Abstract
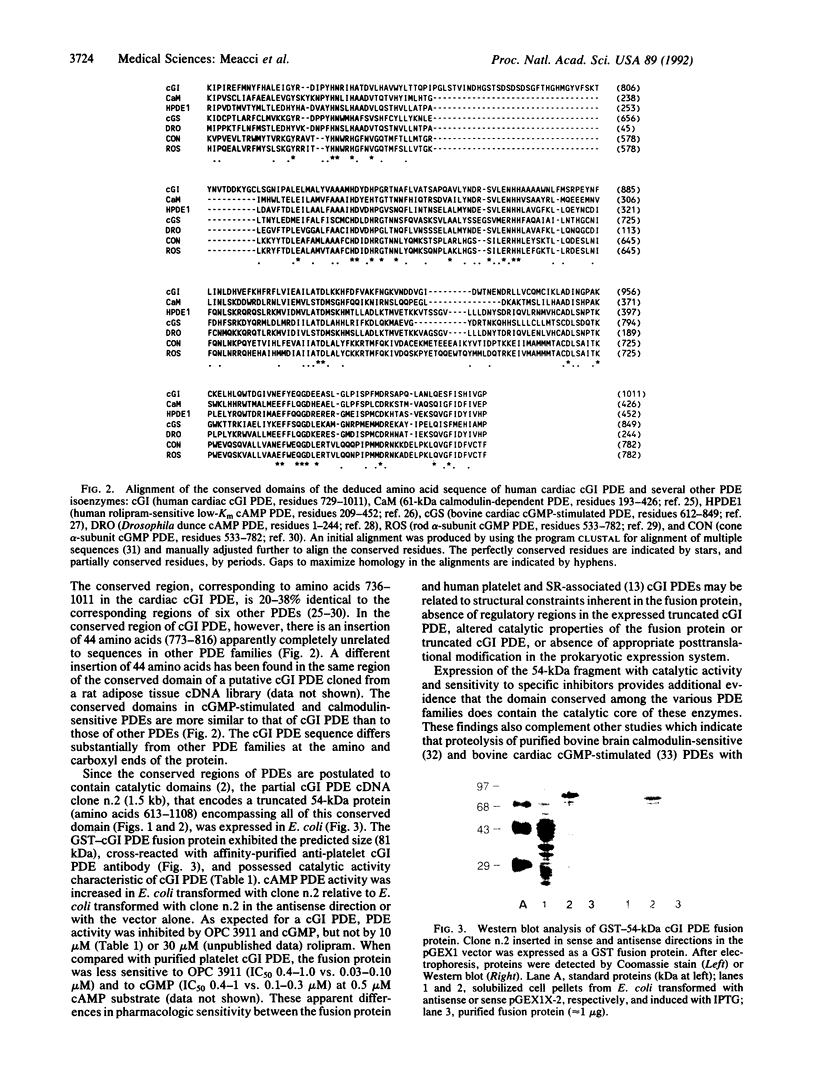
We have cloned a cDNA for a myocardial cGMP-inhibited cAMP phosphodiesterase (cGI PDE) from a human heart cDNA library in lambda Zap II. The open reading frame [3.5 kilobases (kb)] of cDNA clone n.13.2 (7.7 kb) encodes a protein of 125 kDa. In Northern blots of total human ventricle RNA, a single mRNA species (8.3 kb) hybridized with a 4-kb EcoRI restriction fragment of clone n.13.2 cDNA (containing the entire open reading frame). The carboxyl-terminal region of the deduced amino acid sequence of the cGI PDE contains the putative catalytic domain conserved among mammalian PDE families. A partial cDNA clone, n.2, encoding a truncated, 54-kDa cGI PDE containing the conserved domain was expressed as a catalytically active fusion protein in Escherichia coli. cAMP hydrolytic activity was inhibited by cGMP and OPC 3911 but not by rolipram. Thus, this report provides direct proof that the conserved domain contains the catalytic core of cGI PDEs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez R., Banerjee G. L., Bruno J. J., Jones G. L., Littschwager K., Strosberg A. M., Venuti M. C. A potent and selective inhibitor of cyclic AMP phosphodiesterase with potential cardiotonic and antithrombotic properties. Mol Pharmacol. 1986 Jun;29(6):554–560. [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Beier N., Walsh K. A., Beavo J. A. Identification of a conserved domain among cyclic nucleotide phosphodiesterases from diverse species. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9308–9312. doi: 10.1073/pnas.83.24.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Prusti R. K., LeTrong H., Sonnenburg W. K., Mullaney P. J., Walsh K. A., Beavo J. A. Identification of a noncatalytic cGMP-binding domain conserved in both the cGMP-stimulated and photoreceptor cyclic nucleotide phosphodiesterases. Proc Natl Acad Sci U S A. 1990 Jan;87(1):288–292. doi: 10.1073/pnas.87.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. N., Denome S., Davis R. L. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9313–9317. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colucci W. S., Wright R. F., Braunwald E. New positive inotropic agents in the treatment of congestive heart failure. Mechanisms of action and recent clinical developments. 1. N Engl J Med. 1986 Jan 30;314(5):290–299. doi: 10.1056/NEJM198601303140506. [DOI] [PubMed] [Google Scholar]
- Colucci W. S., Wright R. F., Braunwald E. New positive inotropic agents in the treatment of congestive heart failure. Mechanisms of action and recent clinical developments. 2. N Engl J Med. 1986 Feb 6;314(6):349–358. doi: 10.1056/NEJM198602063140605. [DOI] [PubMed] [Google Scholar]
- Degerman E., Manganiello V. C., Newman A. H., Rice K. C., Belfrage P. Purification, properties and polyclonal antibodies for the particulate, low Km cAMP phosphodiesterase from bovine adipose tissue. Second Messengers Phosphoproteins. 1988;12(4):171–182. [PubMed] [Google Scholar]
- Degerman E., Smith C. J., Tornqvist H., Vasta V., Belfrage P., Manganiello V. C. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc Natl Acad Sci U S A. 1990 Jan;87(2):533–537. doi: 10.1073/pnas.87.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. G., Mannarino A. F., Colman R. W. cAMP-mediated phosphorylation of the low-Km cAMP phosphodiesterase markedly stimulates its catalytic activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9071–9075. doi: 10.1073/pnas.85.23.9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. A., Reifsnyder D. H., Gallis B., Cadd G. G., Beavo J. A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol Pharmacol. 1986 May;29(5):506–514. [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Stith-Coleman I. E., Vaughan M. Proteolytic activation of calmodulin-dependent cyclic nucleotide phosphodiesterase. J Biol Chem. 1985 Jul 25;260(15):9009–9015. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Li T. S., Volpp K., Applebury M. L. Bovine cone photoreceptor cGMP phosphodiesterase structure deduced from a cDNA clone. Proc Natl Acad Sci U S A. 1990 Jan;87(1):293–297. doi: 10.1073/pnas.87.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livi G. P., Kmetz P., McHale M. M., Cieslinski L. B., Sathe G. M., Taylor D. P., Davis R. L., Torphy T. J., Balcarek J. M. Cloning and expression of cDNA for a human low-Km, rolipram-sensitive cyclic AMP phosphodiesterase. Mol Cell Biol. 1990 Jun;10(6):2678–2686. doi: 10.1128/mcb.10.6.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka H., Ito M., Nakano T., Naka M., Tanaka T. Effects of amrinone and enoximone on the subclasses of cyclic AMP phosphodiesterase from human heart and kidney. J Cardiovasc Pharmacol. 1990 Feb;15(2):302–307. doi: 10.1097/00005344-199002000-00018. [DOI] [PubMed] [Google Scholar]
- Movsesian M. A., Smith C. J., Krall J., Bristow M. R., Manganiello V. C. Sarcoplasmic reticulum-associated cyclic adenosine 5'-monophosphate phosphodiesterase activity in normal and failing human hearts. J Clin Invest. 1991 Jul;88(1):15–19. doi: 10.1172/JCI115272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C. D., Challiss R. A., Shahid M. Differential modulation of tissue function and therapeutic potential of selective inhibitors of cyclic nucleotide phosphodiesterase isoenzymes. Trends Pharmacol Sci. 1991 Jan;12(1):19–27. doi: 10.1016/0165-6147(91)90484-a. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Gubanov V. V., Khramtsov N. V., Ischenko K. A., Zagranichny V. E., Muradov K. G., Shuvaeva T. M., Lipkin V. M. Cyclic GMP phosphodiesterase from bovine retina. Amino acid sequence of the alpha-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1987 Oct 19;223(1):169–173. doi: 10.1016/0014-5793(87)80530-6. [DOI] [PubMed] [Google Scholar]
- Silver P. J., Allen P., Etzler J. H., Hamel L. T., Bentley R. G., Pagani E. D. Cellular distribution and pharmacological sensitivity of low Km cyclic nucleotide phosphodiesterase isozymes in human cardiac muscle from normal and cardiomyopathic subjects. Second Messengers Phosphoproteins. 1990;13(1):13–25. [PubMed] [Google Scholar]
- Smith C. J., Vasta V., Degerman E., Belfrage P., Manganiello V. C. Hormone-sensitive cyclic GMP-inhibited cyclic AMP phosphodiesterase in rat adipocytes. Regulation of insulin- and cAMP-dependent activation by phosphorylation. J Biol Chem. 1991 Jul 15;266(20):13385–13390. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stroop S. D., Charbonneau H., Beavo J. A. Direct photolabeling of the cGMP-stimulated cyclic nucleotide phosphodiesterase. J Biol Chem. 1989 Aug 15;264(23):13718–13725. [PubMed] [Google Scholar]
- Trong H. L., Beier N., Sonnenburg W. K., Stroop S. D., Walsh K. A., Beavo J. A., Charbonneau H. Amino acid sequence of the cyclic GMP stimulated cyclic nucleotide phosphodiesterase from bovine heart. Biochemistry. 1990 Nov 6;29(44):10280–10288. doi: 10.1021/bi00496a018. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Kobylarz-Singer D. C., Quade M. M., Steffen R. P., Kaplan H. R. Multiple molecular forms of phosphodiesterase and the regulation of cardiac muscle contractility. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(7):513–527. [PubMed] [Google Scholar]