Abstract
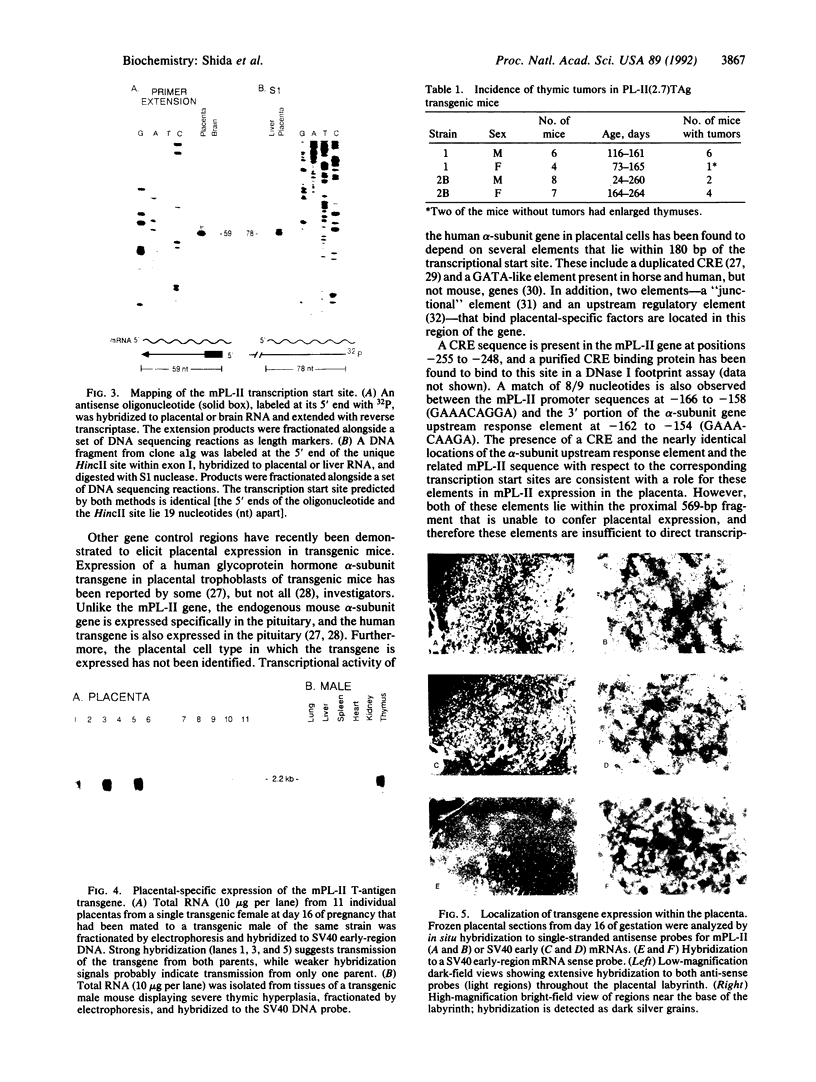
The gene for mouse placental lactogen II (mPL-II) has been isolated and characterized. This gene contains five exons, with a transcription start site 59 nucleotides upstream of the translation initiation ATG. Introduction of a DNA construct containing 2.7 kilobases of sequence upstream of the mPL-II transcription initiation site directed the synthesis of a linked coding region for the simian virus 40 large and small tumor antigens in placental trophoblast giant cells of transgenic mice. The pattern of simian virus 40 transgene expression in the placenta was indistinguishable from that of the endogenous mPL-II gene. In contrast, the first 569 base pairs upstream of the transcription start site proved insufficient to direct placental expression. Thus, one or more elements required for placental trophoblast giant cell expression have been localized to a region between -2700 and -569 of the mPL-II gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B., Kennedy G. C., Nilson J. H. A cis-acting element located between the cAMP response elements and CCAAT box augments cell-specific expression of the glycoprotein hormone alpha subunit gene. J Biol Chem. 1990 Dec 15;265(35):21874–21880. [PubMed] [Google Scholar]
- Barkley M. S., Bradford G. E., Geschwind I. I. The pattern of plasma prolactin concentration during the first half of mouse gestation. Biol Reprod. 1978 Sep;19(2):291–296. doi: 10.1095/biolreprod19.2.291. [DOI] [PubMed] [Google Scholar]
- Barta A., Richards R. I., Baxter J. D., Shine J. Primary structure and evolution of rat growth hormone gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4867–4871. doi: 10.1073/pnas.78.8.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokar J. A., Keri R. A., Farmerie T. A., Fenstermaker R. A., Andersen B., Hamernik D. L., Yun J., Wagner T., Nilson J. H. Expression of the glycoprotein hormone alpha-subunit gene in the placenta requires a functional cyclic AMP response element, whereas a different cis-acting element mediates pituitary-specific expression. Mol Cell Biol. 1989 Nov;9(11):5113–5122. doi: 10.1128/mcb.9.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteri F. M., van der Putten H., Wong D. F., Sauvage C. A., Evans R. M. Unexpected thymic hyperplasia in transgenic mice harboring a neuronal promoter fused with simian virus 40 large T antigen. Mol Cell Biol. 1987 Sep;7(9):3178–3184. doi: 10.1128/mcb.7.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colosi P., Marr G., Lopez J., Haro L., Ogren L., Talamantes F. Isolation, purification, and characterization of mouse placental lactogen. Proc Natl Acad Sci U S A. 1982 Feb;79(3):771–775. doi: 10.1073/pnas.79.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosi P., Swiergiel J. J., Wilder E. L., Oviedo A., Linzer D. I. Characterization of proliferin-related protein. Mol Endocrinol. 1988 Jun;2(6):579–586. doi: 10.1210/mend-2-6-579. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Baxter J. D. Structural analysis of the prolactin gene suggests a separate origin for its 5' end. Nature. 1982 Jun 17;297(5867):603–606. doi: 10.1038/297603a0. [DOI] [PubMed] [Google Scholar]
- Delegeane A. M., Ferland L. H., Mellon P. L. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Mol Cell Biol. 1987 Nov;7(11):3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria T. N., Ogren L., Talamantes F., Linzer D. I., Soares M. J. Localization of placental lactogen-I in trophoblast giant cells of the mouse placenta. Biol Reprod. 1991 Feb;44(2):327–331. doi: 10.1095/biolreprod44.2.327. [DOI] [PubMed] [Google Scholar]
- Faria T. N., Soares M. J. Trophoblast cell differentiation: establishment, characterization, and modulation of a rat trophoblast cell line expressing members of the placental prolactin family. Endocrinology. 1991 Dec;129(6):2895–2906. doi: 10.1210/endo-129-6-2895. [DOI] [PubMed] [Google Scholar]
- Fox N., Solter D. Expression and regulation of the pituitary- and placenta-specific human glycoprotein hormone alpha-subunit gene is restricted to the pituitary in transgenic mice. Mol Cell Biol. 1988 Dec;8(12):5470–5476. doi: 10.1128/mcb.8.12.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hall J., Talamantes F. Immunocytochemical localization of mouse placental lactogen in the mouse placenta. J Histochem Cytochem. 1984 Apr;32(4):379–382. doi: 10.1177/32.4.6368678. [DOI] [PubMed] [Google Scholar]
- Harigaya T., Smith W. C., Talamantes F. Hepatic placental lactogen receptors during pregnancy in the mouse. Endocrinology. 1988 Apr;122(4):1366–1372. doi: 10.1210/endo-122-4-1366. [DOI] [PubMed] [Google Scholar]
- Jackson-Grusby L. L., Pravtcheva D., Ruddle F. H., Linzer D. I. Chromosomal mapping of the prolactin/growth hormone gene family in the mouse. Endocrinology. 1988 Jun;122(6):2462–2466. doi: 10.1210/endo-122-6-2462. [DOI] [PubMed] [Google Scholar]
- Jackson L. L., Colosi P., Talamantes F., Linzer D. I. Molecular cloning of mouse placental lactogen cDNA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8496–8500. doi: 10.1073/pnas.83.22.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson J. L., Powers A. C., Gallagher G. D., Habener J. F. Enhancer and promoter element interactions dictate cyclic adenosine monophosphate mediated and cell-specific expression of the glycoprotein hormone alpha-gene. Mol Endocrinol. 1989 May;3(5):763–772. doi: 10.1210/mend-3-5-763. [DOI] [PubMed] [Google Scholar]
- Lee S. J., Talamantes F., Wilder E., Linzer D. I., Nathans D. Trophoblastic giant cells of the mouse placenta as the site of proliferin synthesis. Endocrinology. 1988 May;122(5):1761–1768. doi: 10.1210/endo-122-5-1761. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Lee S. J., Ogren L., Talamantes F., Nathans D. Identification of proliferin mRNA and protein in mouse placenta. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4356–4359. doi: 10.1073/pnas.82.13.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Mordacq J. C. Transcriptional regulation of proliferin gene expression in response to serum in transfected mouse cells. EMBO J. 1987 Aug;6(8):2281–2288. doi: 10.1002/j.1460-2075.1987.tb02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. A new member of the prolactin-growth hormone gene family expressed in mouse placenta. EMBO J. 1985 Jun;4(6):1419–1423. doi: 10.1002/j.1460-2075.1985.tb03796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordacq J. C., Linzer D. I. Co-localization of elements required for phorbol ester stimulation and glucocorticoid repression of proliferin gene expression. Genes Dev. 1989 Jun;3(6):760–769. doi: 10.1101/gad.3.6.760. [DOI] [PubMed] [Google Scholar]
- Ogren L., Southard J. N., Colosi P., Linzer D. I., Talamantes F. Mouse placental lactogen-I: RIA and gestational profile in maternal serum. Endocrinology. 1989 Nov;125(5):2253–2257. doi: 10.1210/endo-125-5-2253. [DOI] [PubMed] [Google Scholar]
- Ogren L., Talamantes F. Prolactins of pregnancy and their cellular source. Int Rev Cytol. 1988;112:1–65. doi: 10.1016/s0074-7696(08)62005-7. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H. The human growth hormone gene family: nucleotide sequences show recent divergence and predict a new polypeptide hormone. DNA. 1982;1(3):239–249. doi: 10.1089/dna.1.1982.1.239. [DOI] [PubMed] [Google Scholar]
- Soares M. J., Colosi P., Ogren L., Talamantes F. Identification and partial characterization of a lactogen from the midpregnant mouse conceptus. Endocrinology. 1983 Apr;112(4):1313–1317. doi: 10.1210/endo-112-4-1313. [DOI] [PubMed] [Google Scholar]
- Steger D. J., Altschmied J., Büscher M., Mellon P. L. Evolution of placenta-specific gene expression: comparison of the equine and human gonadotropin alpha-subunit genes. Mol Endocrinol. 1991 Feb;5(2):243–255. doi: 10.1210/mend-5-2-243. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. H., Fitzpatrick S. L., Saunders G. F. Human placental lactogen transcriptional enhancer. Tissue specificity and binding with specific proteins. J Biol Chem. 1990 Aug 5;265(22):12940–12948. [PubMed] [Google Scholar]
- Woodruff T. K., Meunier H., Jones P. B., Hsueh A. J., Mayo K. E. Rat inhibin: molecular cloning of alpha- and beta-subunit complementary deoxyribonucleic acids and expression in the ovary. Mol Endocrinol. 1987 Aug;1(8):561–568. doi: 10.1210/mend-1-8-561. [DOI] [PubMed] [Google Scholar]