Abstract
Purpose
To examine anthropometric and maturational characteristics at diagnosis in pediatric idiopathic intracranial hypertension (IIH).
Design
Retrospective, international, multisite study.
Participants
Pediatric patients (2–18 years of age at diagnosis) with IIH.
Main Outcome Measures
Body mass index (BMI), height, and weight Z-scores; sexual maturation.
Methods
Cases of IIH were identified retrospectively based on diagnostic code, pediatric neuroophthalmologist databases, or both and updated diagnostic criteria (2013) were applied to confirm definite IIH. Anthropometric measurements were converted into age- and gender-specific height, weight, and BMI Z-scores CDC 2000 growth charts. When available, sexual maturation was noted.
Results
Two hundred thirty-three cases of definite IIH were identified across 8 sites. In boys, a moderate association between age and BMI Z-scores was noted (Pearson’s correlation coefficient, 0.50; 95% confidence interval [CI], 0.30–0.66; P < 0.001; n = 72), and in girls, a weak association was noted (Pearson’s correlation coefficient, 0.34; 95% CI, 0.20–0.47; P < 0.001; n = 161). The average patient was more likely to be overweight at diagnosis at age 6.7 years in girls and 8.7 years in boys, and obese at diagnosis at age 12.5 years in girls and 12.4 years in boys. Compared with age- and gender-matched reference values, early adolescent patients were taller for age (P = 0.002 in girls and P = 0.02 in boys). Data on Tanner staging, menarchal status, or both were available in 25% of cases (n = 57/233). Prepubertal participants (n = 12) had lower average BMI Z-scores (0.95±1.98) compared with pubertal participants (n = 45; 1.92±0.60), but this result did not reach statistical significance (P = 0.09).
Conclusions
With updated diagnostic criteria and pediatric-specific assessments, the present study identifies 3 subgroups of pediatric IIH: a young group that is not overweight, an early adolescent group that is either overweight or obese, and a late adolescent group that is mostly obese. Data also suggest that the early adolescent group with IIH may be taller than age- and gender-matched reference values. Understanding these features of pediatric IIH may help to illuminate the complex pathogenesis of this condition.
Pseudotumor cerebri syndrome encompasses the constellation of symptoms caused by elevated intracranial pressure of unclear cause with normal brain parenchyma and cerebrospinal fluid constituents.1 When there is no identifiable secondary cause of pseudotumor cerebri syndrome, such as prior exposure to tetracyclines or vitamin A, the condition is termed primary pseudotumor cerebri syndrome or idiopathic intracranial hypertension (IIH).1 Idiopathic intracranial hypertension can occur in both pediatric and adult populations. Pediatric IIH shares some, but not all, features of adult IIH. Previous studies have suggested an influence of age, gender, and pubertal status on the epidemiologic features of pediatric IIH.2–6 Both female gender and obesity seem to be associated more strongly with IIH in older, but not younger, pediatric patients.2,7 In addition, the clinical presentation of pediatric IIH also may vary with age, with a greater number of young children presenting after a routine encounter without symptoms as compared with adolescents.2,5,8
Previous studies examining the role of excess body weight in the presentation of pediatric IIH were limited by the lack of standardized, pediatric-appropriate measurements. For example, investigators have described the relationship between body weight and IIH by defining obesity based on percentage of ideal body weight.2 The United States Centers for Disease Control and Prevention (CDC) defines pediatric obesity based on body mass index (BMI) and, more specifically, BMI percentiles or Z-scores.9 Unlike adults, definitions of being overweight and obese in children are not defined by absolute BMI because this varies with age. Rather, the CDC uses BMI percentiles or Z-scores in the pediatric population: a BMI percentile of 85% or more (equivalent BMI Z-score, ≥1.04) is considered overweight, whereas a BMI percentile of 95% or more (equivalent BMI Z-score, ≥1.64) is considered obese.9
Pubertal status also may contribute to the presentation and prognosis of this condition. Indeed, it has been suggested that children diagnosed with IIH when they are within their pubertal years (estimated as 11–14 years of age) are less likely to obtain excellent visual outcomes.6 Some previous studies examining the influence of pubertal status on the presentation of pediatric IIH also have assumed that all children younger than certain age threshold were prepubertal (e.g., patients younger than 11 years in one study).5 Given the wide range of typical ages at pubertal onset in children, pubertal status should be measured explicitly when attempting to evaluate its role in pediatric IIH. Pubertal status is ascertained most accurately by the development of secondary sexual characteristics (i.e., breast development in girls and testicular enlargement in boys).10,11
The purposes of this study were (1) to identify and confirm cases of pediatric IIH through consistent and rigorous application of the recently revised diagnostic criteria1 and (2) to examine the relationship between pediatric IIH, anthropometric features, and the development of secondary sexual characteristics. To address these issues, a multisite international study was undertaken to provide an adequate sample of pediatric patients with this relatively uncommon disorder.
Methods
This study was a retrospective chart review involving 8 international tertiary medical centers with expertise in pediatric IIH. Institutional review board/ethics committee approval was obtained by each site. The data coordinating center was located at The Children’s Hospital of Philadelphia. This protocol was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia (no. 13-010158).
At each site, patient charts were identified via an electronic medical record search for International Classification of Diseases, Ninth Edition, code 348.2, physician patient databases, or both. For this pediatric study, only patients 2 to 18 years of age at diagnosis were included in the study because we considered the pathophysiologic features of this diagnosis in infancy to be distinct. Retrospective data were collected on patients seen between July 1993 and April 2013 for all sites except for the University of Arkansas, where data were collected between July 1993 and December 2014. Cases of definite IIH were verified through manual chart review according to the revised diagnostic criteria for IIH.1 Cases that did not fulfill all of the criteria for definite IIH were not included in the study. Reasons for exclusion were (1) medical records lacking details of diagnostic studies (e.g., lumbar punctures not completed, cerebrospinal fluid results or opening pressure not recorded) or (2) magnetic resonance venography studies were not performed for atypical patients (i.e., boys, nonobese girls). Although venogram studies were necessary for many cases of IIH, so-called typical IIH patients (i.e., obese and female) did not require a magnetic resonance venography study for inclusion.1 Cases were not included if there was a confirmed or suspected cause of secondary pseudotumor cerebri syndrome, aside from obesity, such as tetracycline use. Only patients fulfilling all diagnostic criteria for definite IIH were collected and included in subsequent analysis. Of note, patients fulfilling diagnostic criteria for probable IIH, that is, those patients who fulfilled all diagnostic criteria for definite IIH except for requirement of elevated opening pressure on lumbar puncture,1 were not included in subsequent analysis.
In cases of definite IIH, age, gender, height measurements, and weight measurements at the time of diagnosis were collected. Pediatric obesity was defined using BMI Z-score. The BMI Z-scores were calculated from anthropometric data according to United States CDC 2000 growth standards.9 According to the CDC, in children between 24 and 36 months of age, BMI Z-scores may be calculated based on stature, determined either by height or recumbent length. In our dataset, only 1 patient was younger than 36 months. In that patient, the method for determining stature is unknown. For all other patients, height was measured and documented. Overweight and obese were defined according to the CDC classifications of overweight (BMI Z-score, ≥1.04 and <1.64) and obese (BMI Z-score, ≥1.64) in children. Severe obesity was defined as the lower value of either BMI of 120% or more of the 95th percentile or more than 35 kg/m2.12 Study data were collected and managed using research electronic data capture tools13 hosted at The Children’s Hospital of Philadelphia.
Data on the development of secondary sexual characteristics were available in 57 patients with definite IIH. Data were collected on Tanner staging (breast, pubic hair, and testicular development, where appropriate14) as well as menarchal status, obtained within 3 months of diagnosis of IIH. Data were collected by patient self-report (menarchal status), after physical examination by a physician (Tanner stage), or both.15 Pubertal status was defined as prepubertal if there was a documented Tanner staging of 1 for breast or testicular volume in girls and boys, respectively. Pubertal status was defined as pubertal if there was documented menarche, Tanner staging (breast) of 2 or more in girls, or both, or if testicular volume was 4 ml or more in boys.
Pearson’s correlation analyses were used for examining the bivariate associations between normally distributed outcomes of interest. The relationships between anthropometric features and age were assessed using linear prediction models, with 95% confidence intervals (CIs). Analysis of the influence of gender on the anthropometric features of IIH was completed by linear regression analysis and chi-square testing. One-sample t tests were used to compare mean Z-scores against hypothesized mean Z-scores (e.g., of theoretical or actual age- and gender-matched reference values). Statistical significance was defined as a 2-sided P value of <0.05. Analyses were performed in Stata software version 13.1 (Stata Corp, College Station, TX).
Results
In total, 233 cases of definite IIH were identified from 8 international sites. Participants had a mean age ± standard deviation of 12.1±4.0 years and were predominately girls (69% girls; n = 161). Participants had a mean ± standard deviation BMI Z-score of 1.55±1.18, which indicated the average body habitus as overweight according to the CDC definition in the pediatric population. Compared with girls, boys were younger and had lower BMI Z-scores (see Table 1). Pubertal data were available in 57 patients with definite IIH (Table 2).
Table 1.
Pediatric Idiopathic Intracranial Hypertension Multicenter Cohort: Overall Demographic and Anthropometric Features of Patients
| Girls | Boys | |
|---|---|---|
| Age (range) at presentation, yrs |
13.1±3.5 (2.3–18.0) | 9.95±4.14 (3.0–17.9)* |
| Height (Z-score), m |
1.52±0.17 (0.17±1.29) | 1.40±0.26 (0.25±1.35)† |
| Weight (Z-score), kg |
72.6±29.1 (1.68±1.14) | 54.6±38.8 (1.28±1.51)* |
| BMI (Z-score), kg/m2 |
30.2±9.6 (1.70±1.07) | 24.7±11.4 (1.24±1.36)* |
BMI = body mass index.
Values are means ± standard deviations unless otherwise indicated.
P < 0.05.
Not significant.
Table 2.
Pediatric Idiopathic Intracranial Hypertension Multisite Cohort: Comparison of Demographic and Anthropometric Features in Prepubertal and Pubertal Patients with Known Pubertal Status
| Prepubertal (n = 12) |
Pubertal (n = 45) |
Not Reported (n = 176) |
|
|---|---|---|---|
| Gender (girls:boys) |
10:2 | 43:2 | 108:68 |
| Age (yrs) | 9.0±2.7 | 14.8±2.4 | 11.6±4.1 |
| BMI Z-score | 0.95±1.98 | 1.92±0.60* | 1.51±1.21 |
| Weight Z-score | 0.73±2.12 | 1.89±0.71* | 1.53±1.30 |
| Height Z-score | 0.13±1.60 | 0.03±1.24† | 0.24±1.31 |
BMI = body mass index.
Values are means ± standard deviations unless otherwise indicated.
P < 0.10, suggesting a possible association (F = 2.93, P =0.09 and F = 3.49, P = 0.07, when comparing BMI and weight Z-scores in prepubertal vs. pubertal subjects, analysis of variance).
No statistically significant differences between height Z-scores seen in prepubertal vs. pubertal patients (F = 0.43, P = 0.51, analysis of variance).
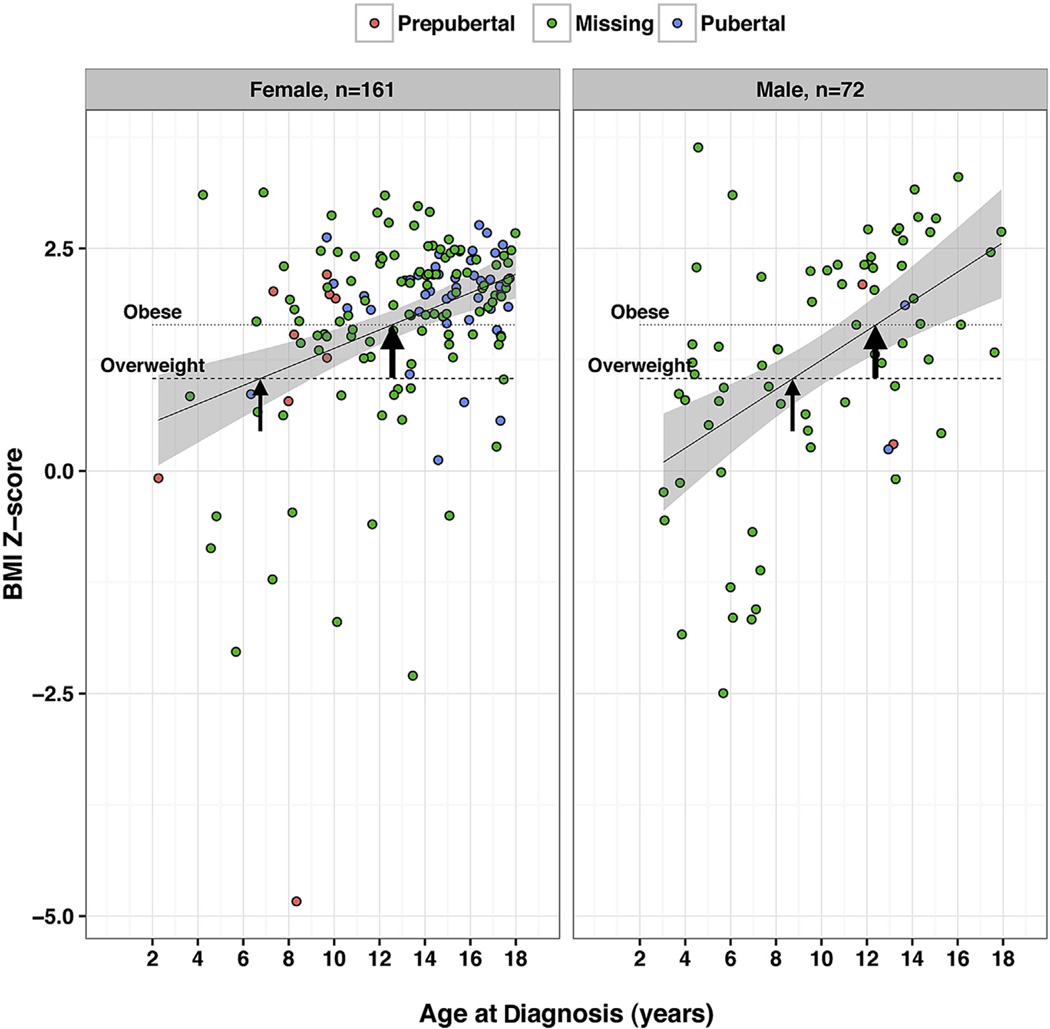
In both boys and girls, we observed an overall positive relationship between age and BMI Z-score at IIH diagnosis (Fig 1). Specifically in boys, a moderate association was noted (Pearson’s correlation coefficient, 0.50; 95% CI, 0.30–0.66; P < 0.001; n = 72), and in girls, a weak association was noted (Pearson’s correlation coefficient, 0.34; 95% CI, 0.20–0.47; P < 0.001; n = 161). In the youngest boys and girls with IIH, average BMI Z-score was within the normal range at diagnosis. After age 6.7 years in girls and 8.7 years in boys, the average BMI Z-score began to exceed 1.04, the threshold for overweight. After age 12.5 years in girls and 12.4 years in boys, the average BMI Z-score exceeded 1.64, the threshold for obesity. The proportion of boys decreased significantly with age at diagnosis of IIH (P < 0.001); boys accounted for 64% (n = 27/42) of participants younger than 8 years, 32% (n = 32/99) of participants 8 to 13 years of age, and 14% (n = 13/92) of participants older than 13 years. We did not detect an influence of gender on the relationship between BMI Z-scores and age at diagnosis (test of interaction between gender and age on BMI-Z in linear regression model; P = 0.71). Overall, in younger age groups, BMI Z-score typically was not in the overweight or obese range. In contrast, mean BMI Z-score was in the overweight and obese ranges in participants at early and late adolescent ages. Stated differently, the proportion of participants with defined pediatric obesity (BMI Z-score, ≥1.64) increased with age in both girls and boys. In girls younger than 6.7 years, 22% of BMI Z-scores were 1.64 or more (n = 2/9), whereas among girls between 6.7 and 12.5 years of age and among girls older than 12.5 years, 59% (n = 30/51) and 77% (n = 78/101) of BMI Z-scores, respectively, were 1.64 or more (P = 0.001). In boys younger than 8.7 years, 13% of BMI Z-scores were 1.64 or more (n = 4/30), whereas among boys between 8.7 and 12.4 years of age and older than 12.4 years, 72% (n = 13/18) and 63% (n = 15/24) of BMI Z-scores, respectively, were 1.64 or more (P < 0.0005). The proportion with severe obesity in boys was 28% (n = 20/72) and that in girls was 37% (n = 59/161; P = 0.19).
Figure 1.
Dot distribution plots showing the relationship between body mass index (BMI) Z-score and age at diagnosis of pediatric idiopathic intracranial hypertension (IIH). In both girls and boys, there is a positive relationship between BMI Z-score and age at diagnosis of definite pediatric IIH. Specifically, in boys, a moderate association was noted (Pearson’s correlation coefficient, 0.50; 95% confidence interval [CI], 0.30–0.66; P < 0.001; n = 72), and in girls, a weak association was noted (Pearson’s correlation coefficient, 0.34; 95% CI, 0.20–0.47; P < 0.001; n = 161). In both groups, the circles represent data obtained from individual participants, whereas the color of the circle represents pubertal status (“missing” indicates that pubertal information was not available). The line represents the results of a linear regression of BMI-Z with 95% CIs of the mean (Stata software; Stata Corp, College Station, TX). Horizontal reference lines indicate the Centers for Disease Control and Prevention (CDC)-defined BMI Z-scores for overweight and obesity features in the pediatric population. The thin arrows indicate overweight thresholds and thick arrows indicate obese thresholds. The overweight threshold indicates the intersection between the plotted linear regression relationship and the BMI Z-score of 1.04 (CDC definition of overweight). The obese threshold indicates the intersection between the linear regression relationship and the BMI Z-score of 1.64 (CDC definition of obese). The overweight threshold occurred at 6.7 years in girls and 8.7 years in boys. The obese threshold occurred at 12.5 years in girls and 12.4 years in boys.
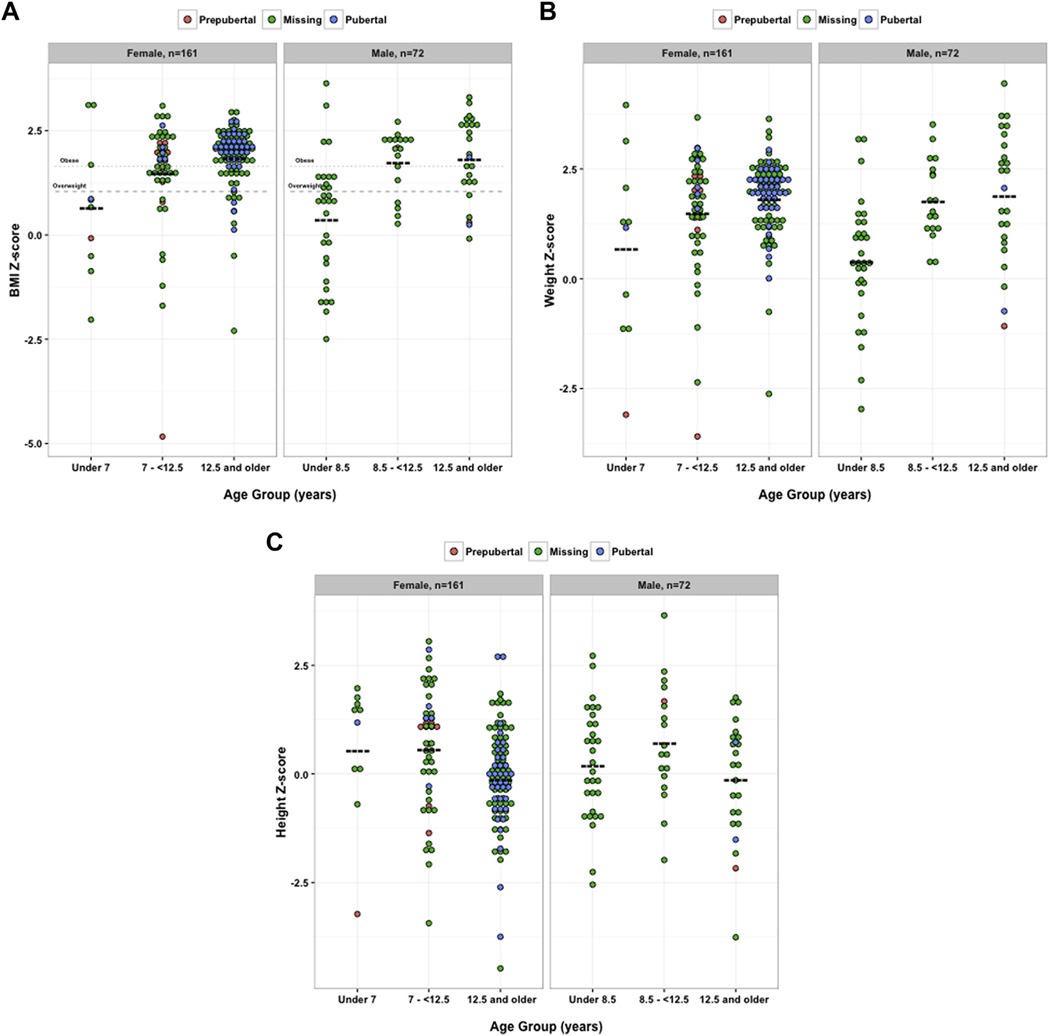
Thus, we have illustrated that there are distinct differences in the anthropometric features of pediatric participants with IIH, depending on the age of diagnosis. Body mass index reflects the relationship between weight and height, both of which change during puberty in complex ways that can depend on both gender and adiposity.16 To delineate better the role of growth patterns in pediatric IIH, we next considered height and weight Z-scores separately. We used gender-specific overweight and obese thresholds, as defined via the regression analyses in Figure 1. Based on the anthropometric features seen in this population of participants with pediatric IIH, these empirically determined age cutoffs helped to define 3 subgroups of pediatric IIH. For girls, we considered children younger than 7 years, early adolescents between 7 and 12.5 years of age, and later adolescents 12.5 years of age or older. For boys, we considered children younger than 8.5 years, early adolescents between 8.5 and 12.5 years of age, and later adolescents 12.5 years of age or older.
Illustrated with dot distribution plots, there were positive relationships between the defined age categories of IIH and both BMI Z-scores and weight Z-scores in both girls and boys (Fig 2A, B). In contrast, height Z-scores were statistically higher in the early adolescent age category for both girls and boys when compared with the theoretical population average height Z-score of 0 (Fig 2C; by 1-sample t test). Compared with the observed average height Z-score of 0.2 in a nationally representative sample,17 height Z-values in the early adolescent group remained statistically significant when considering girls, and there was a trend for significance in boys (using 1-sample t test analyses to compare mean height Z-scores against a predicted mean Z-score of 0.2017: in girls younger than 7 years, P = 0.47; in girls 7–12.5 years of age, P = 0.048; in girls older than 12.5 years, P = 0.01; in boys younger than 8.5 years, P = 0.88; in boys 8.5–12.5 years of age, P = 0.089; and in boys older than 12.5 years, P = 0.29). These observations may indicate early linear growth acceleration in the early adolescent age group.
Figure 2.
Dot distribution plots showing the distinct differences in anthropometric features seen between defined age categories in participants with pediatric idiopathic intracranial hypertension (IIH). Distribution plots illustrate body mass index (BMI), weight, or height Z-scores, seen in defined age categories of participants with pediatric IIH. The age cutoffs were defined empirically, using the regression analysis in Figure 1. For girls, the categories were young children younger than 7 years, early adolescents between 7 and 12.5 years of age, and older adolescents 12.5 years of age or older. For boys, the categories were young children younger than 8.5 years, early adolescents between 8.5 and 12.5 years of age, and older adolescents 12.5 years of age or older. In both boys and girls with pediatric IIH, (A) BMI Z-scores and (B) weight Z-scores increased in older age categories (P = 0.0001 in girls and P < 0.0001 in boys, analysis of variance of BMI Z-scores; and P = 0.0015 in girls and P < 0.0001 in boys, analysis of variance of weight Z-scores). C, In girls and boys with pediatric IIH, only height Z-scores measured in the early adolescent categories were higher than the age- and gender-matched hypothesized values. Statistical comparisons used 1-way t test analyses to compare mean Z-scores against a hypothesized mean Z-score of 0.00 (e.g., age- and gender-matched normative data). In girls, P = 0.28 in those younger than 7 years, P = 0.004 in those 7 to 12.5 years, and P = 0.45 in those 12.5 years of age or older. In boys, P = 0.35 in those younger than 8.5 years, P = 0.03 in those 8.5 to 12.5 years, and P = 0.71 in those 12.5 years of age or older. In (A), (B), and (C), circles represent individual data points, whereas the color of the circle indicates pubertal status (“missing” indicates that pubertal information was not available). Boldface, dashed lines indicate mean values.
Pubertal data were available in 57 patients with definite IIH, representing 25% of the total number of patients accrued. We applied standardized definitions of pubertal status, obtained within 3 months of diagnosis of IIH. As outlined in Table 2, 21% (n = 12) were prepubertal and 79% (n = 45) were pubertal at the time of the diagnosis of IIH. Compared with the prepubertal population, BMI Z-scores and weight Z-scores were higher in the pubertal population, but did not reach statistical significance in this small subsample. Conversely, height Z-scores were not statistically different between prepubertal and pubertal groups. Pubertal status may influence anthropometric features at presentation of pediatric IIH, although a careful prospective collection of pubertal stage information would be needed to establish this in a larger sample.
Discussion
In this study, we used pediatric-specific standards to examine the influence of anthropometric features and sexual maturation on the development of pediatric IIH. We included more than 200 clinical cases of definite IIH using the recently revised criteria for IIH.1 To our knowledge, this is the largest study examining clinical characteristics at presentation of pediatric IIH. Moreover, height and weight measurements were collected from the electronic medical record, and the subanalysis of pubertal status was based on physical examination data by physicians. The CDC recommends the use of BMI Z-values or BMI percentile values to define excess weight in children. In this study, BMI Z-values were used as the metric of pediatric weight status. In contrast to BMI percentile, BMI Z-score is a continuous variable that limits clustering of data points at higher values and lends itself to further statistical analysis, including regression plots.
Previous studies on the influence of anthropometric features and pubertal status on IIH have been limited. First, many did not apply pediatric-specific measurements of anthropometric features.2 Moreover, there are also gender differences to consider in the pediatric population, with specific BMI growth curves for boys and girls.9 A recent study into the anthropometric features of pediatric IIH did use appropriate BMI percentiles to characterize the frequency of overweight and obesity features in the pediatric population with IIH, with overall directionally similar findings.18 However, IIH diagnoses were supported using former diagnostic criteria for pediatric IIH,18 in contrast to the application of the recently revised criteria used in the present study.1 In the present, larger study, we assembled a cohort from a diverse range of sites and included more detailed analyses of height. Finally, previous studies examining the influence of pubertal status on the presentation of IIH have used age-dependent assumptions of puberty,5,6 which do not account for the wide range of physiologic variation in pubertal timing. The use of arbitrary cutoff values may lead to limited insights, and in the present study, we included a subanalysis of directly measured pubertal stage.
This study thus confirms and extends previous insights into pediatric IIH. We confirmed that there is a positive relationship between BMI Z-score and age at diagnosis of pediatric IIH. Using standardized definitions of pediatric obesity accepted at that time, Balcer et al2 evaluated anthropometric data from 40 participants and illustrated the presence of 2 distinct populations of pediatric IIH, namely a younger, equally male and female population and an older, predominately obese and female population. Other studies have illustrated similar findings.18 The present study does support an increasing prevalence of pediatric obesity in older children with IIH. We also identified that the proportion of boys is higher at younger age groups; however, there is no significant effect of gender on the relationship between BMI Z-score and age at diagnosis of IIH.
Our study revealed a new observation that there are multiple subgroups of pediatric IIH as defined by anthropometric thresholds of overweight and obesity features. Specifically, in young children (less than approximately 7 years of age in girls and less than 8.5 years of age in boys), BMI and height Z-scores are similar to age- and gender-based reference standards. In early adolescence, BMI Z-scores cluster between the overweight and obese thresholds, whereas height Z-scores are greater than age and gender-matched reference standards. Finally, in late adolescence (older than approximately 12.5 years of age in girls and boys), average BMI Z-scores are well above the overweight and obese thresholds, and height Z-scores again are similar to age- and gender-matched reference standards. In this cross-sectional study, it remains unclear if these 3 subgroups indicate an underlying progression of pediatric IIH or if these represent distinct subgroups of the disease with unique clinical features and, possibly, underlying pathophysiology. It is also unclear if these 3 subgroups are defined best by anthropometric features, as completed in this study, or if other developmental processes, possibly the development of secondary sexual characteristics, better define these groups. Further longitudinal studies that include explicit measurements of secondary sexual characteristics are required to extend these observations. Moreover, further studies are also needed to determine whether an obesity-related linear growth acceleration, a feature seen in overweight pediatric participants without IIH, drives the taller stature in the early adolescent group.19,20
By characterizing subsets of pediatric IIH, the aim is that governing anthropometric features within each group may be defined. In turn, insights may be gained into potential endocrine foundations of this condition and, ultimately, an understanding of its overall pathogenesis. In prepubertal children younger than 8 years, we suggest that factors other than adiposity may contribute to the presentation of disease. Indeed, examples exist in the literature of secondary pediatric pseudotumor cerebri syndrome in nonobese participants. These include exposure to recombinant growth hormone for various conditions, including growth hormone deficiency, Turner syndrome, and renal disease,21,22 as well as pediatric participants with secondary aldosteronism, possibly related to renal structural or transport dysfunction (e.g., SLC12A3 gene mutation, renal congenital hypoplasia, or genetic renal tubular disorders).23 In the present study, no evidence was found for these disorders on detailed review of the electronic records, but no consistent screening paradigm was applied. Development of an evidence-based screening paradigm for prepubertal children with pseudotumor cerebri syndrome may be an important focus of future investigations.
In the early and late adolescent subgroups, adiposity clearly is associated with IIH. Pediatric adiposity has complex pathophysiologic factors, and as such, many potential factors are likely contributing to the presentation of disease.24 The observation that there is a taller height Z-score in the early adolescent subgroup of pediatric IIH indicates that, at this age, participants are taller than age- and gender-matched reference standards. Increased adiposity has been associated with increased linear growth acceleration in early adolescence in otherwise healthy children.19,20 In general, obese children tend to grow quickly before puberty and may have less growth during puberty as a result. The possibility exists that the increased linear growth acceleration seen in the early adolescent population with IIH is reflecting effects of weight status and not clearly related to IIH per se. However, it may be that factors that influence linear growth acceleration in young adults with overweight and obese status also may influence the development of IIH. Indeed, the secretion and action of growth hormone has been posited to differ in obese individuals, and growth hormone has been implicated in the pathogenesis of pseudotumor cerebri syndrome.25,26 Gonadal hormones also may contribute to the pathogenesis of IIH, particularly in the early and late adolescent, and more likely pubertal, age groups. Indeed, exposure to exogenous (e.g., levonorgestrel27 and emergency contraceptives28) and endogenous (e.g., pregnancy29,30 and polycystic ovary syndrome31) estrogens have been associated with IIH. Moreover, increased androgens, namely basal testosterone and androstenedione, have been linked with a younger age at presentation of IIH in an adult female population.32 Prospective studies, including measurements of adrenal and gonadal steroids, would be useful to understand how these affect IIH pathogenesis around the time of adrenarche and puberty.
Finally, in this study, data were obtained on pubertal status in one quarter of the cases analyzed. Our findings are consistent with previous suggestions that prepubertal participants are of normal weight, whereas participants who have entered puberty demonstrate higher BMI and weight Z-scores at the time of IIH diagnosis.2 These data are strengthened by the use of the documented Tanner staging, notation of menstrual status, or both. The most notable limitation is the incomplete information on pubertal status, making this set of secondary analyses more susceptible to selection bias. We clearly indicated pubertal status in the figures, such that the potential role of selection bias in the results obtained can be visualized directly.
Additional limitations to these data exist. We operated under the assumption that the data collected for the present study accurately represent the population of pediatric IIH as a whole. Retrospective studies are limited by incomplete medical records as well as recall and selection bias. Differences also may exist in data collection technique and documentation in a multisite study. However, in the present study, having collected more than 200 clinical cases of definite IIH, all from sites with expertise in this condition, and each case being rigorously vetted with standard case report forms, we do not anticipate that these differences would have produced systematic errors.
In summary, this study identified 3 subgroups of pediatric IIH based on anthropometric thresholds of pediatric overweight and obesity features. The present study also highlighted the importance of considering sexual maturation when investigating potential developmentally specific mechanisms of IIH. Defining key events that influence the risk for pediatric IIH and its relationship with obesity may provide insight into the endocrine factors that predispose individuals to this poorly understood condition.
Acknowledgments
The authors thank Marianne R. Chilutti and Evanette K. Burrows, the Children’s Hospital of Philadelphia Center for Biomedical Informatics, for their help in designing and building the IIH patient database on Research Electronic Data Capture.
Supported in part by the National Institute of General Medical Sciences, National Institutes of Health, Bethesda, Maryland (grant nos.: 5-K12-DK-94723-2 [S.E.M.] and K23-DK-102659-01 [S.E.M.]); and Boston Children’s Hospital, Boston, Massachusetts (G.H.).
Abbreviations and Acronyms
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- IIH
idiopathic intracranial hypertension
Footnotes
Presented in part at: North American Neuro-Ophthalmology Society Annual Meetings, March 2014 and February 2015.
Financial Disclosure(s):
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Author Contributions:
Conception and design: Sheldon, Paley, McCormack, Liu
Analysis and interpretation: Sheldon, Paley, Xiao, Kesler, Eyal, Ko, Boisvert, Avery, Salpietro, Phillips, Heidary, McCormack, Liu
Data collection: Sheldon, Paley, Kesler, Eyal, Ko, Boisvert, Avery, Salpietro, Phillips, Heidary, McCormack, Liu
Obtained funding: none
Overall responsibility: Sheldon, Paley, McCormack, Liu
References
- 1.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1–7. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- 2.Balcer LJ, Liu GT, Forman S, et al. Idiopathic intracranial hypertension: relation of age and obesity in children. Neurology. 1999;52(4):870–872. doi: 10.1212/wnl.52.4.870. [DOI] [PubMed] [Google Scholar]
- 3.Ko MW, Liu GT. Pediatric idiopathic intracranial hypertension (pseudotumor cerebri) Horm Res Paediatr. 2010;74(6):381–389. doi: 10.1159/000321180. [DOI] [PubMed] [Google Scholar]
- 4.Rangwala LM, Liu GT. Pediatric idiopathic intracranial hypertension. Surv Ophthalmol. 2007;52(6):597–617. doi: 10.1016/j.survophthal.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Cinciripini GS, Donahue S, Borchert MS. Idiopathic intracranial hypertension in prepubertal pediatric patients: characteristics, treatment, and outcome. Am J Ophthalmol. 1999;127(2):178–182. doi: 10.1016/s0002-9394(98)00386-9. [DOI] [PubMed] [Google Scholar]
- 6.Stiebel-Kalish H, Kalish Y, Lusky M, et al. Puberty as a risk factor for less favorable visual outcome in idiopathic intracranial hypertension. Am J Ophthalmol. 2006;142(2):279–283. doi: 10.1016/j.ajo.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 7.Gordon K. Pediatric pseudotumor cerebri: descriptive epidemiology. Can J Neurol Sci. 1997;24(3):219–221. doi: 10.1017/s031716710002182x. [DOI] [PubMed] [Google Scholar]
- 8.Bassan H, Berkner L, Stolovitch C. Asymptomatic idiopathic intracranial hypertension in children. Acta Neurol Scand. 2008;118:251–255. doi: 10.1111/j.1600-0404.2008.01007.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 10.Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings Network. Pediatrics. 1997;99(4):505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 11.Herman-Giddens ME, Steffes J, Harris D, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. 2012;130(5):e1058–e1068. doi: 10.1542/peds.2011-3291. [DOI] [PubMed] [Google Scholar]
- 12.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanner JM. Normal growth and techniques of growth assessment. Clin Endocrinol Metab. 1986;15(3):411–451. doi: 10.1016/s0300-595x(86)80005-6. [DOI] [PubMed] [Google Scholar]
- 15.Dorn LD, Sontag-Padilla LM, Pabst S, et al. Longitudinal reliability of self-reported age at menarche in adolescent girls: variability across time and setting. Dev Psychol. 2013;49(6):1187–1193. doi: 10.1037/a0029424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crocker MK, Stern EA, Sedaka NM, et al. Sexual dimorphisms in the associations of BMI and body fat with indices of pubertal development in girls and boys. J Clin Endocrinol Metab. 2014;99(8):E1519–E1529. doi: 10.1210/jc.2014-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber DR, Leonard MB, Shults J, Zemel BS. A comparison of fat and lean body mass index to BMI for the identification of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab. 2014;99(9):3208–3216. doi: 10.1210/jc.2014-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brara SM, Koebnick C, Porter AH, Langer-Gould A. Pediatric idiopathic intracranial hypertension and extreme childhood obesity. J Pediatr. 2012;161(4):602–607. doi: 10.1016/j.jpeds.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Q, Karlberg J. BMI in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res. 2001;49(2):244–251. doi: 10.1203/00006450-200102000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Minuto F, Barreca A, Del Monte P, et al. Spontaneous growth hormone and somatomedin-C/insulin-like growth factor-I secretion in obese subjects during puberty. J Endocrinol Invest. 1988;11(7):489–495. doi: 10.1007/BF03350166. [DOI] [PubMed] [Google Scholar]
- 21.Reeves GD, Doyle DA. Growth hormone treatment and pseudotumor cerebri: coincidence or close relationship? J Pediatr Endocrinol Metab. 2002;15(Suppl 2):723–730. doi: 10.1515/jpem.2002.15.s2.723. [DOI] [PubMed] [Google Scholar]
- 22.Darendeliler F, Karagiannis G, Wilton P. Headache, idiopathic intracranial hypertension and slipped capital femoral epiphysis during growth hormone treatment: a safety update from the KIGS database. Horm Res. 2007;68(Suppl 5):41–47. doi: 10.1159/000110474. [DOI] [PubMed] [Google Scholar]
- 23.Khan MU, Khalid H, Salpietro V, Weber KT. Idiopathic intracranial hypertension associated with either primary or secondary aldosteronism. Am J Med Sci. 2013;346(3):194–198. doi: 10.1097/MAJ.0b013e31826e3635. [DOI] [PubMed] [Google Scholar]
- 24.Sheldon CA, Kwon YJ, Liu GT, McCormack SE. An integrated mechanism of pediatric pseudotumor cerebri syndrome: evidence of bioenergetic and hormonal regulation of cerebrospinal fluid dynamics. Pediatr Res. 2015;77(2):282–289. doi: 10.1038/pr.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menucci MB, Burman KD. Endocrine changes in obesity. In: De Groot LJ, Beck-Peccoz P, Chrousos G, et al., editors. Endotext. South Dartmouth, MA: MDText.com, Inc; 2000. [Google Scholar]
- 26.Malozowski S, Tanner LA, Wysowski D, Fleming GA. Growth hormone, insulin-like growth factor I, and benign intracranial hypertension. N Engl J Med. 1993;329(9):665–666. doi: 10.1056/NEJM199308263290917. [DOI] [PubMed] [Google Scholar]
- 27.Alder JB, Fraunfelder FT, Edwards R. Levonorgestrel implants and intracranial hypertension [letter] N Engl J Med. 1995;332:1720–1721. [PubMed] [Google Scholar]
- 28.Ivancic R, Pfadenhauer K. Pseudotumor cerebri after hormonal emergency contraception. Eur Neurol. 2004;52(2):120. doi: 10.1159/000080269. [DOI] [PubMed] [Google Scholar]
- 29.Digre KB, Varner MW, Corbett JJ. Pseudotumor cerebri and pregnancy. Neurology. 1984;34:721–729. doi: 10.1212/wnl.34.6.721. [DOI] [PubMed] [Google Scholar]
- 30.Kesler A, Kupferminc M. Idiopathic intracranial hypertension and pregnancy. Clin Obstet Gynecol. 2013;56(2):389–396. doi: 10.1097/GRF.0b013e31828f2701. [DOI] [PubMed] [Google Scholar]
- 31.Glueck CJ, Aregawi D, Goldenberg N, et al. Idiopathic intracranial hypertension, polycystic-ovary syndrome, and thrombophilia. J Lab Clin Med. 2005;145(2):72–82. doi: 10.1016/j.lab.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Klein A, Stern N, Osher E, et al. Hyperandrogenism is associated with earlier age of onset of idiopathic intracranial hypertension in women. Curr Eye Res. 2013;38(9):972–976. doi: 10.3109/02713683.2013.799214. [DOI] [PubMed] [Google Scholar]