Abstract
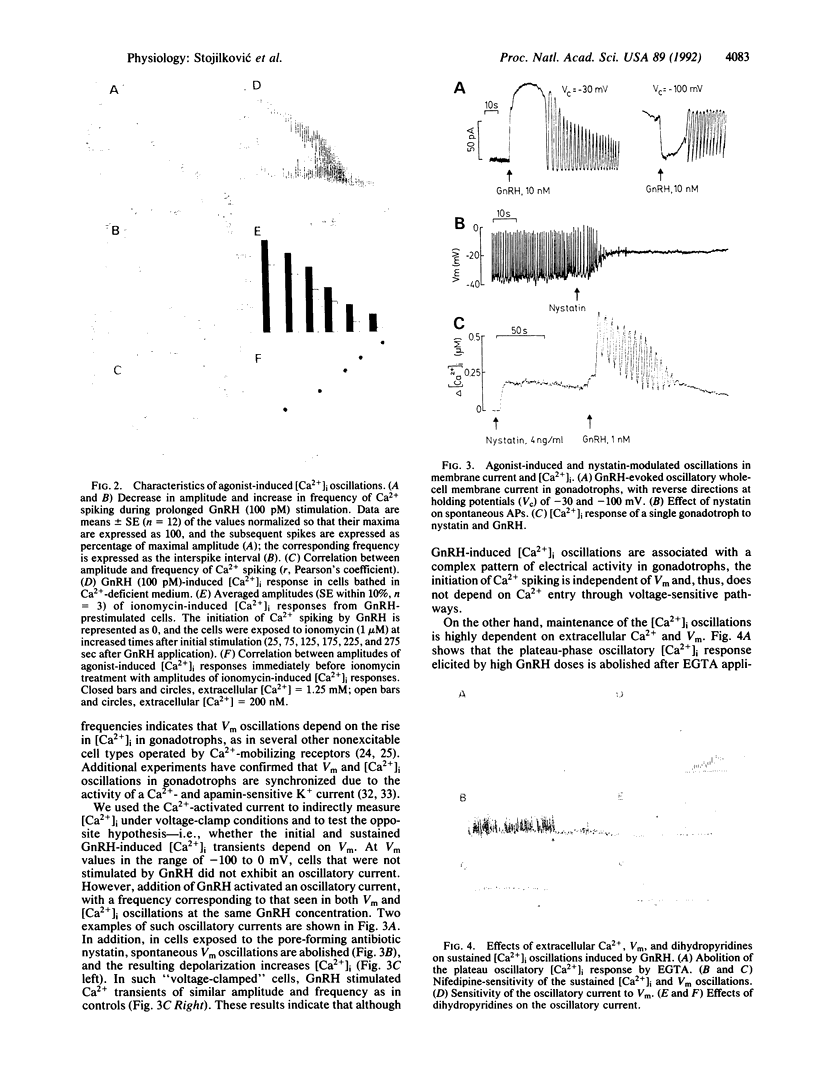
Pituitary gonadotrophs exhibit spontaneous low-amplitude fluctuations in cytoplasmic calcium concentration ([Ca2+]i) due to intermittent firing of nifedipine-sensitive action potentials. The hypothalamic neuropeptide, gonadotropin-releasing hormone, terminates such spontaneous [Ca2+]i transients and plasma-membrane electrical activity and initiates high-amplitude [Ca2+]i oscillations and concomitant oscillations in membrane potential (Vm). The onset of agonist-induced [Ca2+]i oscillations is not dependent on Vm or extracellular Ca2+ but is associated with plasma-membrane hyperpolarization interrupted by regular waves of depolarization with firing of action potentials at the peak of each wave. The Vm and Ca2+ oscillations are interdependent during continued gonadotropin-releasing hormone action (greater than 3-5 min), when sustained Ca2+ entry is necessary for the maintenance of [Ca2+]i spiking. The initial and sustained agonist-induced Ca2+ transients and Vm oscillations are abolished by blockade of endoplasmic reticulum Ca(2+)-ATPase, consistent with the role of Ca2+ re-uptake by internal stores in the oscillatory response during both phases. Such a pattern of synchronization of electrical activity and Ca2+ spiking in cells regulated by Ca(2+)-mobilizing receptors shows that the operation of the cytoplasmic oscillator can be integrated with a plasma-membrane oscillator to provide a long-lasting signal during sustained agonist stimulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammälä C., Larsson O., Berggren P. O., Bokvist K., Juntti-Berggren L., Kindmark H., Rorsman P. Inositol trisphosphate-dependent periodic activation of a Ca(2+)-activated K+ conductance in glucose-stimulated pancreatic beta-cells. Nature. 1991 Oct 31;353(6347):849–852. doi: 10.1038/353849a0. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bird G. S., Rossier M. F., Hughes A. R., Shears S. B., Armstrong D. L., Putney J. W., Jr Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991 Jul 11;352(6331):162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- Chang J. P., Morgan R. O., Catt K. J. Dependence of secretory responses to gonadotropin-releasing hormone on diacylglycerol metabolism. Studies with a diacylglycerol lipase inhibitor, RHC 80267. J Biol Chem. 1988 Dec 15;263(35):18614–18620. [PubMed] [Google Scholar]
- Gray P. T. Oscillations of free cytosolic calcium evoked by cholinergic and catecholaminergic agonists in rat parotid acinar cells. J Physiol. 1988 Dec;406:35–53. doi: 10.1113/jphysiol.1988.sp017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holl R. W., Thorner M. O., Leong D. A. Intracellular calcium concentration and growth hormone secretion in individual somatotropes: effects of growth hormone-releasing factor and somatostatin. Endocrinology. 1988 Jun;122(6):2927–2932. doi: 10.1210/endo-122-6-2927. [DOI] [PubMed] [Google Scholar]
- Holl R. W., Thorner M. O., Mandell G. L., Sullivan J. A., Sinha Y. N., Leong D. A. Spontaneous oscillations of intracellular calcium and growth hormone secretion. J Biol Chem. 1988 Jul 15;263(20):9682–9685. [PubMed] [Google Scholar]
- Iida T., Stojilković S. S., Izumi S., Catt K. J. Spontaneous and agonist-induced calcium oscillations in pituitary gonadotrophs. Mol Endocrinol. 1991 Jul;5(7):949–958. doi: 10.1210/mend-5-7-949. [DOI] [PubMed] [Google Scholar]
- Leong D. A. A complex mechanism of facilitation in pituitary ACTH cells: recent single-cell studies. J Exp Biol. 1988 Sep;139:151–168. doi: 10.1242/jeb.139.1.151. [DOI] [PubMed] [Google Scholar]
- Leong D. A., Thorner M. O. A potential code of luteinizing hormone-releasing hormone-induced calcium ion responses in the regulation of luteinizing hormone secretion among individual gonadotropes. J Biol Chem. 1991 May 15;266(14):9016–9022. [PubMed] [Google Scholar]
- Malgaroli A., Vallar L., Elahi F. R., Pozzan T., Spada A., Meldolesi J. Dopamine inhibits cytosolic Ca2+ increases in rat lactotroph cells. Evidence of a dual mechanism of action. J Biol Chem. 1987 Oct 15;262(29):13920–13927. [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Morgan R. O., Chang J. P., Catt K. J. Novel aspects of gonadotropin-releasing hormone action on inositol polyphosphate metabolism in cultured pituitary gonadotrophs. J Biol Chem. 1987 Jan 25;262(3):1166–1171. [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Sand O. Electrophysiology of excitable endocrine cells. Physiol Rev. 1986 Oct;66(4):887–952. doi: 10.1152/physrev.1986.66.4.887. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Localized all-or-none calcium liberation by inositol trisphosphate. Science. 1990 Nov 16;250(4983):977–979. doi: 10.1126/science.2237441. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Shangold G. A., Murphy S. N., Miller R. J. Gonadotropin-releasing hormone-induced Ca2+ transients in single identified gonadotropes require both intracellular Ca2+ mobilization and Ca2+ influx. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6566–6570. doi: 10.1073/pnas.85.17.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilković S. S., Iida T., Merelli F., Catt K. J. Calcium signaling and secretory responses in endothelin-stimulated anterior pituitary cells. Mol Pharmacol. 1991 Jun;39(6):762–770. [PubMed] [Google Scholar]
- Stojilković S. S., Izumi S., Catt K. J. Participation of voltage-sensitive calcium channels in pituitary hormone release. J Biol Chem. 1988 Sep 15;263(26):13054–13061. [PubMed] [Google Scholar]
- Stojilković S. S., Merelli F., Iida T., Krsmanović L. Z., Catt K. J. Endothelin stimulation of cytosolic calcium and gonadotropin secretion in anterior pituitary cells. Science. 1990 Jun 29;248(4963):1663–1666. doi: 10.1126/science.2163546. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Catecholamines of supposed inhibitory hypophysiotrophic function suppress action potentials in prolactin cells. Nature. 1978 Dec 21;276(5690):832–834. doi: 10.1038/276832a0. [DOI] [PubMed] [Google Scholar]
- Tse A., Hille B. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992 Jan 24;255(5043):462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Romey G., Lazdunski M. Vasopressin modulates the spontaneous electrical activity in aortic cells (line A7r5) by acting on three different types of ionic channels. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9365–9369. doi: 10.1073/pnas.85.23.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakui M., Potter B. V., Petersen O. H. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989 May 25;339(6222):317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- White R. E., Schonbrunn A., Armstrong D. L. Somatostatin stimulates Ca(2+)-activated K+ channels through protein dephosphorylation. Nature. 1991 Jun 13;351(6327):570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- Zhao H., Loessberg P. A., Sachs G., Muallem S. Regulation of intracellular Ca2+ oscillation in AR42J cells. J Biol Chem. 1990 Dec 5;265(34):20856–20862. [PubMed] [Google Scholar]