Abstract
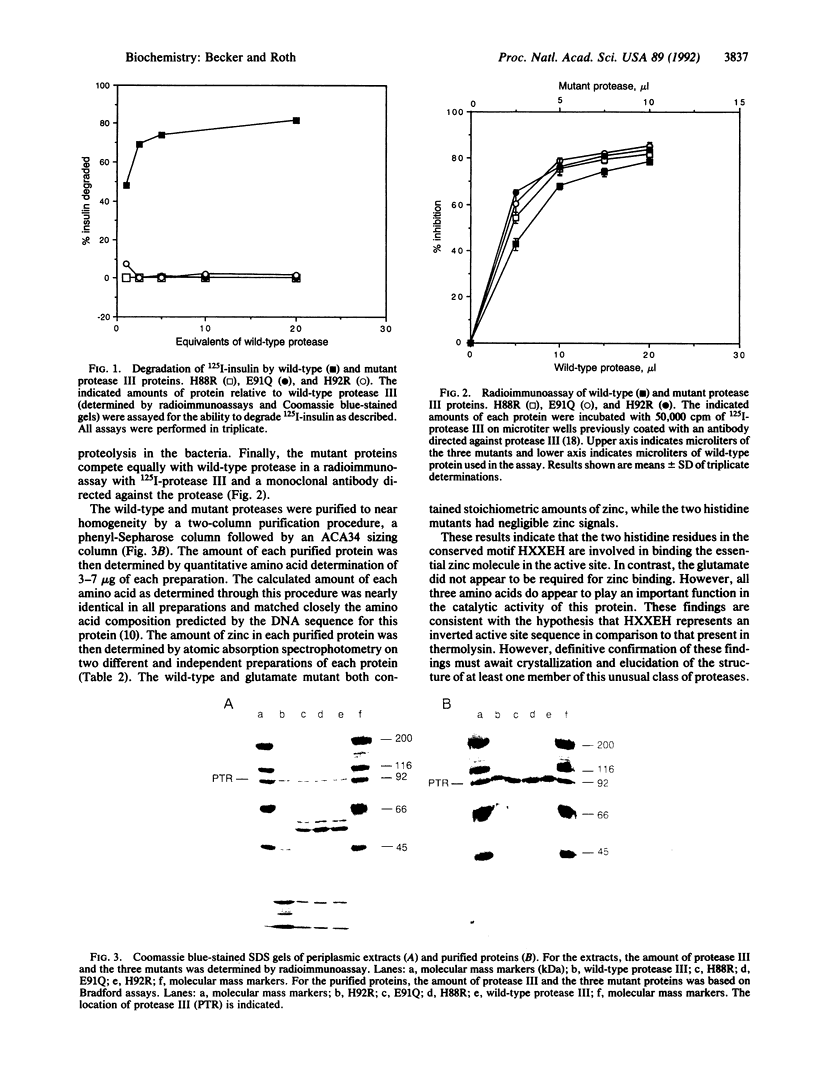
An unusual active site has been identified in a family of zinc metalloendopeptidases that includes bacterial protease III and the human and Drosophila insulin-degrading enzymes. All of these enzymes have been characterized as metalloendopeptidases and purified protease III has been shown to contain stoichiometric levels of zinc. However, all three proteases lack the consensus sequence (HEXXH) described in the active site of other zinc metalloendopeptidases. Instead, these proteases contain an inversion of this motif, HXXEH. To determine whether this region could represent the active site in these proteins, the two histidines in protease III were individually mutated to arginine and the glutamate was mutated to glutamine. All three mutants were devoid of proteolytic activity toward an exogenous substrate, insulin, as compared to the wild-type protease. Three lines of evidence indicate that this loss of activity in the mutants is not due to distortion of the three-dimensional structure of the protein: (i) the mutants are secreted into the periplasmic space and chromatograph normally; (ii) all three mutants are expressed at levels nearly identical to wild-type protein and do not appear to have an increased susceptibility to proteolysis in the bacteria; and (iii) the mutants compete equally with wild-type protein in a radioimmunoassay. The purified wild-type and glutamate mutants were found to contain stoichiometric amounts of zinc by atomic absorption spectrophotometry, whereas both histidine mutants had negligible zinc signals. These findings are consistent with this region being the active site in this protein, with the histidine residues coordinating the essential zinc atom and the glutamate involved in catalysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affholter J. A., Fried V. A., Roth R. A. Human insulin-degrading enzyme shares structural and functional homologies with E. coli protease III. Science. 1988 Dec 9;242(4884):1415–1418. doi: 10.1126/science.3059494. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Butler P. E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Zipser D. Purification and characterization of protease III from Escherichia coli. J Biol Chem. 1979 Jun 10;254(11):4698–4706. [PubMed] [Google Scholar]
- Devault A., Lazure C., Nault C., Le Moual H., Seidah N. G., Chrétien M., Kahn P., Powell J., Mallet J., Beaumont A. Amino acid sequence of rabbit kidney neutral endopeptidase 24.11 (enkephalinase) deduced from a complementary DNA. EMBO J. 1987 May;6(5):1317–1322. doi: 10.1002/j.1460-2075.1987.tb02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A., Nault C., Zollinger M., Fournie-Zaluski M. C., Roques B. P., Crine P., Boileau G. Expression of neutral endopeptidase (enkephalinase) in heterologous COS-1 cells. Characterization of the recombinant enzyme and evidence for a glutamic acid residue at the active site. J Biol Chem. 1988 Mar 15;263(8):4033–4040. [PubMed] [Google Scholar]
- Devault A., Sales V., Nault C., Beaumont A., Roques B., Crine P., Boileau G. Exploration of the catalytic site of endopeptidase 24.11 by site-directed mutagenesis. Histidine residues 583 and 587 are essential for catalysis. FEBS Lett. 1988 Apr 11;231(1):54–58. doi: 10.1016/0014-5793(88)80701-4. [DOI] [PubMed] [Google Scholar]
- Ding L., Becker A. B., Suzuki A., Roth R. A. Comparison of the enzymatic and biochemical properties of human insulin-degrading enzyme and Escherichia coli protease III. J Biol Chem. 1992 Feb 5;267(4):2414–2420. [PubMed] [Google Scholar]
- Duckworth W. C. Insulin degradation: mechanisms, products, and significance. Endocr Rev. 1988 Aug;9(3):319–345. doi: 10.1210/edrv-9-3-319. [DOI] [PubMed] [Google Scholar]
- Dumermuth E., Sterchi E. E., Jiang W. P., Wolz R. L., Bond J. S., Flannery A. V., Beynon R. J. The astacin family of metalloendopeptidases. J Biol Chem. 1991 Nov 15;266(32):21381–21385. [PubMed] [Google Scholar]
- Dykstra C. C., Kushner S. R. Physical characterization of the cloned protease III gene from Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):1055–1059. doi: 10.1128/jb.163.3.1055-1059.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farach H. A., Jr, Mundy D. I., Strittmatter W. J., Lennarz W. J. Evidence for the involvement of metalloendoproteases in the acrosome reaction in sea urchin sperm. J Biol Chem. 1987 Apr 25;262(12):5483–5487. [PubMed] [Google Scholar]
- Finch P. W., Wilson R. E., Brown K., Hickson I. D., Emmerson P. T. Complete nucleotide sequence of the Escherichia coli ptr gene encoding protease III. Nucleic Acids Res. 1986 Oct 10;14(19):7695–7703. doi: 10.1093/nar/14.19.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli V., Yang M. J., Suda K., Lustig A., Schatz G. The MAS-encoded processing protease of yeast mitochondria. Overproduction and characterization of its two nonidentical subunits. J Biol Chem. 1990 Nov 5;265(31):19216–19222. [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Hartl F. U., Neupert W. Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science. 1990 Feb 23;247(4945):930–938. doi: 10.1126/science.2406905. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Bouvier J., Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989 Jan 2;242(2):211–214. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
- Kayalar C., Wong W. T. Metalloendoprotease inhibitors which block the differentiation of L6 myoblasts inhibit insulin degradation by the endogenous insulin-degrading enzyme. J Biol Chem. 1989 May 25;264(15):8928–8934. [PubMed] [Google Scholar]
- Kuo W. L., Gehm B. D., Rosner M. R. Cloning and expression of the cDNA for a Drosophila insulin-degrading enzyme. Mol Endocrinol. 1990 Oct;4(10):1580–1591. doi: 10.1210/mend-4-10-1580. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Schofield P. R., Kuang W. J., Seeburg P. H., Mason A. J., Henzel W. J. Molecular cloning and amino acid sequence of rat enkephalinase. Biochem Biophys Res Commun. 1987 Apr 14;144(1):59–66. doi: 10.1016/s0006-291x(87)80475-8. [DOI] [PubMed] [Google Scholar]
- McKerrow J. H. Human fibroblast collagenase contains an amino acid sequence homologous to the zinc-binding site of Serratia protease. J Biol Chem. 1987 May 5;262(13):5943–5943. [PubMed] [Google Scholar]
- Medina J. F., Wetterholm A., Rådmark O., Shapiro R., Haeggström J. Z., Vallee B. L., Samuelsson B. Leukotriene A4 hydrolase: determination of the three zinc-binding ligands by site-directed mutagenesis and zinc analysis. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7620–7624. doi: 10.1073/pnas.88.17.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy D. I., Strittmatter W. J. Requirement for metalloendoprotease in exocytosis: evidence in mast cells and adrenal chromaffin cells. Cell. 1985 Mar;40(3):645–656. doi: 10.1016/0092-8674(85)90213-2. [DOI] [PubMed] [Google Scholar]
- Nakahama K., Yoshimura K., Marumoto R., Kikuchi M., Lee I. S., Hase T., Matsubara H. Cloning and sequencing of Serratia protease gene. Nucleic Acids Res. 1986 Jul 25;14(14):5843–5855. doi: 10.1093/nar/14.14.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath H. Proteolytic processing and physiological regulation. Trends Biochem Sci. 1989 Jul;14(7):268–271. doi: 10.1016/0968-0004(89)90061-3. [DOI] [PubMed] [Google Scholar]
- Rawlings N. D., Barrett A. J. Homologues of insulinase, a new superfamily of metalloendopeptidases. Biochem J. 1991 Apr 15;275(Pt 2):389–391. doi: 10.1042/bj2750389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick N. M., Maloy W. L., Guy H. R., Zasloff M. A novel endopeptidase from Xenopus that recognizes alpha-helical secondary structure. Cell. 1991 Aug 9;66(3):541–554. doi: 10.1016/0092-8674(81)90017-9. [DOI] [PubMed] [Google Scholar]
- Schneider H., Arretz M., Wachter E., Neupert W. Matrix processing peptidase of mitochondria. Structure-function relationships. J Biol Chem. 1990 Jun 15;265(17):9881–9887. [PubMed] [Google Scholar]
- Semple J. W., Ellis J., Delovitch T. L. Processing and presentation of insulin. II. Evidence for intracellular, plasma membrane-associated and extracellular degradation of human insulin by antigen-presenting B cells. J Immunol. 1989 Jun 15;142(12):4184–4193. [PubMed] [Google Scholar]
- Swamy K. H., Goldberg A. L. Subcellular distribution of various proteases in Escherichia coli. J Bacteriol. 1982 Mar;149(3):1027–1033. doi: 10.1128/jb.149.3.1027-1033.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L., Brandt S. R., Olsen C. M., Judd A. K., Almquist R. G. Isolation and characterization of a new atrial peptide-degrading enzyme from bovine kidney. Biochem Biophys Res Commun. 1991 Mar 29;175(3):886–893. doi: 10.1016/0006-291x(91)91648-v. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Wei L., Alhenc-Gelas F., Corvol P., Clauser E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J Biol Chem. 1991 May 15;266(14):9002–9008. [PubMed] [Google Scholar]
- Witte C., Jensen R. E., Yaffe M. P., Schatz G. MAS1, a gene essential for yeast mitochondrial assembly, encodes a subunit of the mitochondrial processing protease. EMBO J. 1988 May;7(5):1439–1447. doi: 10.1002/j.1460-2075.1988.tb02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. J., Geli V., Oppliger W., Suda K., James P., Schatz G. The MAS-encoded processing protease of yeast mitochondria. Interaction of the purified enzyme with signal peptides and a purified precursor protein. J Biol Chem. 1991 Apr 5;266(10):6416–6423. [PubMed] [Google Scholar]