Abstract
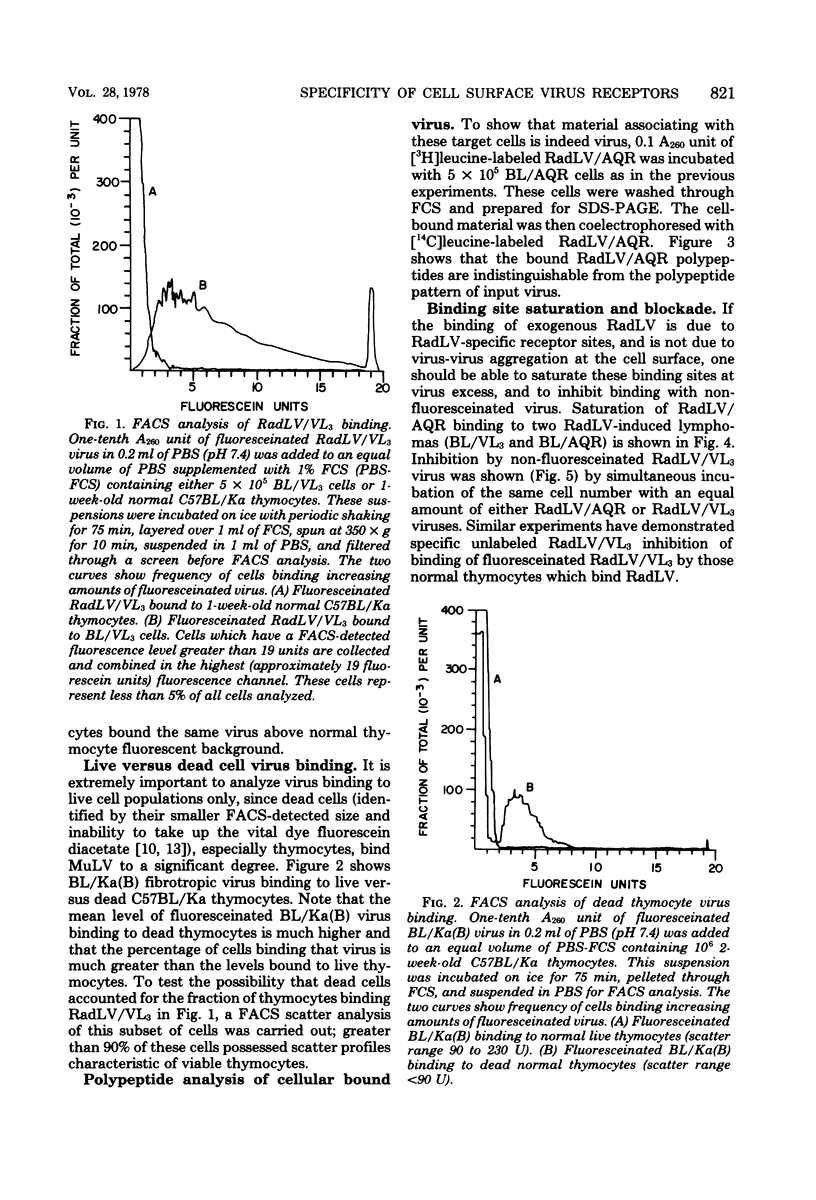
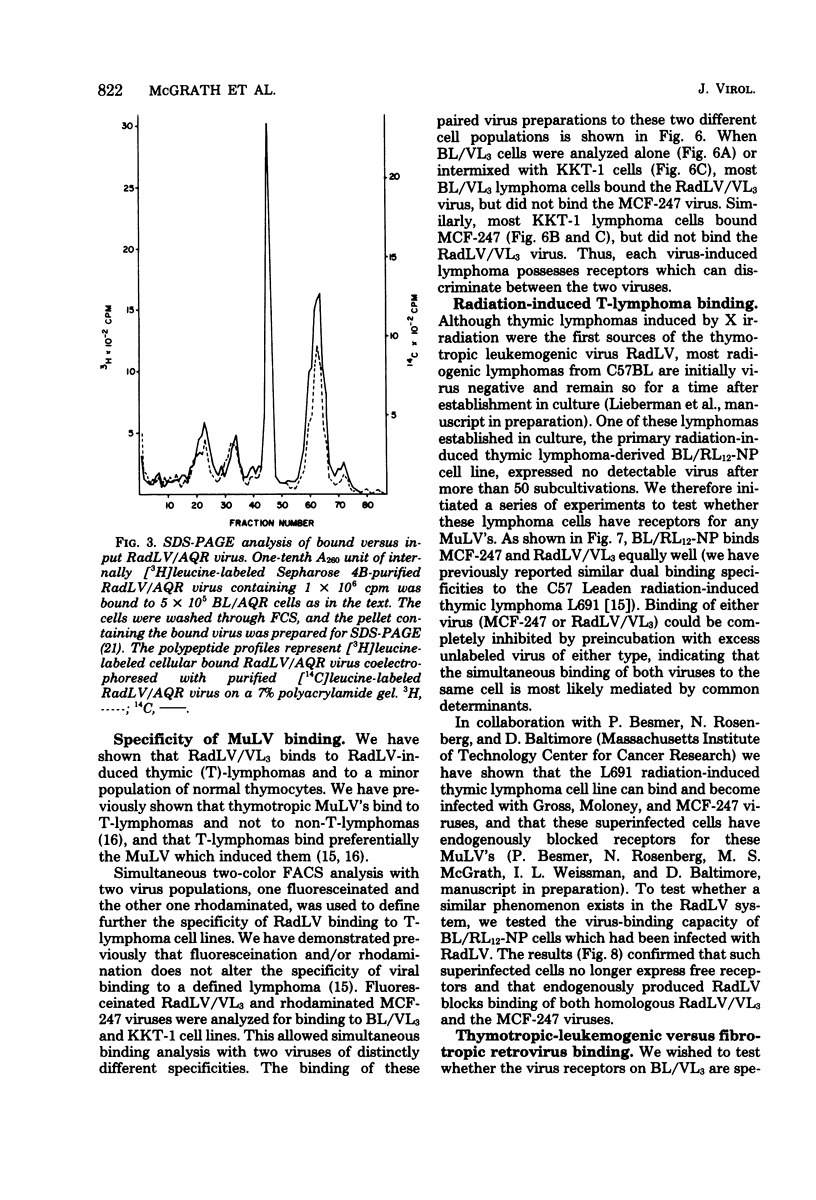
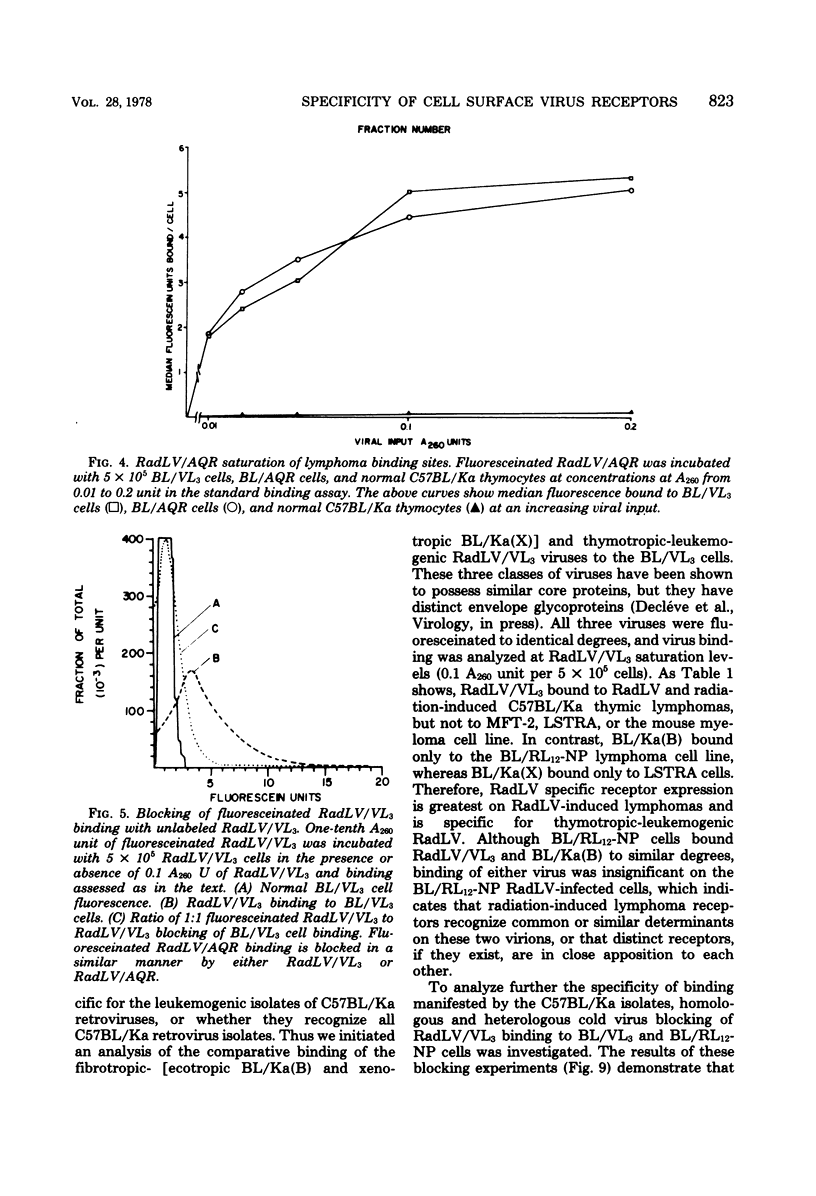
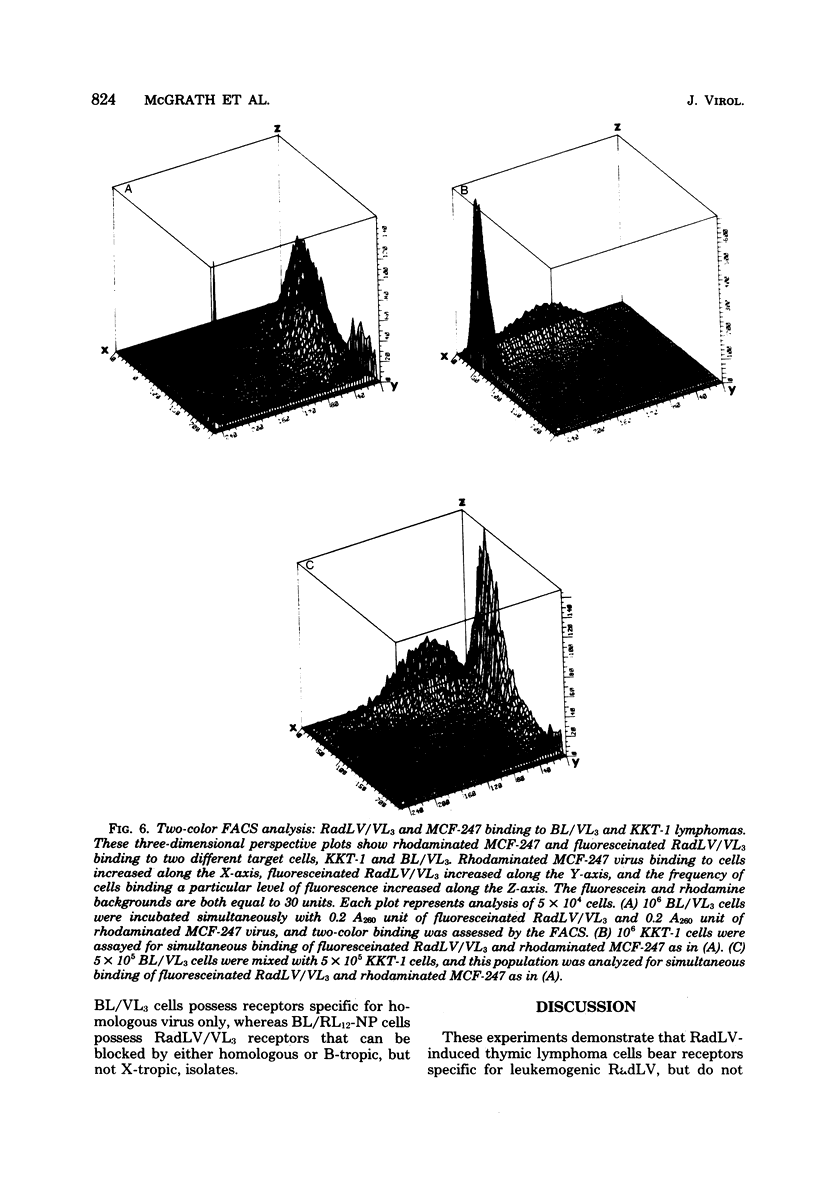
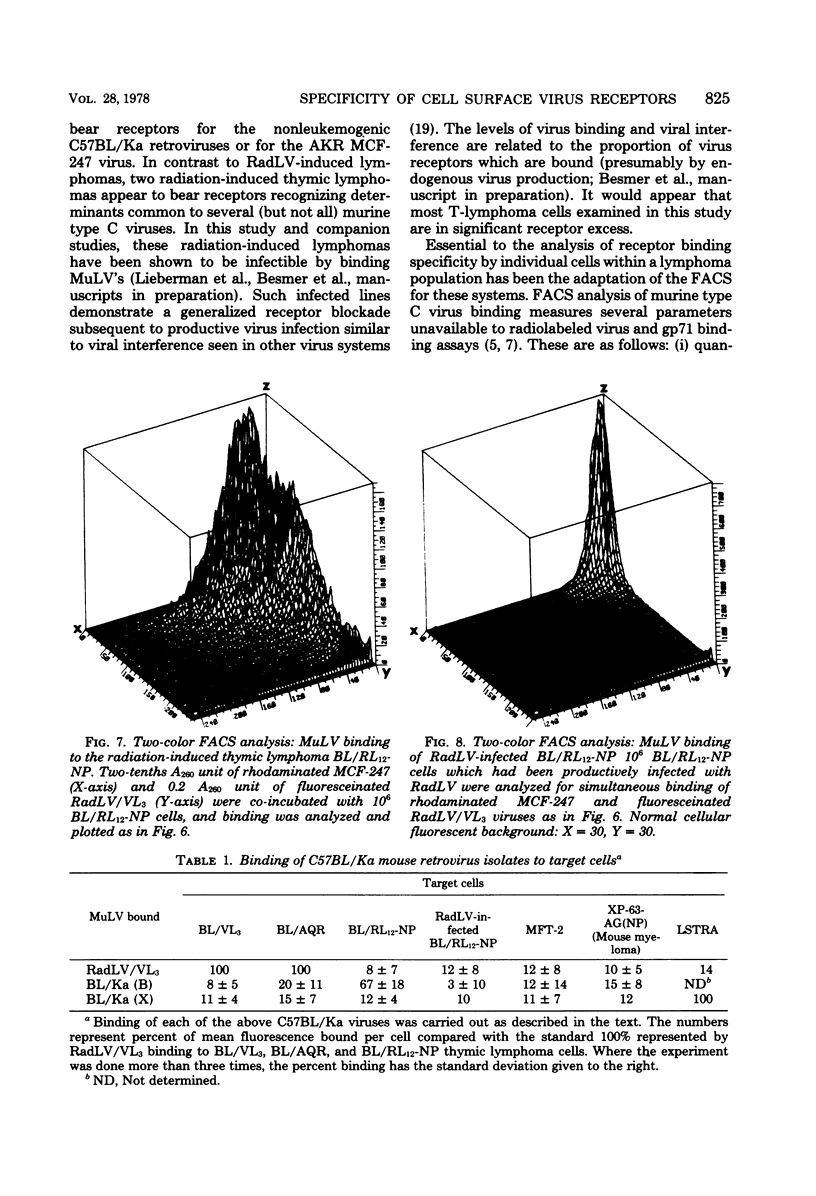
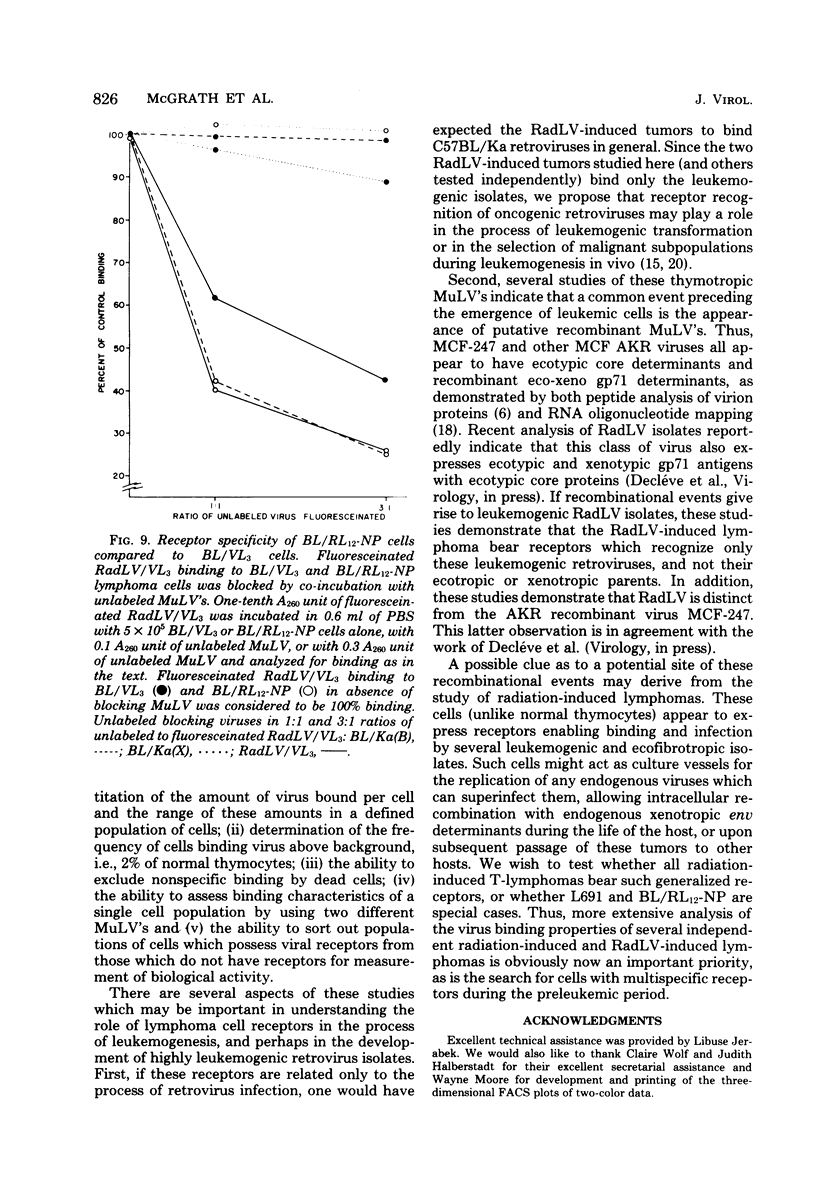
We have developed a system for analysis of murine leukemic virus (MuLV) receptors on the surface of thymic lymphoma cells utilizing the fluorescence-activated cell sorter. The binding of fluoresceinated or rhodaminated MuLV to target cells showed saturation kinetics and was blocked by homologous MuLV, and bound MuLV had a polypeptide profile identical to that of input MuLV. Thymic lymphomas bound specifically the MuLV which induced them, whereas only 0.5 to 2% of normal thymocytes showed equivalent MuLV binding. Simultaneous binding of excess fluoresceinated RadLV and rhodaminated MCF-247 AKR virus to radiation leukemia virus-induced or spontaneous AKR thymic lymphomas demonstrated that even in the presence of both viruses the cells bound preferentially the inducing MuLV. Examination of the C57BL/Ka endogenous viruses showed that radiation leukemia virus-induced thymic lymphomas bind only thymotropic-leukemogenic radiation leukemia virus and not eco- or xenofibrotropic MuLV's. Thus, virus binding in this system involves only leukemogenic isolates of these retroviruses and implies a central role of this receptor-ligand interaction in the processes of leukemic transformation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird S., Raschke W., Weissman I. L. Evidence that MuLV-induced thymic lymphoma cells possess specific cell membrane binding sites for MuLV. Int J Cancer. 1977 Mar 15;19(3):403–413. doi: 10.1002/ijc.2910190319. [DOI] [PubMed] [Google Scholar]
- DeLarco J., Todaro G. J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976 Jul;8(3):365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Declève A., Lieberman M., Ihle J. N., Kaplan H. S. Biological and serological characterization of radiation leukemia virus. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4675–4679. doi: 10.1073/pnas.73.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declève A., Lieberman M., Kaplan H. S. In vivo interaction between RNA viruses isolated from the C57BL/Ka strain of mice. Virology. 1977 Sep;81(2):270–283. doi: 10.1016/0042-6822(77)90144-1. [DOI] [PubMed] [Google Scholar]
- Declève A., Travis M., Weissman I. L., Lieberman M., Kaplan H. S. Focal infection and transformation in situ of thymus cell subclasses by a thymotropic murine leukemia virus. Cancer Res. 1975 Dec;35(12):3585–3595. [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. K., Twardzik D. R., Reed C. D., Weislow O. S., Hellman A. Binding characteristics of Rauscher leukemia virus envelope glycoprotein gp71 to murine lymphoid cells. J Virol. 1977 Dec;24(3):729–735. doi: 10.1128/jvi.24.3.729-735.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Sweet R. G., Herzenberg L. A. Fluorescence-activated cell sorting. Sci Am. 1976 Mar;234(3):108–117. doi: 10.1038/scientificamerican0376-108. [DOI] [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- Loken M. R., Parks D. R., Herzenberg L. A. Two-color immunofluorescence using a fluorescence-activated cell sorter. J Histochem Cytochem. 1977 Jul;25(7):899–907. doi: 10.1177/25.7.330738. [DOI] [PubMed] [Google Scholar]
- McGrath M., Weissman I. L., Baird S., Raschke W., Decleve A., Lieberman M., Kaplan H. S. Each T-cell lymphoma induced by a particular murine leukemia virus bears surface receptors specific for that virus. Birth Defects Orig Artic Ser. 1978;14(2):349–361. [PubMed] [Google Scholar]
- McGrath M., Witte O., Pincus T., Weissman I. L. Retrovirus purification: method that conserves envelope glycoprotein and maximizes infectivity. J Virol. 1978 Mar;25(3):923–927. doi: 10.1128/jvi.25.3.923-927.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. I. Establishment of interference. Virology. 1966 Aug;29(4):628–641. doi: 10.1016/0042-6822(66)90287-x. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L. Polypeptides of Moloney sarcoma-leukemia virions: their resolution and incorporation into extracellular virions. Virology. 1974 Oct;61(2):575–587. doi: 10.1016/0042-6822(74)90291-8. [DOI] [PubMed] [Google Scholar]


