy, metabolic disorders and neurodegenerative conditions, such as Alzheimer’s disease (50–53).
Experimental Procedures
Cell Culture and Viral Infection.
Primary and immortalized MEFs were derived and cultured as previously described (22). PC12 cells (a kind gift from Shelley Allen, University of Bristol, Bristol, United Kingdom) were maintained in RPMI medium 1640 containing GlutaMAX (Invitrogen), 5% horse serum (Invitrogen) and 10% FBS (Invitrogen) in flasks coated with type I collagen from rat tail (Sigma). For experiments, cells were infected with Ad vectors for 4–6 h.
High Content Microscopy and Analysis.
Cells were immunostained in 96-well black-wall imaging plates (Corning, 3904) as previously described (22) and imaged using an IN Cell Analyzer 2000 (GE Healthcare) microscope using a 10× or 20× objective lens, typically acquiring 500–2000 individual cells (2–6 fields) per well in duplicate or triplicate wells per condition. All exposures were set such that signals were >30 AFU above background and maximal <500 AFU below the maximum dynamic range. S-phase pulse labeling experiments were carried out using 10 μM 5-ethynyl-2′-deoxyuridine (EdU) fluorescent thymidine analog (EdU) for 2 h before cell fixation and detected using a Click-iT EdU Alexa Fluor 647 HCS Assay Kit (Invitrogen), according to manufacturer’s instructions. In multiplex assays, EdU Alexa Fluor 647 detection was carried out before antibody staining. Analysis was performed using IN Cell Developer software (GE Healthcare) and a custom algorithm using DAPI and p-ERK images to define nuclear and cytoplasmic regions, respectively, which were used as a binary mask applied to images from all fluorophores to derive measures on a per cell, per compartment basis.
Details of all other methodology used, including vector manufacture and mathematical modeling, are detailed in SI Materials and Methods.
Acknowledgments
We thank Dr. Simon Cook (Babraham Institute), Dr. Susanne Gebhard (University of Bath), Dr. Owen Sansom (Beatson Institute), and Dr. Albena Dinkova-Kostova (University of Dundee) for critical reading of the manuscript. This work was supported by Cancer Research UK Programme Grant C8227/A12053 (to S.M.K.), Royal Society Research Grant 2010/R2 (to C.J.C.), Medical Research Council Research Grant MR/N020790/1 (to S.M.K. and C.J.C.), a Dundee Cancer Centre Studentship (to A.M.K.), and a Commonwealth Scholarship (to C.B.). T.R. gratefully acknowledges the support of the Royal Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data used to create the figures in this paper are available from the University of Bath data archive (https://doi.org/10.15125/BATH-00317).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614684114/-/DCSupplemental.
References
- 1.Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: Mechanisms for providing signaling specificity. J Cell Sci. 2005;118(Pt 14):2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- 2.Marshall CJ. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 3.Lavoie H, Therrien M. Regulation of RAF protein kinases in ERK signalling. Nat Rev Mol Cell Biol. 2015;16(5):281–298. doi: 10.1038/nrm3979. [DOI] [PubMed] [Google Scholar]
- 4.Dougherty MK, et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17(2):215–224. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 5.Ritt DA, Monson DM, Specht SI, Morrison DK. Impact of feedback phosphorylation and Raf heterodimerization on normal and mutant B-Raf signaling. Mol Cell Biol. 2010;30(3):806–819. doi: 10.1128/MCB.00569-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rushworth LK, Hindley AD, O’Neill E, Kolch W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol Cell Biol. 2006;26(6):2262–2272. doi: 10.1128/MCB.26.6.2262-2272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buday L, Warne PH, Downward J. Downregulation of the Ras activation pathway by MAP kinase phosphorylation of Sos. Oncogene. 1995;11(7):1327–1331. [PubMed] [Google Scholar]
- 8.Zakrzewska M, et al. ERK-mediated phosphorylation of fibroblast growth factor receptor 1 on Ser777 inhibits signaling. Sci Signal. 2013;6(262):ra11. doi: 10.1126/scisignal.2003087. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Huang Y, Jiang J, Frank SJ. ERK-dependent threonine phosphorylation of EGF receptor modulates receptor downregulation and signaling. Cell Signal. 2008;20(11):2145–2155. doi: 10.1016/j.cellsig.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caunt CJ, Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs): Shaping the outcome of MAP kinase signalling. FEBS J. 2013;280(2):489–504. doi: 10.1111/j.1742-4658.2012.08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26(22):3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 12.Caunt CJ, Sale MJ, Smith PD, Cook SJ. MEK1 and MEK2 inhibitors and cancer therapy: The long and winding road. Nat Rev Cancer. 2015;15(10):577–592. doi: 10.1038/nrc4000. [DOI] [PubMed] [Google Scholar]
- 13.Kidger AM, Keyse SM. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs) Semin Cell Dev Biol. 2016;50:125–132. doi: 10.1016/j.semcdb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das Thakur M, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494(7436):251–255. doi: 10.1038/nature11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun C, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508(7494):118–122. doi: 10.1038/nature13121. [DOI] [PubMed] [Google Scholar]
- 16.Deschênes-Simard X, Kottakis F, Meloche S, Ferbeyre G. ERKs in cancer: Friends or foes? Cancer Res. 2014;74(2):412–419. doi: 10.1158/0008-5472.CAN-13-2381. [DOI] [PubMed] [Google Scholar]
- 17.Shojaee S, et al. Erk negative feedback control enables pre-B cell transformation and represents a therapeutic target in acute lymphoblastic leukemia. Cancer Cell. 2015;28(1):114–128. doi: 10.1016/j.ccell.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriceau G, et al. Tunable-combinatorial mechanisms of acquired resistance limit the efficacy of BRAF/MEK cotargeting but result in melanoma drug addiction. Cancer Cell. 2015;27(2):240–256. doi: 10.1016/j.ccell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buffet C, et al. Dual specificity phosphatase 5, a specific negative regulator of ERK signaling, is induced by serum response factor and Elk-1 transcription factor. PLoS One. 2015;10(12):e0145484. doi: 10.1371/journal.pone.0145484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kucharska A, Rushworth LK, Staples C, Morrice NA, Keyse SM. Regulation of the inducible nuclear dual-specificity phosphatase DUSP5 by ERK MAPK. Cell Signal. 2009;21(12):1794–1805. doi: 10.1016/j.cellsig.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Mandl M, Slack DN, Keyse SM. Specific inactivation and nuclear anchoring of extracellular signal-regulated kinase 2 by the inducible dual-specificity protein phosphatase DUSP5. Mol Cell Biol. 2005;25(5):1830–1845. doi: 10.1128/MCB.25.5.1830-1845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rushworth LK, et al. Dual-specificity phosphatase 5 regulates nuclear ERK activity and suppresses skin cancer by inhibiting mutant Harvey-Ras (HRasQ61L)-driven SerpinB2 expression. Proc Natl Acad Sci USA. 2014;111(51):18267–18272. doi: 10.1073/pnas.1420159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin SH, Park SY, Kang GH. Down-regulation of dual-specificity phosphatase 5 in gastric cancer by promoter CpG island hypermethylation and its potential role in carcinogenesis. Am J Pathol. 2013;182(4):1275–1285. doi: 10.1016/j.ajpath.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Cai C, et al. Down-regulation of dual-specificity phosphatase 5 predicts poor prognosis of patients with prostate cancer. Int J Clin Exp Med. 2015;8(3):4186–4194. [PMC free article] [PubMed] [Google Scholar]
- 25.Montero-Conde C, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3(5):520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pratilas CA, et al. (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci USA. 2009;106(11):4519–4524. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yun J, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325(5947):1555–1559. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volmat V, Camps M, Arkinstall S, Pouysségur J, Lenormand P. The nucleus, a site for signal termination by sequestration and inactivation of p42/p44 MAP kinases. J Cell Sci. 2001;114(Pt 19):3433–3443. doi: 10.1242/jcs.114.19.3433. [DOI] [PubMed] [Google Scholar]
- 29.Nakakuki T, et al. Ligand-specific c-Fos expression emerges from the spatiotemporal control of ErbB network dynamics. Cell. 2010;141(5):884–896. doi: 10.1016/j.cell.2010.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturm OE, et al. The mammalian MAPK/ERK pathway exhibits properties of a negative feedback amplifier. Sci Signal. 2010;3(153):ra90. doi: 10.1126/scisignal.2001212. [DOI] [PubMed] [Google Scholar]
- 31.Omerovic J, Clague MJ, Prior IA. Phosphatome profiling reveals PTPN2, PTPRJ and PTEN as potent negative regulators of PKB/Akt activation in Ras-mutated cancer cells. Biochem J. 2010;426(1):65–72. doi: 10.1042/BJ20091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekerot M, et al. Negative-feedback regulation of FGF signalling by DUSP6/MKP-3 is driven by ERK1/2 and mediated by Ets factor binding to a conserved site within the DUSP6/MKP-3 gene promoter. Biochem J. 2008;412(2):287–298. doi: 10.1042/BJ20071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groom LA, Sneddon AA, Alessi DR, Dowd S, Keyse SM. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 1996;15(14):3621–3632. [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsson M, Mathers J, Dickinson RJ, Mandl M, Keyse SM. Both nuclear-cytoplasmic shuttling of the dual specificity phosphatase MKP-3 and its ability to anchor MAP kinase in the cytoplasm are mediated by a conserved nuclear export signal. J Biol Chem. 2004;279(40):41882–41891. doi: 10.1074/jbc.M406720200. [DOI] [PubMed] [Google Scholar]
- 35.Marchetti S, et al. Extracellular signal-regulated kinases phosphorylate mitogen-activated protein kinase phosphatase 3/DUSP6 at serines 159 and 197, two sites critical for its proteasomal degradation. Mol Cell Biol. 2005;25(2):854–864. doi: 10.1128/MCB.25.2.854-864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fritsche-Guenther R, et al. Strong negative feedback from Erk to Raf confers robustness to MAPK signalling. Mol Syst Biol. 2011;7(489):489. doi: 10.1038/msb.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen KJ, Moyer CB, Janes KA. Network architecture predisposes an enzyme to either pharmacologic or genetic targeting. Cell Syst. 2016;2(2):112–121. doi: 10.1016/j.cels.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuels ML, Weber MJ, Bishop JM, McMahon M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol Cell Biol. 1993;13(10):6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic Ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12(19):2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woods D, et al. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17(9):5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunet A, et al. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18(3):664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 44.Cuevas BD, Abell AN, Johnson GL. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 2007;26(22):3159–3171. doi: 10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- 45.Friday BB, et al. BRAF V600E disrupts AZD6244-induced abrogation of negative feedback pathways between extracellular signal-regulated kinase and Raf proteins. Cancer Res. 2008;68(15):6145–6153. doi: 10.1158/0008-5472.CAN-08-1430. [DOI] [PubMed] [Google Scholar]
- 46.Lito P, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22(5):668–682. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrero A, et al. Small molecule inhibition of erk dimerization prevents tumorigenesis by RAS-ERK pathway oncogenes. Cancer Cell. 2015;28(2):170–182. doi: 10.1016/j.ccell.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Michailovici I, et al. Nuclear to cytoplasmic shuttling of ERK promotes differentiation of muscle stem/progenitor cells. Development. 2014;141(13):2611–2620. doi: 10.1242/dev.107078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmes DA, Yeh JH, Yan D, Xu M, Chan AC. Dusp5 negatively regulates IL-33-mediated eosinophil survival and function. EMBO J. 2015;34(2):218–235. doi: 10.15252/embj.201489456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabio G, Davis RJ. TNF and MAP kinase signalling pathways. Semin Immunol. 2014;26(3):237–245. doi: 10.1016/j.smim.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010;277(1):22–29. doi: 10.1111/j.1742-4658.2009.07367.x. [DOI] [PubMed] [Google Scholar]
- 53.Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: Targeting Raf/MEK/ERK1/2-signaling. Int J Biochem Cell Biol. 2009;41(12):2351–2355. doi: 10.1016/j.biocel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Caunt CJ, McArdle CA. Stimulus-induced uncoupling of extracellular signal-regulated kinase phosphorylation from nuclear localization is dependent on docking domain interactions. J Cell Sci. 2010;123(Pt 24):4310–4320. doi: 10.1242/jcs.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 2000;7(12):1034–1038. doi: 10.1038/sj.gt.3301197. [DOI] [PubMed] [Google Scholar]
- 56.Staples CJ, Owens DM, Maier JV, Cato ACB, Keyse SM. Cross-talk between the p38alpha and JNK MAPK pathways mediated by MAP kinase phosphatase-1 determines cellular sensitivity to UV radiation. J Biol Chem. 2010;285(34):25928–25940. doi: 10.1074/jbc.M110.117911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lidke DS, et al. ERK nuclear translocation is dimerization-independent but controlled by the rate of phosphorylation. J Biol Chem. 2010;285(5):3092–3102. doi: 10.1074/jbc.M109.064972. [DOI] [PMC free article] [PubMed] [Google Scholar]