Abstract
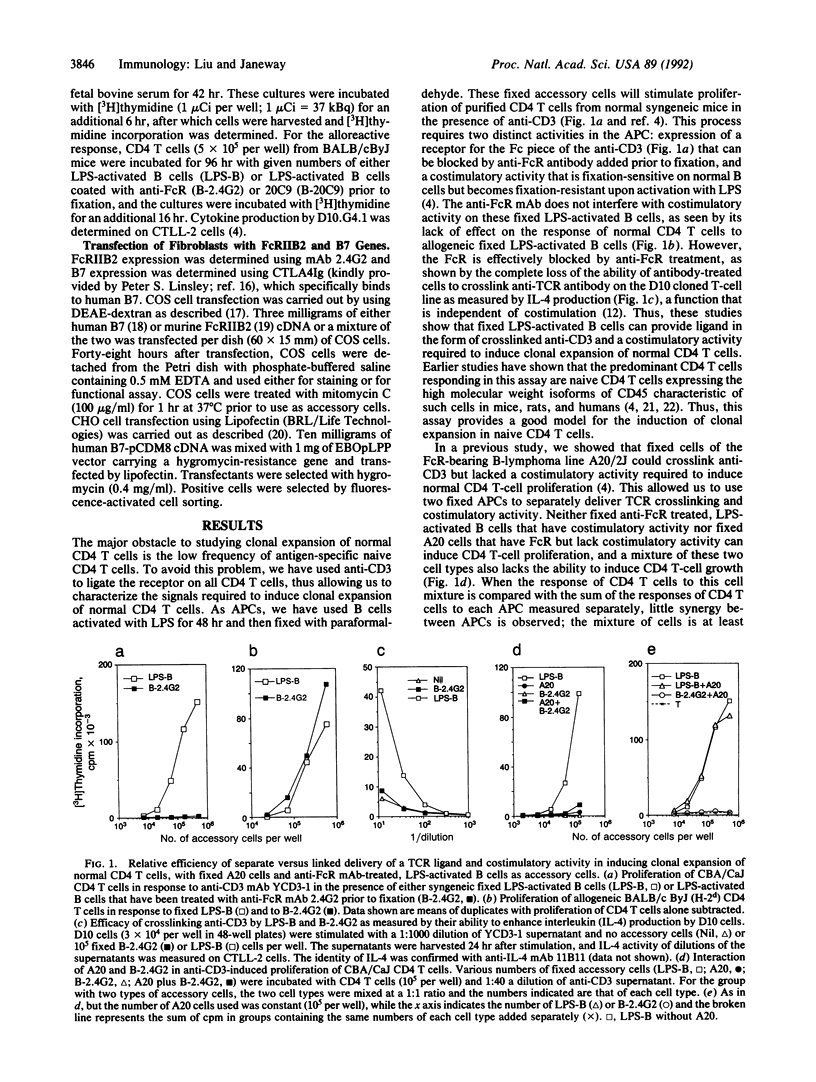
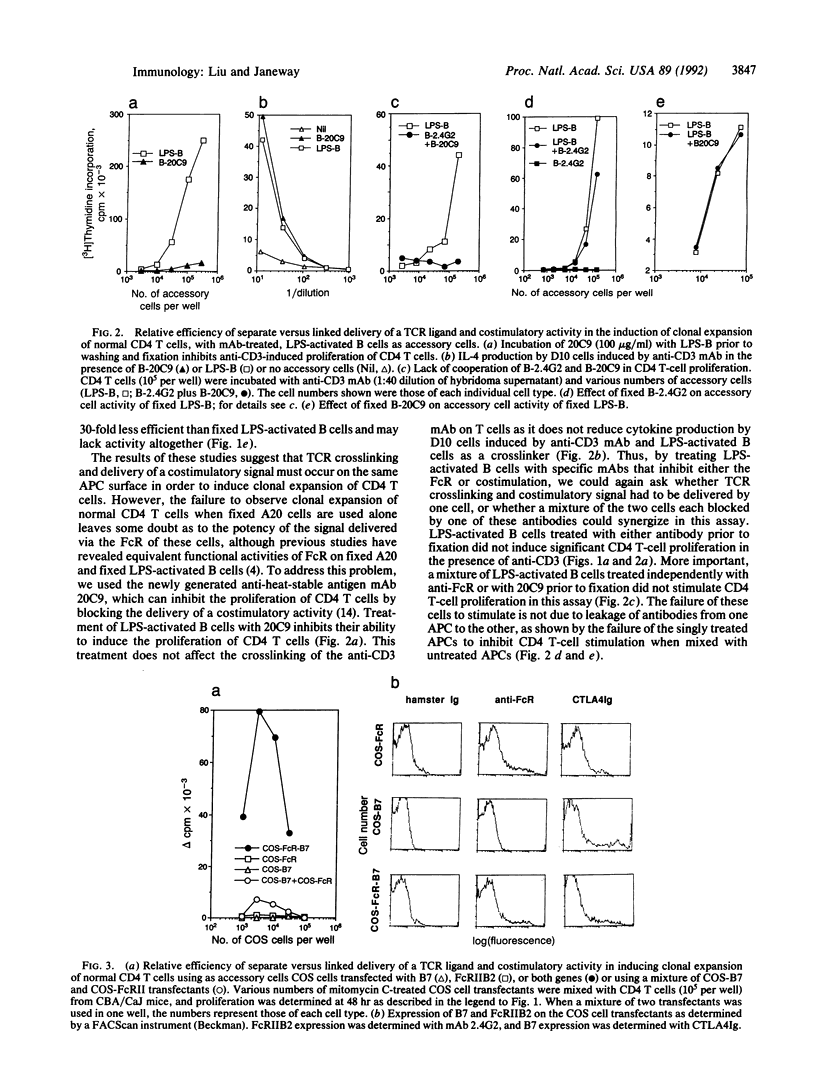
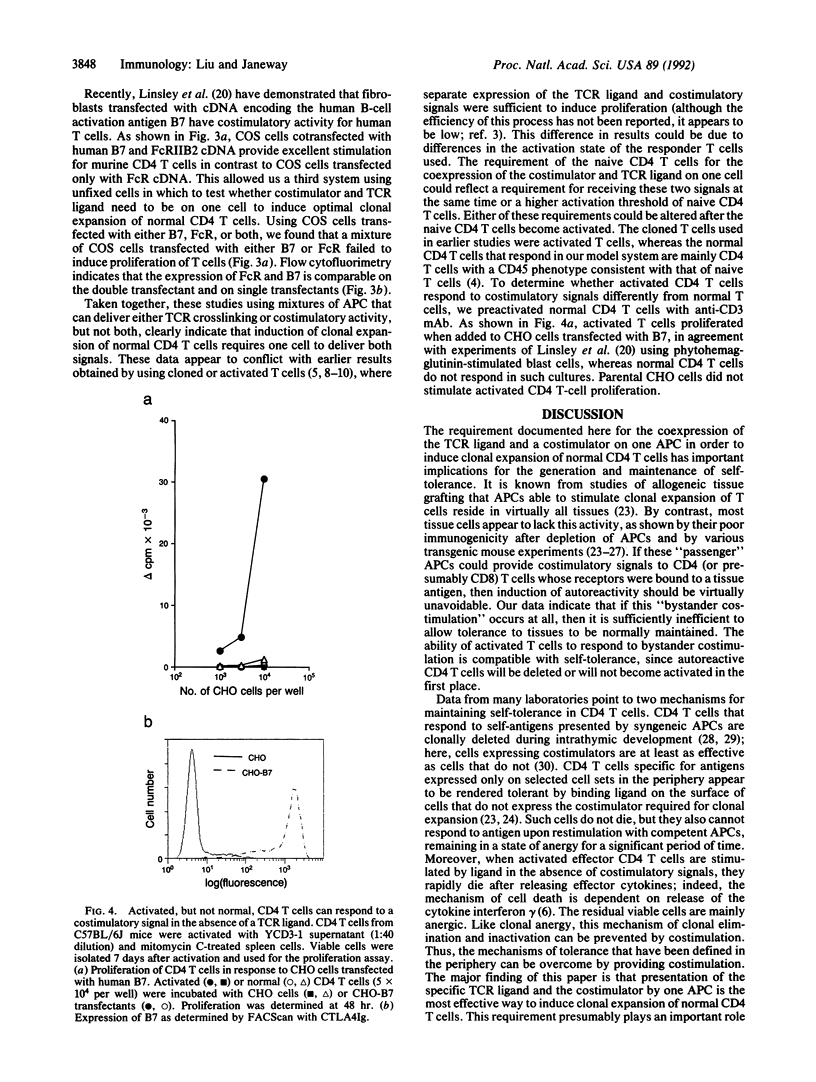
Clonal expansion of naive CD4 T cells is a necessary step in most adaptive immune responses. Two distinct signals are required for clonal expansion to occur, ligation of T-cell receptors by an antigenic peptide bound to self major histocompatibility complex-encoded class II molecules (signal 1) and a costimulatory signal derived from an antigen-presenting cell (signal 2). To study whether these two signals need to be delivered by a single cell in order to induce clonal expansion of normal CD4 T cells, we have used anti-CD3 bound to Fc receptors as a ligand for the T-cell receptor to deliver signal 1 to all CD4T cells, and we have inactivated signal 2 with a newly generated monoclonal antibody or by using Fc receptor-positive cells that lack the costimulator. Costimulation was delivered by cells whose Fc receptors were blocked with anti-Fc receptor monoclonal antibody. Our results indicate that delivery of ligand and costimulator on one cell is at least 30-fold more efficient than separate delivery. No significant clonal expansion was observed when signals 1 and 2 were delivered by different cells. We have also carried out experiments using fibroblast transfectants that can deliver either or both of these two signals. These studies show that separate delivery of these two signals is at least 80-fold less efficient than their combined delivery by one cell. These findings may explain why tissues can express autoantigens and contain active antigen-presenting cells without inducing autoimmunity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J., Campbell I. L., Morahan G., Mandel T. E., Harrison L. C., Miller J. F. Diabetes in transgenic mice resulting from over-expression of class I histocompatibility molecules in pancreatic beta cells. Nature. 1988 Jun 9;333(6173):529–533. doi: 10.1038/333529a0. [DOI] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Lo D., Kanagawa O., Brinster R. L., Flavell R. A. T-cell tolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature. 1989 Nov 30;342(6249):564–566. doi: 10.1038/342564a0. [DOI] [PubMed] [Google Scholar]
- Dianzani U., Luqman M., Rojo J., Yagi J., Baron J. L., Woods A., Janeway C. A., Jr, Bottomly K. Molecular associations on the T cell surface correlate with immunological memory. Eur J Immunol. 1990 Oct;20(10):2249–2257. doi: 10.1002/eji.1830201014. [DOI] [PubMed] [Google Scholar]
- Gilbert K. M., Hoang K. D., Weigle W. O. Th1 and Th2 clones differ in their response to a tolerogenic signal. J Immunol. 1990 Mar 15;144(6):2063–2071. [PubMed] [Google Scholar]
- Janeway C. A., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Ashwell J. D., Schwartz R. H. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988 May 15;140(10):3324–3330. [PubMed] [Google Scholar]
- Jenkins M. K., Pardoll D. M., Mizuguchi J., Quill H., Schwartz R. H. T-cell unresponsiveness in vivo and in vitro: fine specificity of induction and molecular characterization of the unresponsive state. Immunol Rev. 1987 Feb;95:113–135. doi: 10.1111/j.1600-065x.1987.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Lafferty K. J., Prowse S. J., Simeonovic C. J., Warren H. S. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Annu Rev Immunol. 1983;1:143–173. doi: 10.1146/annurev.iy.01.040183.001043. [DOI] [PubMed] [Google Scholar]
- Lewis V. A., Koch T., Plutner H., Mellman I. A complementary DNA clone for a macrophage-lymphocyte Fc receptor. 1986 Nov 27-Dec 3Nature. 324(6095):372–375. doi: 10.1038/324372a0. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990 Dec 1;172(6):1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Microbial induction of co-stimulatory activity for CD4 T-cell growth. Int Immunol. 1991 Apr;3(4):323–332. doi: 10.1093/intimm/3.4.323. [DOI] [PubMed] [Google Scholar]
- Liu Y., Jones B., Aruffo A., Sullivan K. M., Linsley P. S., Janeway C. A., Jr Heat-stable antigen is a costimulatory molecule for CD4 T cell growth. J Exp Med. 1992 Feb 1;175(2):437–445. doi: 10.1084/jem.175.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D., Burkly L. C., Widera G., Cowing C., Flavell R. A., Palmiter R. D., Brinster R. L. Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic beta cells. Cell. 1988 Apr 8;53(1):159–168. doi: 10.1016/0092-8674(88)90497-7. [DOI] [PubMed] [Google Scholar]
- Matzinger P., Guerder S. Does T-cell tolerance require a dedicated antigen-presenting cell? Nature. 1989 Mar 2;338(6210):74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- Mellman I. S., Unkeless J. C. Purificaton of a functional mouse Fc receptor through the use of a monoclonal antibody. J Exp Med. 1980 Oct 1;152(4):1048–1069. doi: 10.1084/jem.152.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten G. R., Germain R. N. Split anergy in a CD8+ T cell: receptor-dependent cytolysis in the absence of interleukin-2 production. Science. 1991 Mar 8;251(4998):1228–1231. doi: 10.1126/science.1900952. [DOI] [PubMed] [Google Scholar]
- Portoles P., Rojo J., Golby A., Bonneville M., Gromkowski S., Greenbaum L., Janeway C. A., Jr, Murphy D. B., Bottomly K. Monoclonal antibodies to murine CD3 epsilon define distinct epitopes, one of which may interact with CD4 during T cell activation. J Immunol. 1989 Jun 15;142(12):4169–4175. [PubMed] [Google Scholar]
- Reichel H., Koeffler H. P., Tobler A., Norman A. W. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(10):3385–3389. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Acquisition of immunologic self-tolerance. Cell. 1989 Jun 30;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Watts T. H., McConnell H. M. Biophysical aspects of antigen recognition by T cells. Annu Rev Immunol. 1987;5:461–475. doi: 10.1146/annurev.iy.05.040187.002333. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Teh H. S., Kisielow P. The thymus selects the useful, neglects the useless and destroys the harmful. Immunol Today. 1989 Feb;10(2):57–61. doi: 10.1016/0167-5699(89)90307-1. [DOI] [PubMed] [Google Scholar]


