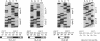Abstract
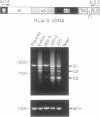
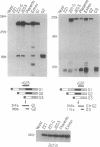
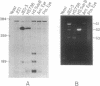
We have investigated HLA-G mRNA expression in cells and tissues expressing the gene. This analysis has demonstrated that the HLA-G primary transcript is alternatively spliced to yield at least three distinct mature mRNAs. Sequencing of the transcripts has shown that the largest mRNA is essentially that previously characterized, encoding a leader sequence, three external domains, a transmembrane region, and a cytoplasmic sequence. Of the two smaller messages, a 900-base mRNA does not include exon 3, resulting in a predicted protein sequence with the alpha 1 and alpha 3 external domains joined. The smallest mRNA results from splicing out exons 3 and 4, connecting the alpha domain directly to the transmembrane sequence. Alternative splicing of HLA-G mRNA was found in placental tissues and in eye tissue as well as in HLA-G-transfected cell lines. In term placental tissue the smallest mRNA appeared to be more abundant than the full-length form, while in a cell line derived from an earlier developmental stage the larger form predominated. Immunoprecipitation of [35S]methionine-labeled cell lysates showed that three different HLA-G proteins were present in transfected cells, with sizes corresponding to those predicted from the three alternative mRNA sequences. These findings are discussed in terms of potential functions of the alternative HLA-G proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Ellis S. A., Palmer M. S., McMichael A. J. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA Class I molecule. J Immunol. 1990 Jan 15;144(2):731–735. [PubMed] [Google Scholar]
- Fahrner K., Hogan B. L., Flavell R. A. Transcription of H-2 and Qa genes in embryonic and adult mice. EMBO J. 1987 May;6(5):1265–1271. doi: 10.1002/j.1460-2075.1987.tb02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty D. E., Koller B. H., Orr H. T. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9145–9149. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty D. E., Pei J., Lipsky B., Hansen J. A., Taillon-Miller P., Bronson S. K., Chaplin D. D. Cloning and physical mapping of the HLA class I region spanning the HLA-E-to-HLA-F interval by using yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2669–2673. doi: 10.1073/pnas.89.7.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty D. E., Wei X. H., Orr H. T., Koller B. H. Human leukocyte antigen F (HLA-F). An expressed HLA gene composed of a class I coding sequence linked to a novel transcribed repetitive element. J Exp Med. 1990 Jan 1;171(1):1–18. doi: 10.1084/jem.171.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Geraghty D. E., Shimizu Y., DeMars R., Orr H. T. HLA-E. A novel HLA class I gene expressed in resting T lymphocytes. J Immunol. 1988 Aug 1;141(3):897–904. [PubMed] [Google Scholar]
- Kovats S., Main E. K., Librach C., Stubblebine M., Fisher S. J., DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990 Apr 13;248(4952):220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- Krangel M. S. Secretion of HLA-A and -B antigens via an alternative RNA splicing pathway. J Exp Med. 1986 May 1;163(5):1173–1190. doi: 10.1084/jem.163.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizardi P. M. Methods for the preparation of messenger RNA. Methods Enzymol. 1983;96:24–38. doi: 10.1016/s0076-6879(83)96006-8. [DOI] [PubMed] [Google Scholar]
- McCluskey J., Boyd L. F., Maloy W. L., Coligan J. E., Margulies D. H. Alternative processing of H-2Dd pre-mRNAs results in membrane expression of differentially phosphorylated protein products. EMBO J. 1986 Oct;5(10):2477–2483. doi: 10.1002/j.1460-2075.1986.tb04524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Noonan K. E., Roninson I. B. mRNA phenotyping by enzymatic amplification of randomly primed cDNA. Nucleic Acids Res. 1988 Nov 11;16(21):10366–10366. doi: 10.1093/nar/16.21.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. W., McMichael A. J., Stirrat G. M., Sunderland C. A., Ting A. Class 1 major histocompatibility complex antigens on human extra-villous trophoblast. Immunology. 1984 Jul;52(3):457–468. [PMC free article] [PubMed] [Google Scholar]
- Salter R. D., Benjamin R. J., Wesley P. K., Buxton S. E., Garrett T. P., Clayberger C., Krensky A. M., Norment A. M., Littman D. R., Parham P. A binding site for the T-cell co-receptor CD8 on the alpha 3 domain of HLA-A2. Nature. 1990 May 3;345(6270):41–46. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- Sanders S. K., Giblin P. A., Kavathas P. Cell-cell adhesion mediated by CD8 and human histocompatibility leukocyte antigen G, a nonclassical major histocompatibility complex class 1 molecule on cytotrophoblasts. J Exp Med. 1991 Sep 1;174(3):737–740. doi: 10.1084/jem.174.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Geraghty D. E., Koller B. H., Orr H. T., DeMars R. Transfer and expression of three cloned human non-HLA-A,B,C class I major histocompatibility complex genes in mutant lymphoblastoid cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):227–231. doi: 10.1073/pnas.85.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla H., Swaroop A., Srivastava R., Weissman S. M. The mRNA of a human class I gene HLA G/HLA 6.0 exhibits a restricted pattern of expression. Nucleic Acids Res. 1990 Apr 25;18(8):2189–2189. doi: 10.1093/nar/18.8.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroynowski I. Molecules related to class-I major histocompatibility complex antigens. Annu Rev Immunol. 1990;8:501–530. doi: 10.1146/annurev.iy.08.040190.002441. [DOI] [PubMed] [Google Scholar]
- Ulker N., Lewis K. D., Hood L. E., Stroynowski I. Activated T cells transcribe an alternatively spliced mRNA encoding a soluble form of Qa-2 antigen. EMBO J. 1990 Dec;9(12):3839–3847. doi: 10.1002/j.1460-2075.1990.tb07602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X. H., Orr H. T. Differential expression of HLA-E, HLA-F, and HLA-G transcripts in human tissue. Hum Immunol. 1990 Oct;29(2):131–142. doi: 10.1016/0198-8859(90)90076-2. [DOI] [PubMed] [Google Scholar]
- Wenger R. H., Nielsen P. J. Reannealing of artificial heteroduplexes generated during PCR-mediated isotyping. Trends Genet. 1991 Jun;7(6):178–178. doi: 10.1016/0168-9525(91)90430-x. [DOI] [PubMed] [Google Scholar]