Abstract
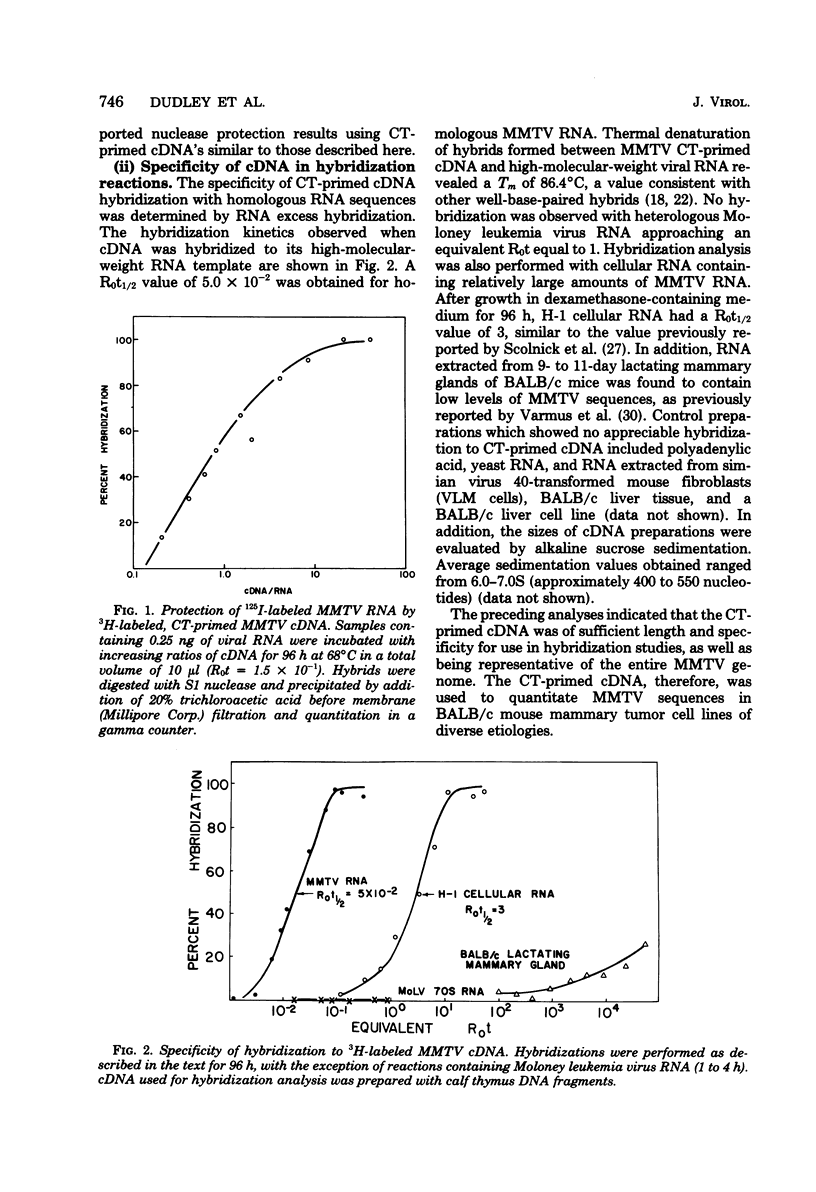
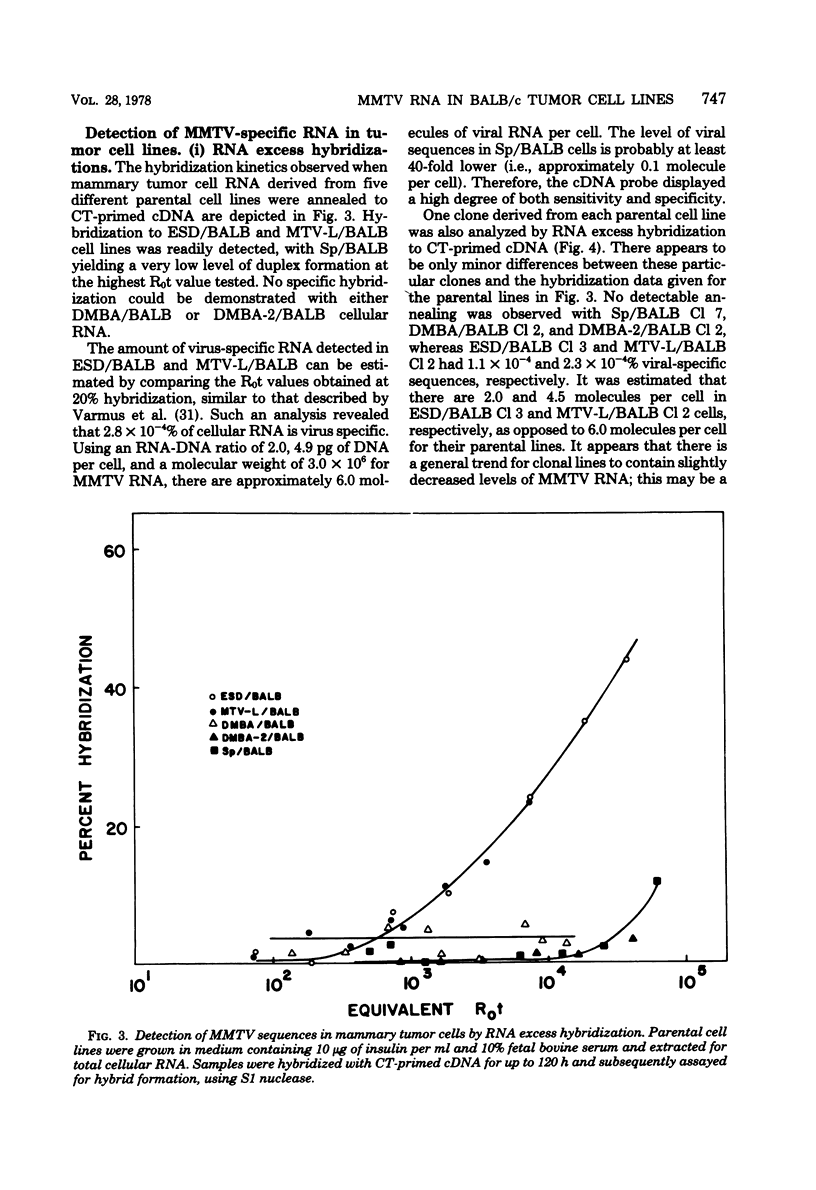
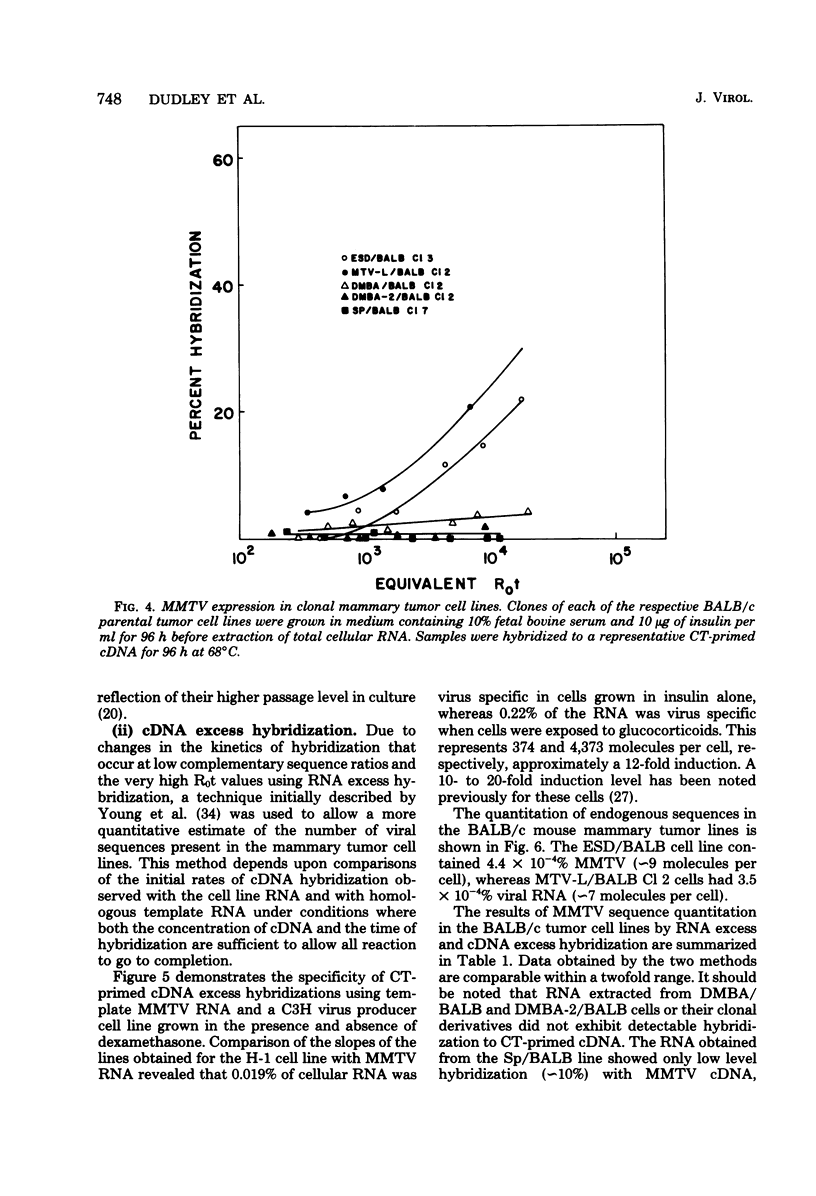
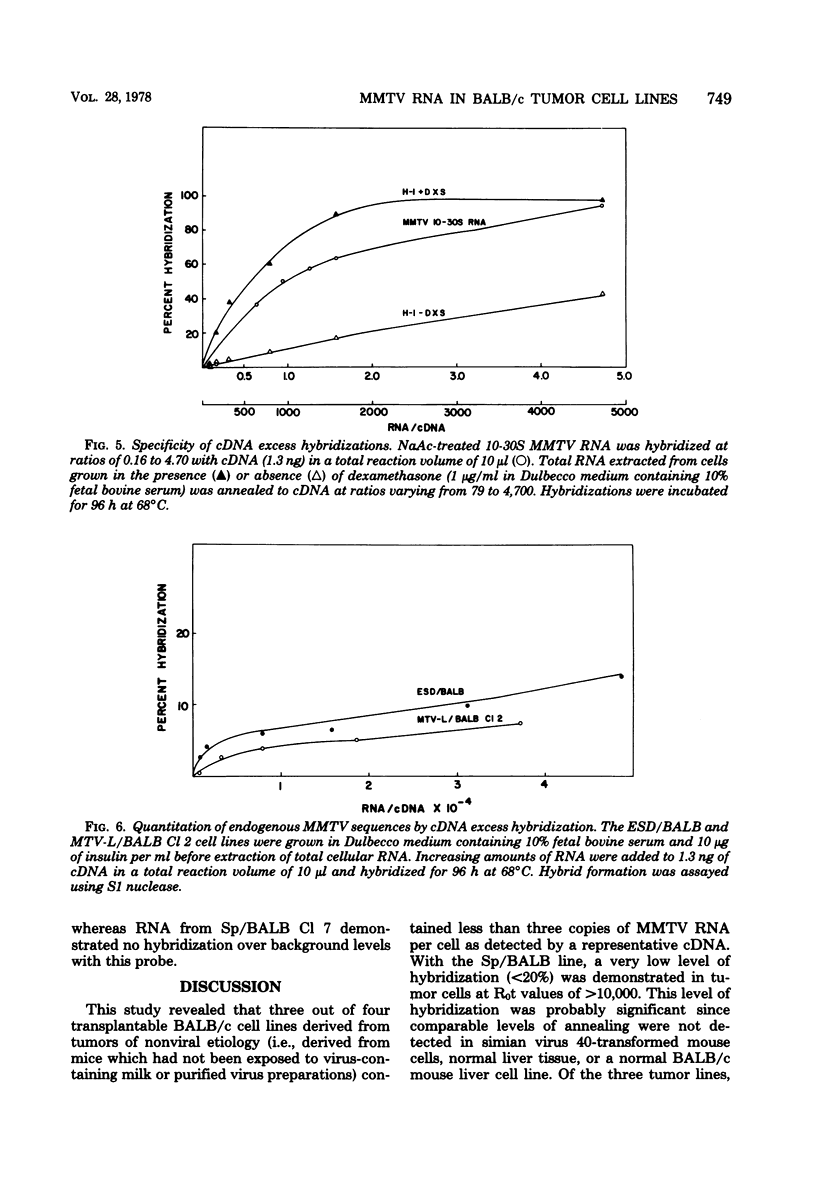
A complementary DNA (cDNA) probe to mouse mammary tumor virus (MMTV) RNA was synthesized using calf thymus DNA oligonucleotides as a random primer. This probe was then used to study the expression of MMTV RNA in cell lines from BALB/c tumors induced in vivo either spontaneously or in response to viral, chemical, or hormonal stimuli. The cDNA had a length of approximately 400 to 500 nucleotides and specifically hybridized to MMTV RNA and BALB/c lactating mammary gland RNA, but not to Moloney leukemia virus RNA. Calf thymus DNA-primed cDNA could protect 50% of iodinated MMTV RNA from S1 nuclease digestion at cDNA-RNA ratios of 1:1 and 90% of labeled viral RNA at ratios of 10:1. Thermal denaturation of MMTV RNA-cDNA hybrids yielded a Tm of 88.5°C, indicative of a well-base-paired duplex. Screening of mouse mammary tumor cells for MMTV sequences revealed that three out of five lines of BALB/c origin had undetectable levels of viral RNA (<three molecules per cell) by RNA excess hybridization. Two of the three “virus-negative” cell lines were derived from tumors induced by the chemical carcinogen 7,12-dimethylbenz(α)anthracene, whereas the third tumor occurred spontaneously. Two lines from tumors induced by either viral (mammary tumor virus) or hormonal (17-β-estradiol) stimulus contained between three and nine molecules of MMTV RNA per cell by both RNA excess and cDNA excess hybridization. Clonal derivatives of these tumor lines had levels of viral RNA comparable to those of their parental lines. Therefore, it appears that the presence of detectable MMTV RNA sequences is not a necessary requirement for the maintenance of all murine mammary gland neoplasias.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentvelzen P., Daams J. H., Hageman P., Calafat J. Genetic transmission of viruses that incite mammary tumor in mice. Proc Natl Acad Sci U S A. 1970 Sep;67(1):377–384. doi: 10.1073/pnas.67.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Dudley J. P., Medina D. Comparison of the growth properties in vitro and transplantability of continuous mouse mammary tumor cell lines and clonal derivatives. Cancer Res. 1977 Jun;37(6):1892–1900. [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Cox R. F. Estrogen withdrawal in chick oviduct. Selective loss of high abundance classes of polyadenylated messenger RNA. Biochemistry. 1977 Jul 26;16(15):3433–3443. doi: 10.1021/bi00634a022. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Nayak D. P. Expression of endogenous retroviral genes in leukemic guinea pig cells. J Virol. 1977 Aug;23(2):263–271. doi: 10.1128/jvi.23.2.263-271.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Fischinger P. J. Nucleotide sequences in mouse DNA and RNA specific for Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3705–3709. doi: 10.1073/pnas.73.10.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz M. J., Elder P. K., Moses H. L. Equivalent expression of endogenous murine leukemia virus-related genes in C3H/10T1/2 cells and chemically transformed derivative cells. Cancer Res. 1978 Mar;38(3):566–569. [PubMed] [Google Scholar]
- Getz M. J., Reiman H. M., Jr, Siegal G. P., Quinlan T. J., Proper J., Elder P. K., Moses H. L. Gene expression in chemically transformed mouse embryo cells: selective enhancement of the expression of C type RNA tumor virus genes. Cell. 1977 Aug;11(4):909–921. doi: 10.1016/0092-8674(77)90302-6. [DOI] [PubMed] [Google Scholar]
- Harris S. E., Rosen J. M., Means A. R., O'Malley B. W. Use of a specific probe for ovalbumin messenger RNA to quantitate estrogen-induced gene transcripts. Biochemistry. 1975 May 20;14(10):2072–2081. doi: 10.1021/bi00681a006. [DOI] [PubMed] [Google Scholar]
- Hilgers J., Bentvelzen P. Interaction between viral and genetic factors in murine mammary cancer. Adv Cancer Res. 1978;26:143–195. doi: 10.1016/s0065-230x(08)60087-1. [DOI] [PubMed] [Google Scholar]
- Links J., Calafat J., Buijs F., Tol O. Simultaneous chemical induction of MTV and MLV in vitro. Eur J Cancer. 1977 Jun;13(6):557–587. [PubMed] [Google Scholar]
- Medina D., DeOme K. B. Effects of various oncogenic agents on tumor-producing capabilities of series D BALB-c mammary nodule outgrowth lines. J Natl Cancer Inst. 1970 Aug;45(2):353–363. [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. VI. Tumor-producing capabilities of mammary dysplasias in BALB/cCrgl mice. J Natl Cancer Inst. 1976 Nov;57(5):1185–1189. doi: 10.1093/jnci/57.5.1185. [DOI] [PubMed] [Google Scholar]
- Medina D. Preneoplastic lesions in murine mammary cancer. Cancer Res. 1976 Jul;36(7 Pt 2):2589–2595. [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., Woo S. L., Robberson D. L., O'Malley B. W. The synthesis and properties of the complete complementary DNA transcript of ovalbumin mRNA. Biochemistry. 1976 Jan 13;15(1):223–233. doi: 10.1021/bi00646a034. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Hubbell E. S., Goldberg R. J., O'Neill F. J., Scolnick E. M. High frequency variation in mammary tumor virus expression in cell culture. Cell. 1976 May;8(1):87–93. doi: 10.1016/0092-8674(76)90189-6. [DOI] [PubMed] [Google Scholar]
- Prensky W., Steffensen D. M., Hughes W. L. The use of iodinated RNA for gene localization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1860–1864. doi: 10.1073/pnas.70.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. M., Barker S. W. Quantitation of casein messenger ribonucleic acid sequences using a specific complementary DNA hybridization probe. Biochemistry. 1976 Nov 30;15(24):5272–5280. doi: 10.1021/bi00669a012. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Comstock J. P. Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry. 1975 Jul;14(13):2895–2903. doi: 10.1021/bi00684a016. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Holder J. W., Means A. R., O'Malley B. W. Preparation and preliminary characterization of purified ovalbumin messenger RNA from the hen oviduct. Biochemistry. 1975 Jan 14;14(1):69–78. doi: 10.1021/bi00672a012. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Pomenti A. A., Dion A. S. Replication of mouse mammary tumor virus in tissue culture. II. Kinetics of virus production and the effect of RNA and protein inhibitors on viral synthesis. Virology. 1977 Mar;77(1):31–44. doi: 10.1016/0042-6822(77)90403-2. [DOI] [PubMed] [Google Scholar]
- Schlom J., Michalides R., Kufe D., Hehlmann R., Spiegelman S., Bentvelzen P., Hageman P. A comparative study of the biologic and molecular basis of murine mammary carcinoma: a model for human breast cancer. J Natl Cancer Inst. 1973 Aug;51(2):541–551. [PubMed] [Google Scholar]
- Scolnick E. M., Young H. A., Parks W. P. Biochemical and physiological mechanisms in glucocorticoid hormone induction of mouse mammary tumor virus. Virology. 1976 Jan;69(1):148–156. doi: 10.1016/0042-6822(76)90202-6. [DOI] [PubMed] [Google Scholar]
- Timmermans A., Bentvelzen P., Hageman P. C., Calat J. Activation of a mammary tumour virus in O20 strain mice by x-irradiation and urethane. J Gen Virol. 1969 Jun;4(4):619–621. doi: 10.1099/0022-1317-4-4-619. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Orenstein J. M., Gebert R., Kaighn M. E., Stadler U. C. Growth and structural properties of epithelial cell cultures established from normal rat liver and chemically induced hepatomas. Cancer Res. 1975 Jan;35(1):253–263. [PubMed] [Google Scholar]
- Woo S. L., Rosen J. M., Liarakos C. D., Choi Y. C., Busch H., Means A. R., O'Malley Physical and chemical characterization of purified ovalbumin messenger RNA. J Biol Chem. 1975 Sep 10;250(17):7027–7039. [PubMed] [Google Scholar]
- Young B. D., Harrison P. R., Gilmour R. S., Birnie G. D., Hell A., Humphries S., Paul J. Kinetic studies of gene frequency. II. Complexity of globin complementary DNA and its hybridization characteristics. J Mol Biol. 1974 Apr 25;84(4):555–568. doi: 10.1016/0022-2836(74)90116-8. [DOI] [PubMed] [Google Scholar]
- Young H. A., Shih T. Y., Scolnick E. M., Parks W. P. Steroid induction of mouse mammary tumor virus: effect upon synthesis and degradation of viral RNA. J Virol. 1977 Jan;21(1):139–146. doi: 10.1128/jvi.21.1.139-146.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


