Abstract
SLC44A2 was discovered as the target of an antibody that causes hearing loss. Knockout mice develop age related hearing loss, loss of sensory cells and spiral ganglion neurons. SLC44A2 has polymorphic sites implicated in human disease. Transfusion related acute lung injury (TRALI) is linked to rs2288904 and genome wide association studies link rs2288904 and rs9797861 to venous thromboembolism (VTE), coronary artery disease and stroke. Here we report linkage disequilibrium of rs2288904 with rs3087969 and the association of these SLC44A2 SNPs with Meniere’s disease severity. Tissue-specific isoform expression differences suggest that the N-terminal domain is linked to different functions in different cell types. Heterozygosity at rs2288904 CGA/CAA and rs3087969 GAT/GAC showed a trend for association with intractable Meniere’s disease compared to less severe disease and to controls. The association of SLC44A2 SNPs with VTE suggests that thrombi affecting cochlear vessels could be a factor in Meniere’s disease.
Keywords: Choline transporter-like protein 2, Solute Carrier protein 44A2, Hearing, vestibular tissue, human gene expression, DNA sequence differences, amino acid polymorphisms, Meniere’s Disease
1.0 Introduction
SLC44A2 was discovered as an antigen expressed in supporting cells in the inner ear1–3. Subsequently this antigen was shown to be a target of antibody-induced hearing loss4–7. Disruption of its function by in vivo antibody binding leads to auditory hair cell death and hearing loss4,5,7. This antigen is also a target of autoantibodies implicated in human autoimmune hearing loss6,8,9. The inner ear supporting cell antigen was identified as CTL2 (later called SLC44A2) by immunoprecipitation and mass spectrometry sequencing10. The SLC44A2 gene resides on human chromosome 19p13.1.
SLC44A2 is a member of the CTL1/SLC44A1-CTL5/SLC44A5 choline transporter-like protein family within the solute carrier (SLC) superfamily of membrane transporter proteins11. While the closely-related SLC44A1 protein has been shown to have a strong role in choline transport or uptake12–18 the function of SLC44A2 is still unclear. Some evidence suggests that SLC44A1 and SLC44A2 act together to transport choline19,20. Alone, the SLC44A2 isoform 2 shows weak choline transport21.
SLC44A2 is expressed in the inner ear of guinea pigs, mice and humans as a 68–70kDa N-glycosylated protein, with a deglycosylated reduced core protein of 62kDa1,5,10. SLC44A2 is also expressed strongly in lung, mucosal epithelium in the tongue, and to varying degrees in liver, heart, colon and kidney tissues, although glycosylation differs greatly across tissues resulting in a molecular mass range of ~62–95kDa depending on the tissue21,22. SLC44A2 is commonly expressed as one of two full-length isoforms transcribed from either promoter 1 (p1) or promoter 2 (p2)21. Isoform 1 (iso1) (704 amino acids) uses an upstream promoter and an alternate exon1a, and isoform 2 (iso2) (706 amino acids) uses a more proximal first exon, exon 1b21. The two isoforms differ by the amino acid sequences encoded by exon1a (10 amino acids) and exon1b (12 amino acids), yet otherwise are identical. The SLC44A2 isoforms are variably expressed in different animal and human tissues.
Several lines of evidence indicate that SLC44A2 function is important for normal homeostasis. We showed that antibody binding to SLC44A2 on supporting cells in the inner ear can disrupt auditory hair cell survival and perception of sound4,5,7. Similarly, knocking out the first outer loop of SLC44A2 (exons 3–10) causes progressive hearing loss, hair cell loss and spiral ganglion loss in mice23.
Meniere’s disease (MD) is a poorly-understood and debilitating disorder of the inner ear with symptoms of rotational dizziness, hearing loss and tinnitus24. Affected individuals often experience episodic bouts of vertigo, with nausea and vomiting and an inability to function normally during such episodes. The symptoms of MD can be often be medically managed by diet and lifestyle changes, or in more severe cases be surgically treated by vestibular nerve section or even labyrinthectomy24. Medical management includes daily diuretic use combined with dietary sodium restriction, along with vestibular suppressant medications for symptomatic relief during acute episodes. In this study, patients whose symptoms did not respond to medical management opted for a surgical approach to control the vertigo.
Due to the role of SLC44A2 in rapid onset hearing loss, we postulated that SLC44A2 might also play a role in Meniere’s disease. We sequenced cDNA from inner ear tissue of a patient with intractable MD and found that the patient was heterozygous at the SLC44A2 SNP rs2288904 (p.152arg/gln). As this polymorphism encodes either a positively-charged arginine or a neutral polar glutamine, we questioned if heterozygous expression might affect the folding or function of the molecules and thereby alter the homeostasis of the inner ear. Subsequent discoveries of other individuals who were heterozygous at c.455CGA/CAA.p.152arg/gln and had severe MD prompted an investigation of the frequency of SLC44A2 allele distribution in medically manageable and surgically managed MD and controls. The controls included tissue samples from inner ear of patients with vestibular schwannoma (VS) (benign tumors of the vestibular nerve that present similar symptoms to MD), and genomic DNA from anonymous normal donors. In addition to rs2288904 another SNP, rs3087969, c.198GAT>GAC p.66asp/asp was analyzed; and SLC44A2 isoform preference in inner ear, human endothelial cells, cell lines and blood samples was investigated.
2.0 Results
2.1 c.455CAA Allele in Linkage Disequilibrium with c.198GAC Allele
In the course of sequencing full-length SLC44A2 (Figure 1A) from inner ear cDNA samples we noted that the polymorphic site at c.455 (rs2288904) appeared to be in linkage disequilibrium with a second polymorphism in SLC44A2 (rs3087969) of c.198GAT/GAC p.66asp/asp. A TaqMan assay (Figure 1B) was used to screen for these polymorphisms in samples from multiple donors. The GAT/GAC SNP is a synonymous or silent polymorphism in which both alleles encode aspartate. The c.198 GAC allele (rs3087969) had the same distribution as the c.455CAA allele in 329/330 cases, indicating that, with the exception of one MD patient, the GAC allele and the CAA allele are always present on the same chromosome (p=1.0 McNemar test) (Table 1). Although SNP rs3087969 is in the NCBI database, the relationship of this SNP with rs2288904 to our knowledge has not been previously reported.
Figure 1.
Genotyping strategies. A. SLC44A2 gene located on Chromosome 19p13.1 has 23 exons with alternate exons 1a and 1b driven by an upstream p1 and a proximal p2 promoter respectively. Exon 1a is 22.9 kb upstream of exon 1b. The p1 and p2 SLC44A2 isoforms code for full-length proteins that differ only in exon1 (a or b). The p1 isoform (iso1) has 704 and the p2 isoform (iso2) has 706 amino acids. To examine the full-length expressed sequences the cDNA primers Iso1F and Iso2F were used with a single reverse primer Iso1/2R followed by sequencing with a set of overlapping sequencing primers (as reported in Nair et al. 200410). Focal genotyping of rs2288904 was done initially with DNA primers E5–7F and E5–7R followed by sequencing of the PCR product (Figure 1A). Subsequent genotyping of rs2288904 (c.455CGA/CAA) and rs3087969 (c.198GAT/GAC) was carried out using TaqMan assays with DNA as illustrated in Figure 1B.
Table 1.
Polymorphisms CAA at rs 2288904 and GAC at rs 3087969 in CTL2/SLC44A2 are on the same chromosome
| Alleles (rs2288904) Arginine/Glutamine |
CGA/CGA Arginine |
CGA/CAA Arg/Glut |
CAA/CAA Glutamine |
Total |
|---|---|---|---|---|
| Meniere’s Disease | 21 (65.6%) | 11 (34.3%) | 0 | 32 (100%) |
| Meniere’s Disease** | 0 | 1 (100%) | 0 | 1 (100%) |
| Vestibular Schwannoma | 23(60.5%) | 15 (39.5%) | 0 | 38 (100%) |
| UMSCC cell lines* | 55 (74.3%) | 15 (20.2%) | 4 (5.4%) | 74 |
| Control samples* | 119 (64.3%) | 57 (30.8%) | 9 (4.9%) | 185 (100%) |
| Total | 219 (66%) | 98 (30%) | 13 (3.9%) | 330 (100%) |
|
Alleles (rs3087969) Silent polymorphism |
GAT/GAT Aspartate |
GAT/GAC Aspartate |
GAC/GAC Aspartate |
Total |
| Meniere’s Disease | 21 (65.6%) | 11 (34.4%) | 0 | 32 (100%) |
| Meniere’s Disease** | 1 (100%) | 0 | 0 | 1 (100%) |
| Vestibular Schwannoma | 23 (60.5%) | 15 (39.5%) | 0 | 38 (100%) |
| UMSCC cell lines*** | 55 (74.3%) | 15 (20.2%) | 4 (5.4%) | 74 |
| Control samples | 119 (64.3%) | 57 (30.8%) | 9 (4.9%) | 185 (100%) |
| Total | 218 (64.1%) | 99 (30%) | 13 (3.9%) | 330 (100%) |
Control samples are genomic DNA from blood samples of North American Caucasian males and females living in Michigan (18 years or older).
In one Meniere’s disease patient only CGA/GAT was expressed in inner ear cDNA. However, the genomic DNA was heterozygous at rs2288904 (CGA/CAA) (p.152) but was homozygous for GAT at rs3087969 (p.62). This is the only case in which CAA and GAC were not linked and probably represents a rare crossover or a chromosome rearrangement in this patient or her lineage.
UMSCC head and neck cancer cell lines established from male and female patients treated at the University of Michigan and represent the same catchment area as the otology patients and the control population. All UMSCC cell lines used in this study have unique genotypes as determined by Profiler Plus assessment of 10 polymorphic alleles and all match the genotype of the donor whenever genomic DNA was available.
2.2 Allele Frequencies of SNP rs2288904 at SLC44A2 c.455
The distribution of allele frequencies for the SNP (rs2288904) at c.455 is reported to be 60% CGA, 36% CGA/CAA and 4% CAA of alleles in the North American population of European descent (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?searchType=adhoc_search&type=rs&rs=rs2288904); however, as more genomes from broader ancestral groups are being added these numbers are shifting.
We first sequenced full-length SLC44A2 cDNA from 15 inner ear vestibular tissue samples (6 MD and 9 VS patients) and found that 4/6 (67%) MD patients expressed CGA/CAA in inner ear cDNA whereas only 2/9 (22%) VS patients were heterozygous. We then examined genotypes from additional subjects and normal controls. TaqMan assays (Figure 1B) were performed with inner ear cDNA and/or genomic DNA from blood of 46 MD (35 surgically treated, 11 medically managed) patients, 70 VS patients, and 185 normal genomic DNA samples from a panel of North American control volunteers collected for a different study (RN and JTE). Of surgically treated Meniere’s disease patients 15/35 (43%) were heterozygous CGA/CAA (R/Q) at rs2288904 whereas only 3/11 (27%) medically managed Meniere’s disease patients were heterozygous (odds ratio: 2.0, 95% C.I. = [0.45, 8.84]). The distribution of genotyping results is shown in Table 2. Similarly, the frequency of heterozygosity in the surgically managed MD cases was more common than among normal controls (odds ratio 1.68 (95% C.I.=[0.80, 3.53]). Among the patients with vestibular schwannoma, 27/70 (39%) were heterozygous, 42/70 (60%) were homozygous for the common CGA allele encoding arginine and 1 individual was homozygous for the rare CAA allele encoding glutamine (p=0.11 comparing all MD patients to VS). Genotyping of the normal controls at codon 152 revealed approximately 64% CGA, 31% CGA/CAA and 5% CAA, (Table 2) which roughly corresponds to the expected distribution.
Table 2.
Allele frequency in CTL2/SLC44A2 at rs2288904
| Alleles (rs2288904) Arginine/Glutamine |
CGA/CGA Arginine |
CGA/CAA Arg/Glut |
CAA/CAA Glutamine |
Total |
|---|---|---|---|---|
|
Meniere’s Disease- Surgical cases |
20 (57%) | 15 (43%) | 0 | 35 |
|
Meniere’s Disease- Non-surgical cases (blood) |
8 (73%) | 3 (27%) | 0 | 11 |
| Total | 28 (61%) | 18 (39%) | 0 | 46 |
|
Vestibular Schwannoma |
42 (60%) | 27 (39%) | 1 (1.4%) | 70 |
|
North American Controls |
119 (64%) | 57 (31%) | 9 (5%) | 185 |
In addition to the inner ear tissues and blood genomic DNA, 74 UM-SCC squamous cancer cell lines, a sample of convenience, were genotyped (Table 1). Of the cancer cell lines (55/74) (74%) contained the common c.455CGA p.152arg allele, 4/74 cell lines (5.4%) had the rare c.455CAA p.152gln allele, but these cell lines had lower than expected incidence of heterozygosity at c.152 (15/74) (20%). This skewed distribution in the tumor cells is likely due to segmental loss of one chromosome in these cancers25. However, these lines were very useful in supporting the linkage disequilibrium between rs2288904 and rs3087969.
2.3 Tissue-specific SLC44A2 Isoform Expression
We previously reported tissue specific differences in isoform 1 and isoform 2 in murine and guinea pig tissues21. In the current study, SLC44A2 isoform expression was evaluated in a total of 97 tissue and blood samples, including 76 inner ear tissue samples (54 VS patients, 21 MD patients, 1 vertigo patient) and 21 lymphocyte samples from peripheral blood of normal hearing volunteers. Additionally cDNA from EBV transformed lymphoblasts from 7 MD patients and 3 normal donors as well as cDNA from 5 SCC cell lines from 3 donors were also assessed for isoform expression.
Among inner ear tissues from VS patients 15/54 (28%) expressed only isoform 1 and the remaining 39/54 (72%) expressed iso1 more strongly than iso2. Of the MD inner ear tissues 11/21 (52%) expressed only iso1, and 10/21 (48%) expressed iso1 more strongly than iso2 (Figure 2). EBV transformed lymphocytes from MD patients and whole blood from normal donors exclusively expressed iso1 (Figure 3A). Human dermal microvascular endothelial cells expressed mostly iso1 mRNA although low levels of iso2 mRNA are also produced (Figure 3B, left).
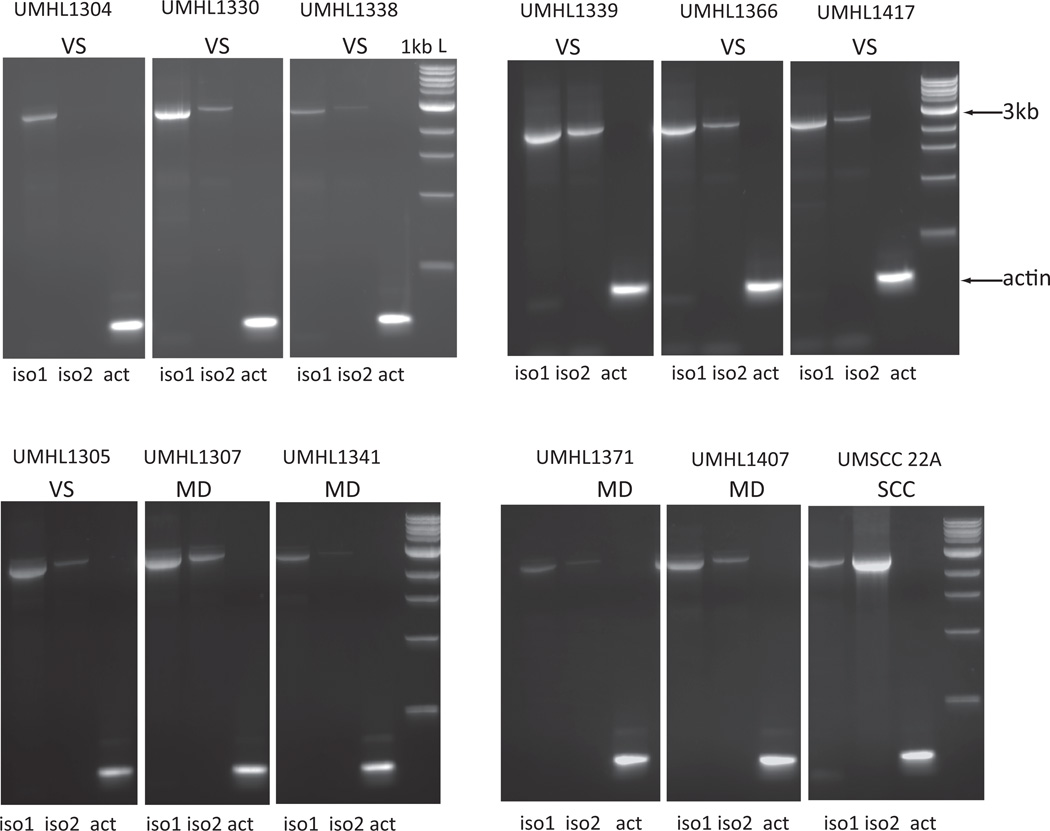
Figure 2.
RT-PCR results in 11 human vestibular tissue samples and one representative human epithelial cell line (UMSCC 22A) showing relative isoform1 and isoform 2 expression. Human inner tissues are indicated by UMHL- (University of Michigan Hearing Loss) and a 4 digit number (i.e. UMHL1304, UMHL1330 etc.). VS=Vestibular schwannoma, MD=Meniere’s disease. Lanes are labeled iso1, iso2 or actin in the representative samples shown. Note that iso1 is more abundant in all samples except in UMSCC 22A in which iso2 is the more abundant isoform. Actin was equally amplified in all cases.
Figure 3.
SLC44A2 iso1 and iso2 expression pattern in blood cells (A) and other human tissues (B). A. Isoform 1 is expressed exclusively human blood cells. Top panel: Proliferating EBV transformed human lymphoblasts from Meniere’s disease patients (MD8, MD10, MD22, MD32, MD33). Middle panel: MD34, MD38 and whole blood samples (Normal 4, and Normal 7 from normal hearing donors). Lower panel: Isoform expression in human endothelial cells, fetal liver, adult liver and adult lung. Human endothelial cells predominantly express iso1 with lower iso2 expression. Fetal and adult liver exhibit weak iso1 and undetectable iso2 expression. In contrast human lung strongly expresses iso1 and iso2 to an equivalent degree. Actin cDNA was used as a control. 1kbL=One Kb Ladder.
Prior studies of cDNA from murine and guinea pig fetal and adult liver and adult lung showed that liver expressed mostly iso1 whereas lung strongly expressed both isoforms in these species21. Similarly, human fetal and adult liver expressed predominantly iso1. In contrast, both iso1 and iso2 are strongly expressed in adult human lung (Figure 3B, right).
We previously reported that SLC44A2 is expressed at the protein level in oral mucosal cells21. Therefore we examined a panel of mucosal squamous cell cancers and transformed oral keratinocytes for SLC44A2 isoform expression using Q-RT-PCR (Figure 4). The squamous cell carcinoma cell lines expressed iso2 more than iso1. Oral keratinocytes immortalized with HPV E6 and E7 (HOK16B) showed low level expression of both isoforms, with iso2 being stronger. Similarly, in neonatal human keratinocytes grown in conditions that favor proliferation and inhibit differentiation (keratinocyte growth medium, EGF, low calcium), iso2 is strongly expressed and iso1 expression is weak. When grown in conditions that favor differentiation (Dulbecco’s Modified Eagle’s Medium, high calcium, fetal calf serum, which favors differentiation), expression of both isoforms is low (Data not shown).
Figure 4.
CTL2/SLC44A2 isoform expression in squamous carcinoma cell lines by quantitative-RT-PCR. The upper panel shows the Q-RT-PCR results for SLC44A2 isoform 1 and 2 expression in head and neck cancer (UM-SCC-14A, -14C, -22A, -22B), cervical cancer (ME-180) and HPV-transformed human oral keratinocyte (HOK-16B) samples. RPLP0 (Ribosomal Protein Large P0) was used as endogenous control. In all of these epithelial cell lines iso2 expression levels are greater than that of iso1. The lower panel shows agarose gel electrophoresis of the Q-RT-PCR products. For each sample, left lane is iso1, middle lane is iso2 and right lane is RPLP0. This data demonstrates that in squamous cell carcinomas and HPV-transformed mucosal cells SLC44A2 iso2 transcripts are more abundant than iso1 transcripts.
3.0 Discussion
Mutations and polymorphisms play an important role in human disease including polymorphisms within the solute carrier family26,27. In the present study, we asked if the c.455G>A p.152arg>gln polymorphism in SLC44A2 could play a role in the severity of Meniere’s disease. In the surgically managed MD cases, 15/35 (43%) were heterozygous at p.152arg/gln, compared to only 3/11 (27%) of medically managed patients. However, the numbers are too low for statistical significance. Establishing an association of allelic imbalance with severe MD will require a multi-institutional study of a larger population of patients graded for severity by well-defined clinical criteria.
Why would heterozygosity for the c.455G>A p.152arg>gln SNP predispose to disease? It is conceivable that the proteins produced from both alleles interact in a way that increases the risk of developing vertigo and other symptoms so severe that surgical intervention is necessary. If the proteins from both alleles are expressed in heterozygous individuals and the resulting molecules combine to form a transporter molecule, different folding of the protein containing arginine and the protein containing glutamine at amino acid 152/154 could result in altered function of the complex. Although Chen et al.28 reported that computational predictions of the p.154arg and p154gln alleles revealed no deleterious structural or functional differences, this type of analysis does not take into account the subtle conformational change that result from these different amino acids. These differences are sufficient to create the HNA3a epitope. In support of subtle changes having an effect on protein conformation, Chen et al.28 and Flesch et al.29 report a missense SNP rs147820753 position 537C>T (p1) p.151leu>phe which immediately precedes rs2288904 and alters HNA-3a antibody reactivity with the epitope involving p.152arg.
SLC44A2 is a complex molecule with a number of polymorphisms, different isoforms and varied glycosylation in different tissues and species differences in isoform preference in the inner ear. Leukocytes and EBV transformed lymphoblasts express only iso1 of SLC44A2. Flesch et al.29 and Bayat et al.30 showed only iso1 expression in neutrophils, which is consistent with our results on lymphocytes. Bayat et al.30 also showed expression of both iso1 and 2 in human microvascular endothelial cells from lung and umbilical blood vessels, and that anti-HNA3a antibodies recognize both iso1 and iso2 SLC44A2 proteins expressed on transfected HEK cells. We confirmed expression of both iso1 and iso2 on cultured human endothelial cells (HUVECs) with expression of iso1 being more predominant.
The role of SLC44A2 (CTL2) in disease is becoming well established. In vivo binding of a murine anti-SLC44A2 antibody induces hearing loss in mice and guinea pigs4,5,7. Patients with rapidly progressive hearing loss often have antibodies reactive with inner ear supporting cells and recombinant human SLC44A28,6. Patients with such antibodies enjoy a better response to corticosteroid treatment9. The murine anti-SLC44A2 antibody binds to N-linked carbohydrate on SLC44A210, whereas patient antibodies bind to the core SLC44A2 protein8. Other antibodies to the molecule can also alter SLC44A2 function. In 2010 two groups reported that transfusion related acute lung injury (TRALI) resulted from antibody interactions with human neutrophil alloantigen HNA-3a/HNA-3b31,32 encoded by the c.455G>A p.152arg>gln polymorphism in SLC44A2 iso1 (or p.154arg>gln in iso2). Individuals homozygous for the rare c.455G>A p.152Q (HNA-3b) can make antibodies against the arginine containing protein encoded by c.455G p.152R allele (HNA-3a). These antibodies bind to and aggregate granulocytes32,33. Bayat et al.34 showed that HNA3a antibody induces neutrophil activation through interaction with endothelial cells34 requiring interaction of SLC44A2, von Willebrand Factor and CD11b/CD1834.
In addition to tissue damage triggered by antibody interaction with SLC44A2 the rs2288904 polymorphism is linked to cardiovascular disease. In 2015, Germain et al.35 identified SLC44A2 as a risk allele (rs2288904, c.455G>A p.152R>Q) for venous thromboembolism (VTE), the third-leading cause of cardiovascular mortality. Similarly, Hinds et al.36 reported linkage of rs9797861 to both VTE and to coronary artery disease and that rs9797861 is in strong linkage disequilibrium with rs2288904 (r2=0.89). Thus, rs2288904, rs3087969 and rs9797861 are all in linkage disequilibrium, which has greatly increased interest in the function and role of SLC44A2 polymorphisms in a range of human disease.
Antibodies to CTL2/SLC44A2 are associated with sudden onset or rapidly progressive hearing loss9,37. Anti-phospholipid syndrome associated with cutaneous and retinal manifestations has been linked to thrombosis and disturbances of microcirculation including that of the inner ear38. This information and the linkage of SLC44A2 polymorphisms including rs2288904, c.455G>A p.152R>Q, with VTE and stroke suggest that thrombosis of the microcirculation of the inner ear could also be a possible etiologic factor in Meniere’s disease by causing unilateral inner ear damage.
In summary, CTL2/SLC44A2 is a complex molecule with alternate splice forms, polymorphisms and varied tissue expression, as well as highly variant glycosylation21. Although its function is not yet confirmed, it is important in maintaining tissue homeostasis since antibodies that bind N-linked carbohydrate residues of SLC44A2 result in loss of outer hair cells and hearing loss in both mice and guinea pig models. Knockout mice lacking the first outer loop (exon 3–10) of this gene develop auditory sensory cell loss, spiral ganglion cell loss and profound hearing loss23. Antibodies to the HNA-3a allele encoded by the c.455CAA allele induce granulocyte aggregation and acute lung injury, including direct disturbance of endothelial cells, production of reactive oxygen species, altered actin stress fibers, increased permeability of endothelial cells and loosening of junctional endothelial cadherin junctions30. Similarly, SLC44A2 HNA-3a (rs2288904) expression in monocytes, macrophages, endothelial cells and whole blood appears to be a risk allele in pathophysiology of venous thrombosis in VTE35 as well as arterial thrombosis in CAD36. In conclusion SLC44A2 plays an important role in hearing, TRALI, VTE, CAD, regulation of cell growth and possibly cancer.
Materials and Methods
Tissue and blood samples
This study was approved by the University of Michigan Medical School Institutional Review Board (IRBMED). All individuals who provided tissue or blood samples gave written informed consent including permission to sequence DNA and RNA from tissues and blood. Fresh inner ear tissues were obtained from consented patients undergoing surgeries that invade the inner ear for removal of VS (70 patients); or for ablation of the vestibular nerve for debilitating and medically non-responsive vertigo (Meniere’s disease)(35 patients). IRB permission to search hospital records for other patients with a diagnosis of MD was obtained and patients who were being managed medically were identified and asked to participate. Blood samples were collected from all consented surgical patients, and from 11 medically managed consented patients with MD. EBV transformation of lymphocytes from fresh blood of some Meniere’s patients was done as described previously.39 Isolated DNA and RNA from tissues, blood or from transformed lymphoblast lines were assessed for expression of SLC44A2. DNA and RNA were also isolated from a collection of human squamous cell carcinoma cell lines40. Permission to establish and study the tumor cell lines, including genetic analysis, was obtained by written informed consent. Blood DNA from a large set of anonymized consented normal North Americans collected for other genetic studies was also studied. Human liver and lung RNA was obtained from Cell Applications, Inc (San Diego, CA). Human Dermal Microvascular Endothelial cells (HDMEC) were kindly provided by Dr. Jacques Nor.
RNA extraction and Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Human inner ear tissue samples were placed in cold RNAlater (Ambion, Austin, TX) immediately following removal and stored in the refrigerator. The samples were transferred to lysis buffer, homogenized with a pestle and sheared with 18-gauge needle 5 times. Total RNA was extracted with the RNeasy Mini and Midi kits (Qiagen, Valencia, CA), respectively. RNA extraction of EBV transformed lymphocytes and fresh or frozen blood was done using the Qiagen Mini kit spin protocol. cDNA synthesis and full-length amplification of SLC44A2 isoform p1 and p2 and sequence analysis were done as described21. DNA was extracted from blood using the Qiagen Mini kit.
Quantitative- RT- PCR
Q-RT-PCR was done as described by Stoll et al.41 Total RNA was extracted and SLC44A2 mRNA transcripts were quantified by Q-RT-PCR relative to the housekeeping gene RPLP0. The SLC44A2 iso1 primers are: GCCATGGAGGACGAGCGGAAAAACG (F) ACAGCCACGTAGCCCACAATG (R) with a 146 bp product. For SLC44A2 iso2 the primers are: GTGCCTCCCTCCAGACTCGG (F) and the same reverse primer with a 223bp product. The same probe, AAGGACCCATTTACAATAGG with 5’FAM reporter and 3’MGB quencher, was used for both isoforms. The assays were run using the ABI Taqman gene expression assay (Applied Biosystems, Foster City, CA). Ribosomal protein large P0 (RPLP0 ABI catalog # Hs 99999902_m1 ABI) was used as an endogenous control41,42 (110bp product size). Expression was determined as fold-change (delta Ct) relative to RPLP0 multiplied by 103 and expressed as percentage fold-change versus RPLP0 = 2−(Ct target −Ct RPLP0).
Genomic DNA Isolation
DNA was isolated from whole blood, EBV immortalized lymphoblasts and UM-SCC cell lines. Cell pellets were flash frozen at −80°C, resuspended in Tris EDTA with 1% sodium dodecyl sulfate and digested overnight at 55°C with proteinase K (New England Biolabs, Ipswich, MA). The DNA was extracted in phenol/chloroform and precipitated in ammonium acetate and ethanol. The precipitated DNA was washed with 70% ethanol, air dried, and resuspended in high performance liquid chromatography–grade H2O40.
Cell Culture
DNA from UM-SCC cell lines established from head and neck cancer patients was also examined for the frequency of the polymorphic alleles. Cell lines were grown in complete Dulbecco’s modified Eagle’s medium (cDMEM; Sigma Chemical Co, St. Louis, MO) containing 2 mM L-glutamine, 1% nonessential amino acids, 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) and 10% fetal bovine serum, in a humidified atmosphere of 5% CO2 at 37°C. All cell lines were free of mycoplasma, as assessed by the MycoAlert Detection Kit (Cambrex, Rockland, ME).
Genotyping Single Nucleotide Polymorphisms in SLC44A2
Gene expression was evaluated using RT-PCR and genotyping was carried out by cDNA sequencing and by TaqMan genotyping assays. The SLC44A2 SNP rs2288904 (CGA arginine/CAA glutamine at amino acid 152 in isoform 1 and 154 in isoform 2) was assessed using genomic DNA with the following primer set covering exons 5–7 (forward ATCACACCATTGCACTCCATCCTG; reverse CTGCAGAGCCTCCCCGTCAC) yielding a 600bp product (Figure 1A) that was sequenced by the Sanger method. The rs3087869 (silent polymorphism GAT/GAC (aspartate/aspartate at amino acid 66 for iso1 and 68 for iso2)) was genotyped using the Taqman assay (Applied Biosystems, Foster City, CA) (Figure 1B).
Statistical Considerations
Odds ratios and 95% confidence intervals [C.I.] were calculated using the standard approach43. Differences between groups were examined using the Pearson Chi Square Test for independence. A two-sided alpha level of less than 0.05 was considered statistically significant. There were no adjustments for multiple comparisons.
Acknowledgments
Support for this project was from NIH NIDCD grant (R01 DC03686, R01 DC02272); The Ruth and Lynn Townsend Fund, Research Center Core Grant NIH NIDCD (P30 DC05188)
References
- 1.Zajic G, Nair TS, Ptok M, et al. Monoclonal antibodies to inner ear antigens: I. Antigens expressed by supporting cells of the guinea pig cochlea. Hear Res. 1991;52:59–71. doi: 10.1016/0378-5955(91)90187-e. [DOI] [PubMed] [Google Scholar]
- 2.Ptok M, Nair TS, Altschuler RA, Schacht J, Carey TE. Monoclonal antibodies to inner ear antigens: II. Antigens expressed in sensory cell stereocilia. Hear Res. 1991;57:79–90. doi: 10.1016/0378-5955(91)90077-m. [DOI] [PubMed] [Google Scholar]
- 3.Ptok M, Nair T, Carey TE, Altschuler RA. Distribution of KHRI 3 epitopes in the inner ear. Hear Res. 1993;66:245–252. doi: 10.1016/0378-5955(93)90144-p. [DOI] [PubMed] [Google Scholar]
- 4.Nair TS, Prieskorn DM, Miller JM, Dolan DF, Raphael Y, Carey TE. KHRI-3 monoclonal antibody-induced damage to the inner ear: antibody staining of nascent scars. Hear Res. 1999;129:50–60. doi: 10.1016/s0378-5955(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 5.Nair TS, Prieskorn DM, Miller JM, Mori A, Gray J, Carey TE. In vivo binding and hearing loss after intracochlear infusion of KHRI-3 antibody. Hear Res. 1997;107:93–101. doi: 10.1016/s0378-5955(97)00024-5. [DOI] [PubMed] [Google Scholar]
- 6.Disher MJ, Ramakrishnan A, Nair TS, et al. Human autoantibodies and monoclonal antibody KHRI-3 bind to a phylogenetically conserved inner-ear-supporting cell antigen. Ann N Y Acad Sci. 1997;830:253–265. doi: 10.1111/j.1749-6632.1997.tb51896.x. [DOI] [PubMed] [Google Scholar]
- 7.Nair TS, Raphael Y, Dolan DF, et al. Monoclonal antibody induced hearing loss. Hear Res. 1995;83:101–113. doi: 10.1016/0378-5955(94)00194-u. [DOI] [PubMed] [Google Scholar]
- 8.Kommareddi PK, Nair TS, Vallurupalli M, et al. Autoantibodies to recombinant human CTL2 in autoimmune hearing loss. Laryngoscope. 2009;119:924–932. doi: 10.1002/lary.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeitoun H, Beckman JG, Arts HA, et al. Corticosteroid response and supporting cell antibody in autoimmune hearing loss. Arch Otolaryngol Head Neck Surg. 2005;131:665–672. doi: 10.1001/archotol.131.8.665. [DOI] [PubMed] [Google Scholar]
- 10.Nair TS, Kozma KE, Hoefling NL, et al. Identification and characterization of choline transporter-like protein 2, an inner ear glycoprotein of 68 and 72 kDa that is the target of antibody-induced hearing loss. J Neurosci. 2004;24:1772–1779. doi: 10.1523/JNEUROSCI.5063-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L, Vasiliou K, Nebert DW. Analysis and update of the human solute carrier (SLC) gene superfamily. Human genomics. 2009;3:195–206. doi: 10.1186/1479-7364-3-2-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Regan S, Traiffort E, Ruat M, Cha N, Compaore D, Meunier FM. An electric lobe suppressor for a yeast choline transport mutation belongs to a new family of transporter-like proteins. Proc Natl Acad Sci U S A. 2000;97:1835–1840. doi: 10.1073/pnas.030339697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Regan S, Meunier FM. Selection and characterization of the choline transport mutation suppressor from Torpedo electric lobe, CTL1. Neurochem Res. 2003;28:551–555. doi: 10.1023/a:1022877524469. [DOI] [PubMed] [Google Scholar]
- 14.Traiffort E, Ruat M, O'Regan S, Meunier FM. Molecular characterization of the family of choline transporter-like proteins and their splice variants. J Neurochem. 2005;92:1116–1125. doi: 10.1111/j.1471-4159.2004.02962.x. [DOI] [PubMed] [Google Scholar]
- 15.Michel V, Bakovic M. The solute carrier 44A1 is a mitochondrial protein and mediates choline transport. FASEB J. 2009;23:2749–2758. doi: 10.1096/fj.08-121491. [DOI] [PubMed] [Google Scholar]
- 16.Lee NY, Choi HM, Kang YS. Choline transport via choline transporter-like protein 1 in conditionally immortalized rat syncytiotrophoblast cell lines TR-TBT. Placenta. 2009;30:368–374. doi: 10.1016/j.placenta.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Fullerton MD, Wagner L, Yuan Z, Bakovic M. Impaired trafficking of choline transporter-like protein-1 at plasma membrane and inhibition of choline transport in THP-1 monocyte-derived macrophages. Am J Physiol Cell Physiol. 2006;290:C1230–C1238. doi: 10.1152/ajpcell.00255.2005. [DOI] [PubMed] [Google Scholar]
- 18.Fujita T, Shimada A, Okada N, Yamamoto A. Functional characterization of Na+-independent choline transport in primary cultures of neurons from mouse cerebral cortex. Neurosci Lett. 2006;393:216–221. doi: 10.1016/j.neulet.2005.09.069. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Fujiwara R, Ishiguro N, et al. Involvement of Choline Transporter-Like Proteins, CTL1 and CTL2, in Glucocorticoid-Induced Acceleration of Phosphatidylcholine Synthesis via Increased Choline Uptake. Biol Pharm Bull. 2010;33:691–696. doi: 10.1248/bpb.33.691. [DOI] [PubMed] [Google Scholar]
- 20.Iwao B, Yara M, Hara N, et al. Functional expression of choline transporter like-protein 1 (CTL1) and CTL2 in human brain microvascular endothelial cells. Neurochem Int. 2016;93:40–50. doi: 10.1016/j.neuint.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Kommareddi PK, Nair TS, Thang LV, et al. Isoforms, Expression, Glycosylation, and Tissue Distribution of CTL2/SLC44A2. Protein J. 2010 doi: 10.1007/s10930-010-9268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Haas M, Muniz-Diaz E, Alonso LG, et al. Neutrophil antigen 5b is carried by a protein, migrating from 70 to 95 kDa, and may be involved in neonatal alloimmune neutropenia. Transfusion. 2000;40:222–227. doi: 10.1046/j.1537-2995.2000.40020222.x. [DOI] [PubMed] [Google Scholar]
- 23.Kommareddi P, Nair T, Kakaraparthi BN, et al. Hair Cell Loss, Spiral Ganglion Degeneration, and Progressive Sensorineural Hearing Loss in Mice with Targeted Deletion of Slc44a2/Ctl2. J Assoc Res Otolaryngol. 2015 doi: 10.1007/s10162-015-0547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sajjadi H, Paparella MM. Meniere's disease. Lancet. 2008;372:406–414. doi: 10.1016/S0140-6736(08)61161-7. [DOI] [PubMed] [Google Scholar]
- 25.Van Dyke DL, Worsham MJ, Benninger MS, et al. Recurrent cytogenetic abnormalities in squamous cell carcinomas of the head and neck region. Genes Chromosomes Cancer. 1994;9:192–206. doi: 10.1002/gcc.2870090308. [DOI] [PubMed] [Google Scholar]
- 26.Durand E, Boutin P, Meyre D, et al. Polymorphisms in the amino acid transporter solute carrier family 6 (neurotransmitter transporter) member 14 gene contribute to polygenic obesity in French Caucasians. Diabetes. 2004;53:2483–2486. doi: 10.2337/diabetes.53.9.2483. [DOI] [PubMed] [Google Scholar]
- 27.Lamason RL, Mohideen MA, Mest JR, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q, Srivastava K, Ardinski SC, et al. Full-length nucleotide sequences of 30 common SLC44A2 alleles encoding human neutrophil antigen-3. Transfusion. 2016;56:729–736. doi: 10.1111/trf.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flesch BK, Reil A, Bux J. Genetic variation of the HNA-3a encoding gene. Transfusion. 2011;51:2391–2397. doi: 10.1111/j.1537-2995.2011.03155.x. [DOI] [PubMed] [Google Scholar]
- 30.Bayat B, Tjahjono Y, Sydykov A, et al. Anti-human neutrophil antigen-3a induced transfusion-related acute lung injury in mice by direct disturbance of lung endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33:2538–2548. doi: 10.1161/ATVBAHA.113.301206. [DOI] [PubMed] [Google Scholar]
- 31.Curtis BR, Cox NJ, Sullivan MJ, et al. The neutrophil alloantigen HNA-3a (5b) is located on choline transporter-like protein 2 and appears to be encoded by an R>Q154 amino acid substitution. Blood. 2010;115:2073–2076. doi: 10.1182/blood-2009-11-248336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greinacher A, Wesche J, Hammer E, et al. Characterization of the human neutrophil alloantigen-3a. Nat Med. 2010;16:45–48. doi: 10.1038/nm.2070. [DOI] [PubMed] [Google Scholar]
- 33.Silliman CC, Curtis BR, Kopko PM, et al. Donor antibodies to HNA-3a implicated in TRALI reactions prime neutrophils and cause PMN-mediated damage to human pulmonary microvascular endothelial cells in a two-event in vitro model. Blood. 2007;109:1752–1755. doi: 10.1182/blood-2006-05-025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayat B, Tjahjono Y, Berghofer H, et al. Choline Transporter-Like Protein-2: New von Willebrand Factor-Binding Partner Involved in Antibody-Mediated Neutrophil Activation and Transfusion-Related Acute Lung Injury. Arterioscler Thromb Vasc Biol. 2015;35:1616–1622. doi: 10.1161/ATVBAHA.115.305259. [DOI] [PubMed] [Google Scholar]
- 35.Germain M, Chasman DI, de Haan H, et al. Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am J Hum Genet. 2015;96:532–542. doi: 10.1016/j.ajhg.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinds DA, Buil A, Ziemek D, et al. Genome-wide association analysis of self-reported events in 6135 individuals and 252 827 controls identifies 8 loci associated with thrombosis. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Billings PB, Keithley EM, Harris JP. Evidence linking the 68 kilodalton antigen identified in preogressive sensorineural hearing loss with heat shock priotein 70. Ann Otol Rhinol laryngol. 1995;104:181–188. doi: 10.1177/000348949510400302. [DOI] [PubMed] [Google Scholar]
- 38.Kessel A, Toubi E. Autoimmune Sensorineural hearing loss. In: Shoenfeld Y, et al., editors. Diagnostic Criteria in Autoimmune Diseases. Totowa, NJ: Humana Press; 2008. pp. 449–453. [Google Scholar]
- 39.Nair RP, Guo SW, Jenisch S, et al. Scanning chromosome 17 for psoriasis susceptibility: lack of evidence for a distal 17q locus. Hum Hered. 1995;45:219–230. doi: 10.1159/000154293. [DOI] [PubMed] [Google Scholar]
- 40.Brenner JC, Graham MP, Kumar B, et al. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010;32:417–426. doi: 10.1002/hed.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoll SW, Johnson JL, Bhasin A, et al. Metalloproteinase-mediated, context-dependent function of amphiregulin and HB-EGF in human keratinocytes and skin. J Invest Dermatol. 2010;130:295–304. doi: 10.1038/jid.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bamias G, Goukos D, Laoudi E, et al. Comparative study of candidate housekeeping genes for quantification of target gene messenger RNA expression by real-time PCR in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2840–2847. doi: 10.1097/01.MIB.0000435440.22484.e8. [DOI] [PubMed] [Google Scholar]
- 43.Gordis L. Epidemiology. Philadelphia: Elsevier; 2009. [Google Scholar]