Abstract
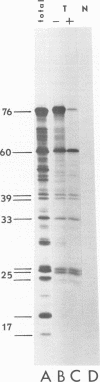
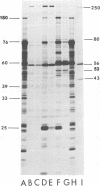
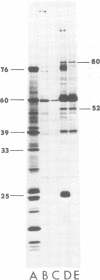
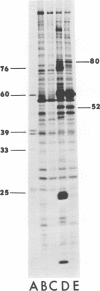
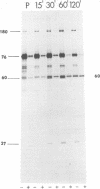
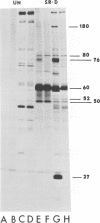
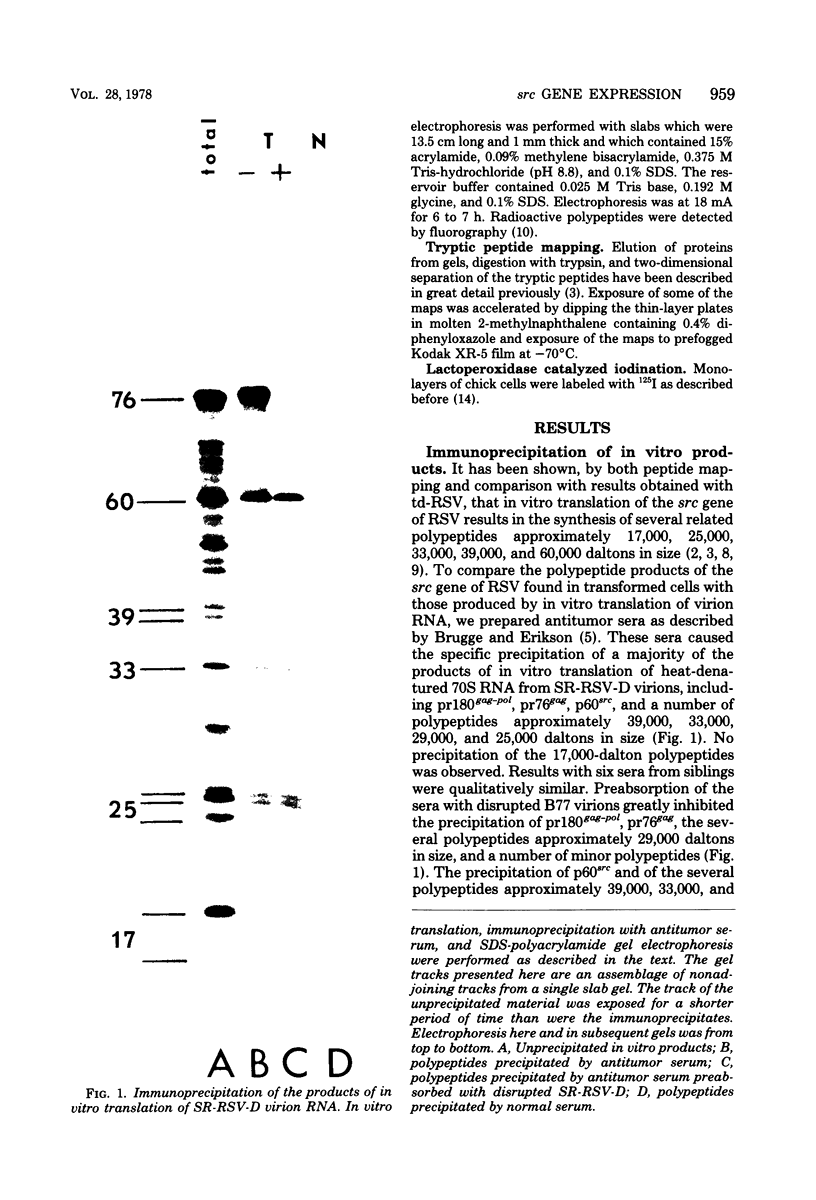
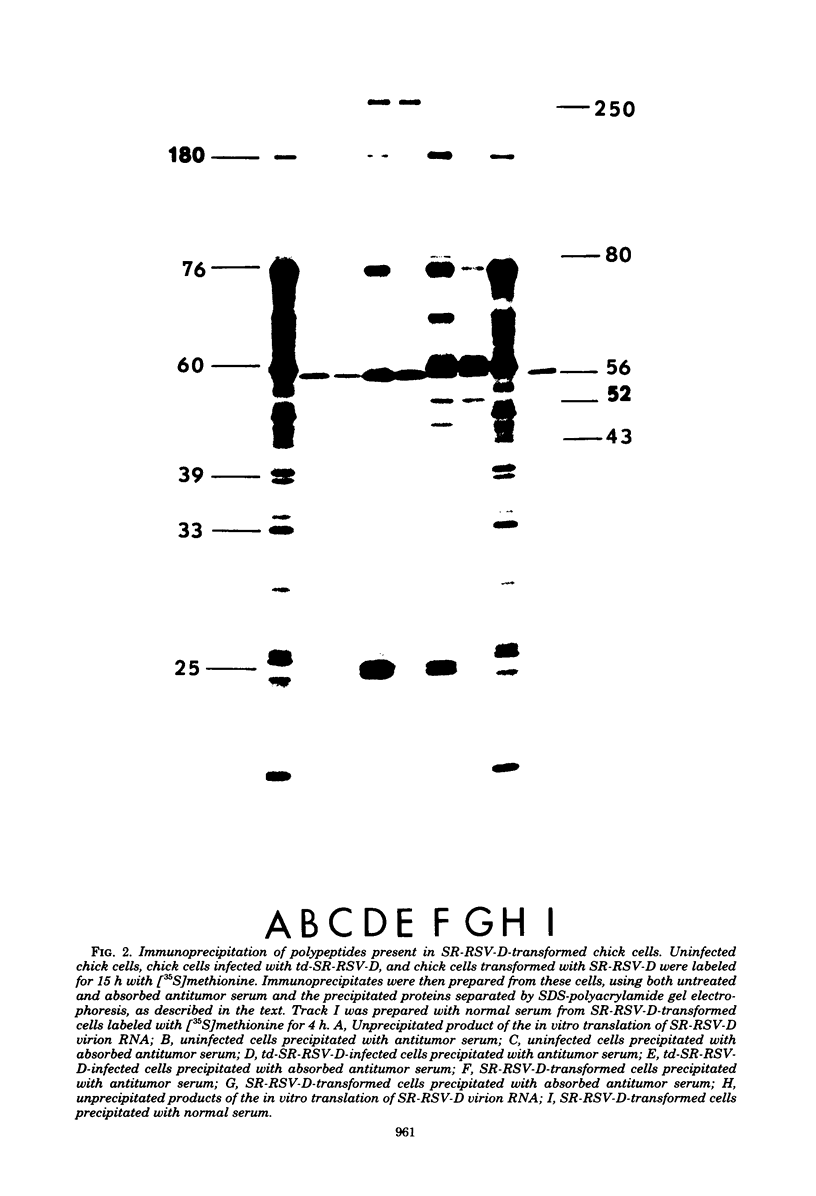
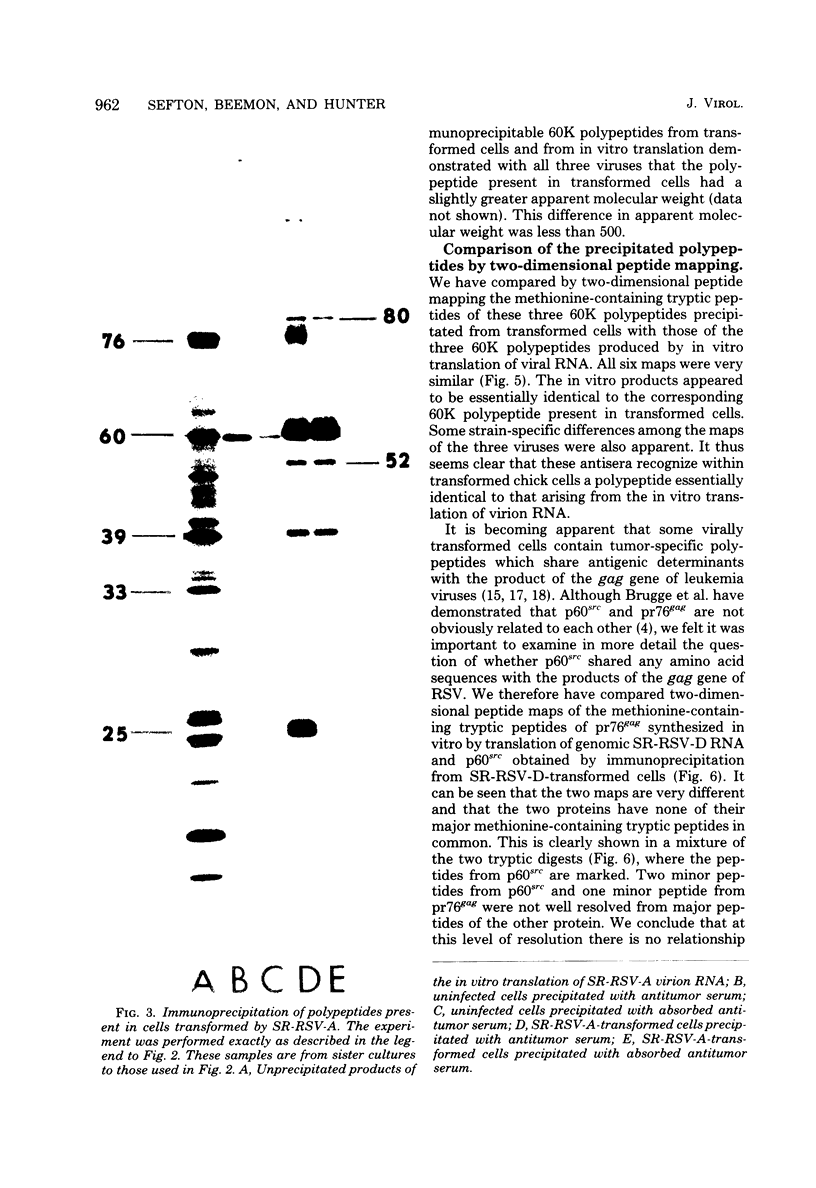
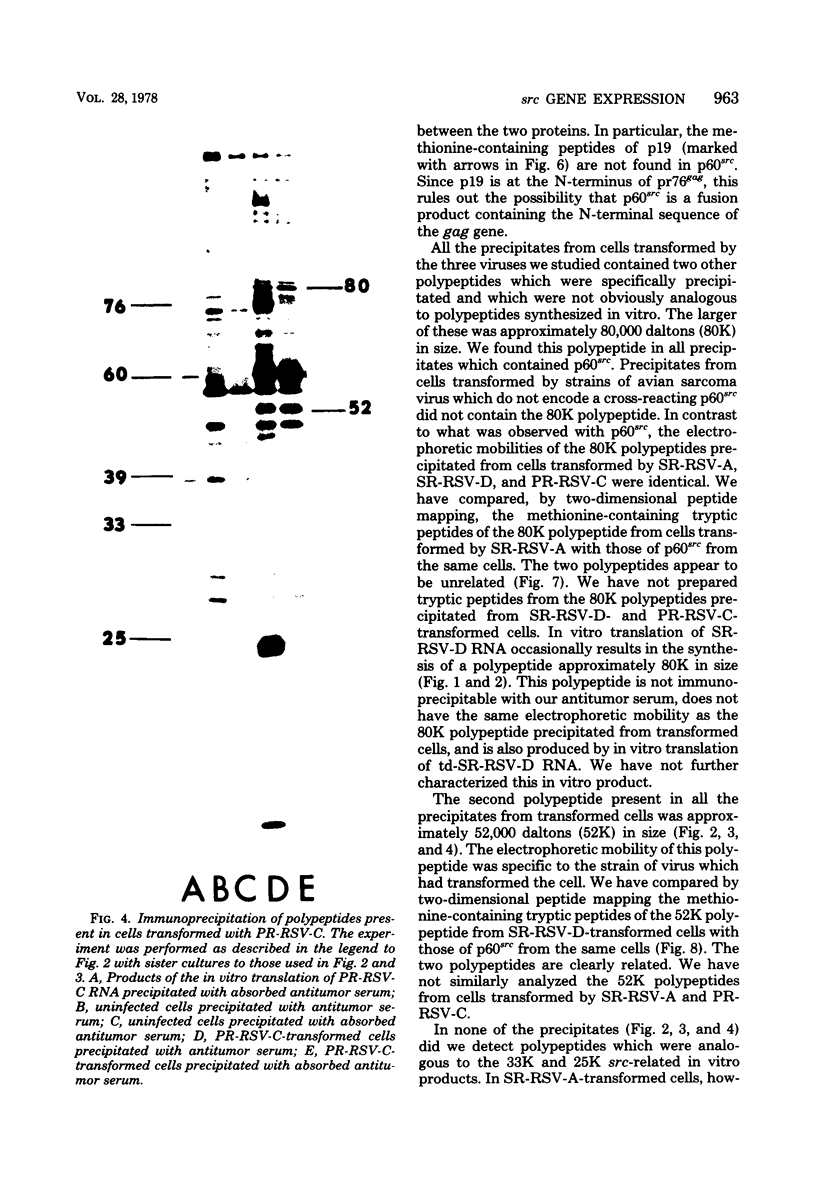
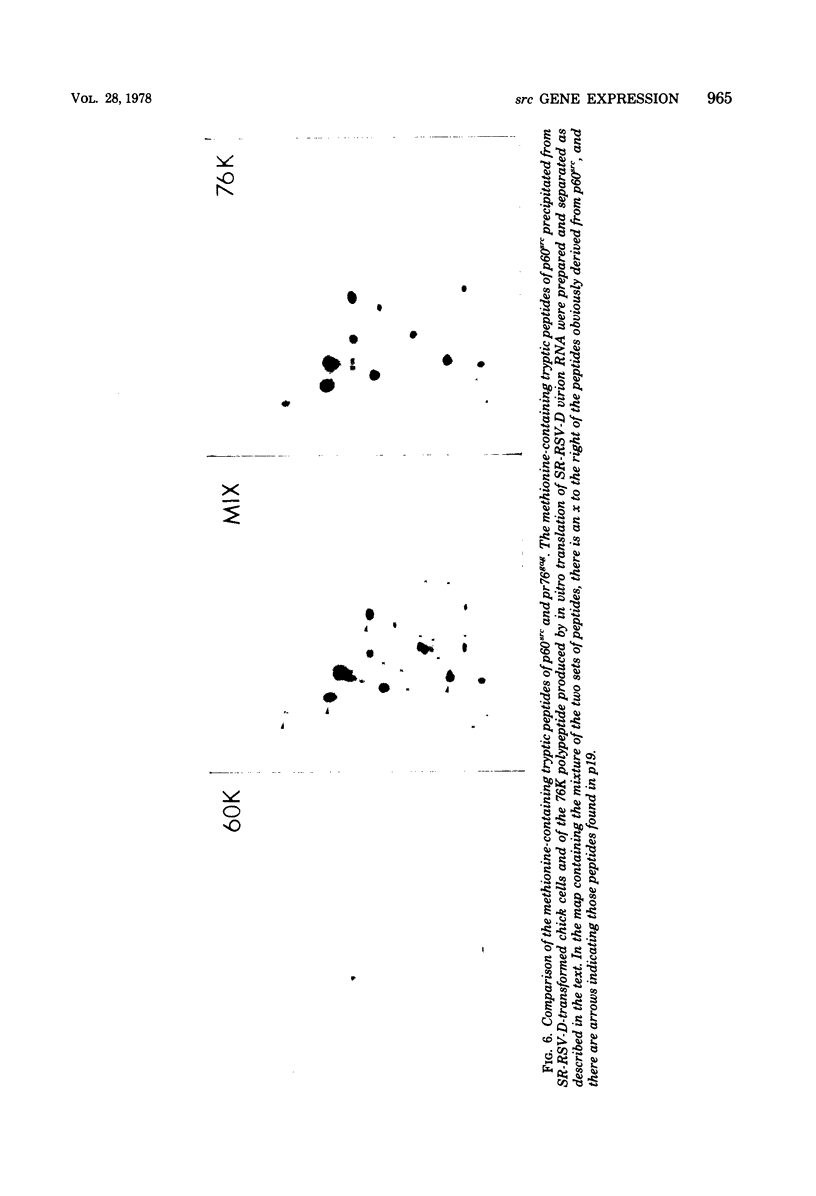
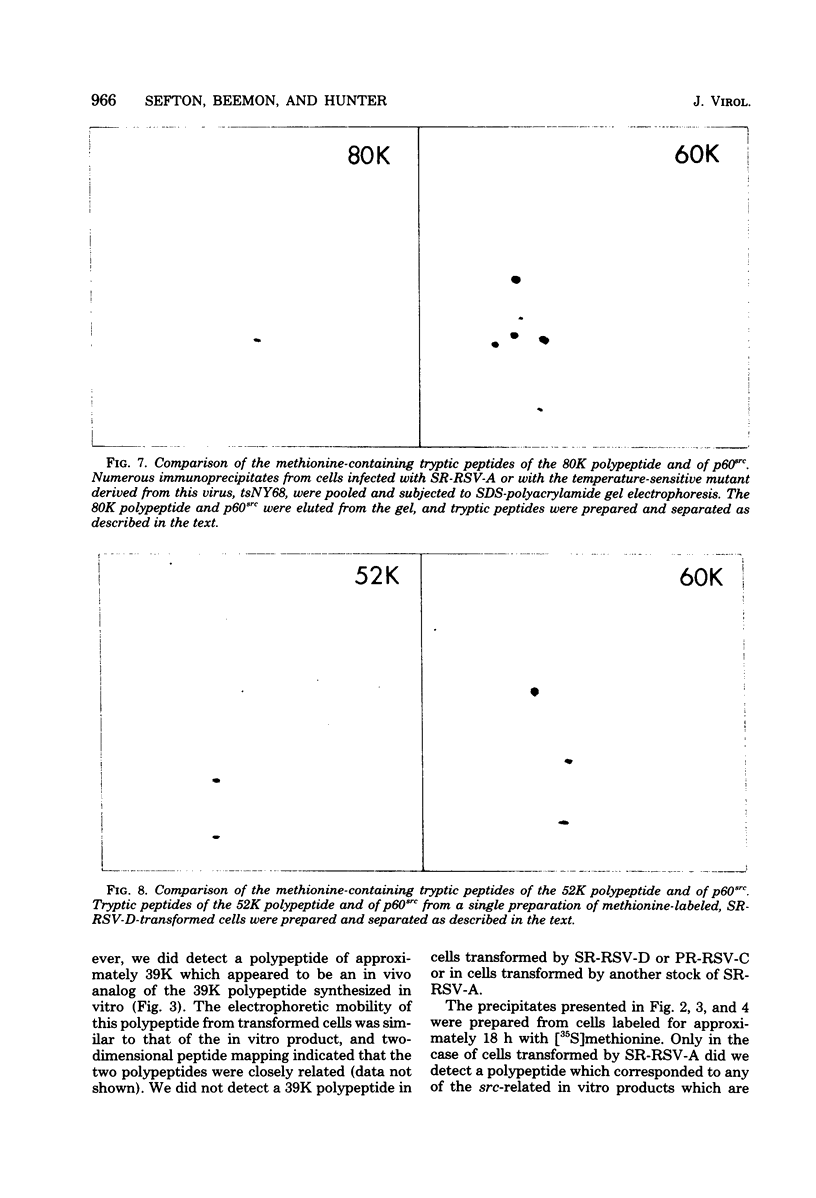
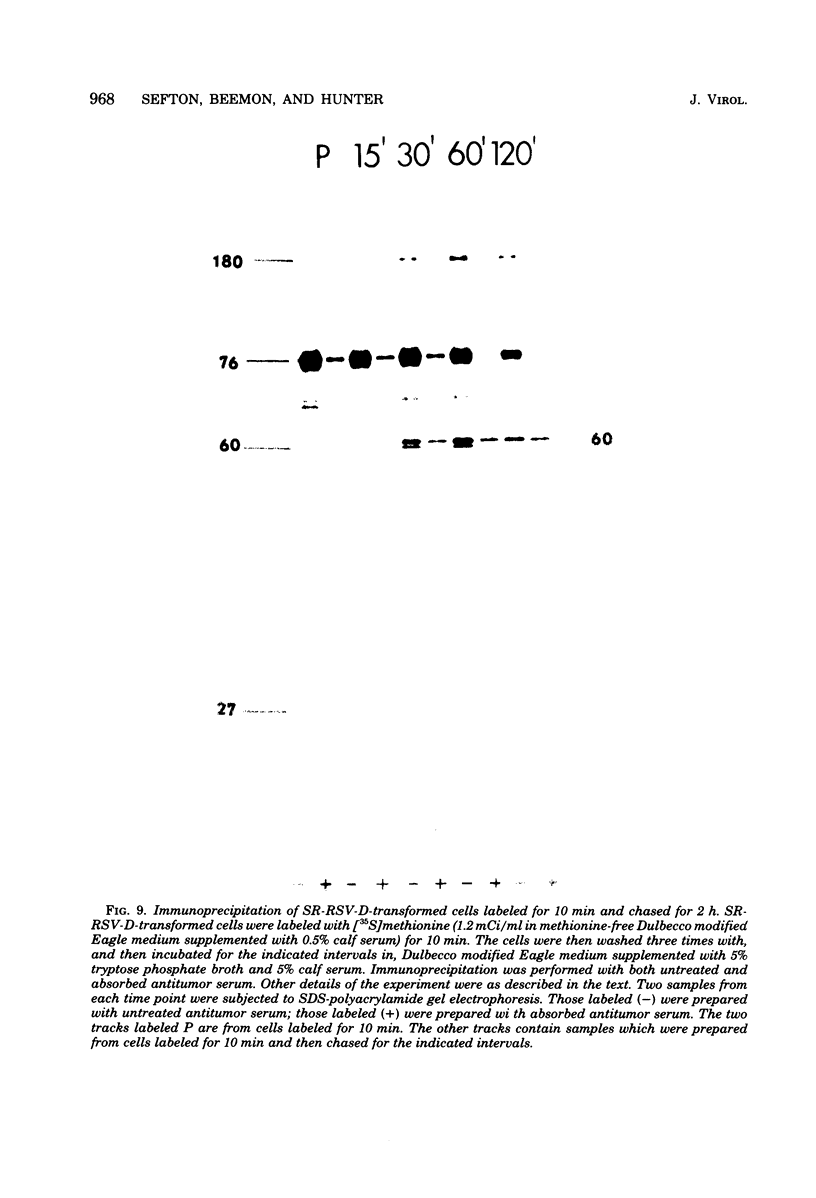
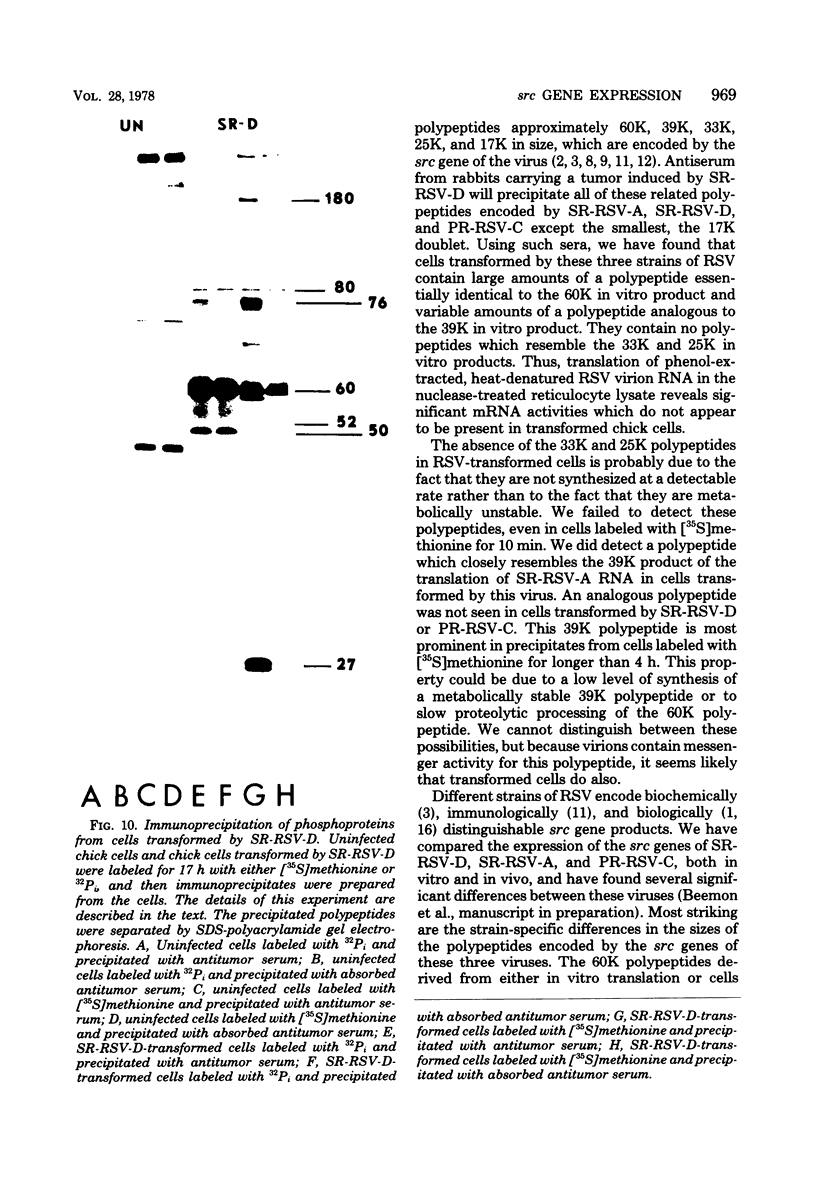
We have compared the polypeptide products of the src gene of several strains of Rous sarcoma virus produced by in vitro translation of heat-denatured 70S virion RNA in the nuclease-treated reticulocyte lysate with those present in chick cells transformed by these viruses. We have done this by immunoprecipitation, using sera from rabbits injected at birth with Schmidt-Ruppin Rous sarcoma virus. In vitro translation results in the synthesis of at least nine polypeptides which appear to be encoded by the src gene. These range in size from 17,000 to 60,000 daltons. The sera from tumor-bearing rabbits precipitated these polypeptides arising from the in vitro translation of RNA from Schmidt-Ruppin Rous sarcoma virus of both subgroup A and subgroup D and from one stock of Prague Rous sarcoma virus of subgroup C. In each case, all of this family of related polypeptides could be precipitated except the smallest, the 17,000-dalton polypeptide. No precipitation of analogous polypeptides resulting from the translation of RNA from other strains of Rous sarcoma virus was observed. Cells transformed by these three strains of Rous sarcoma virus contain easily detectable amounts of a polypeptide, p60src, essentially identical to the 60,000-dalton in vitro product. With one exception, they do not contain significant amounts of polypeptides analogous to the smaller in vitro products which can be precipitated by these sera. Cells transformed by one stock of Schmidt-Ruppin Rous sarcoma virus of subgroup A did contain a 39,000-dalton polypeptide, which was related, by peptide mapping, to the 60,000-dalton polypeptide and was similar in size to a precipitable in vitro product. The 60,000-dalton polypeptide present in transformed cells appeared to be phosphorylated 10 to 25 min after its synthesis, metabolically very stable, and not derived from a precursor polypeptide. All immunoprecipitates from transformed cells which contained p60src also contained an 80,000-dalton phosphoprotein. This polypeptide is unrelated to p60src, as determined by peptide mapping, and may well be a host cell polypeptide which is specifically associated with p60src.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Ray D. A., Brown N. R. Accumulation of water during transformation of cells by an avian sarcoma virus. Cell. 1974 Nov;3(3):307–313. doi: 10.1016/0092-8674(74)90146-9. [DOI] [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T. In vitro translation yields a possible Rous sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3302–3306. doi: 10.1073/pnas.74.8.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J., Erikson E., Collett M. S., Erikson R. I. Peptide analysis of the transformation-specific antigen from avian sarcoma virus-transformed cells. J Virol. 1978 Jun;26(3):773–782. doi: 10.1128/jvi.26.3.773-782.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Shiu R. P., Jay F. T., Levine A. S., Pastan I. Identification of a transformation-specific protein induced by a Rous sarcoma virus. Cell. 1978 Mar;13(3):527–534. doi: 10.1016/0092-8674(78)90326-4. [DOI] [PubMed] [Google Scholar]
- Kamine J., Buchanan J. M. Cell-free synthesis of two proteins unique to RNA of transforming virions of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1977 May;74(5):2011–2015. doi: 10.1073/pnas.74.5.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamine J., Burr J. G., Buchanan J. M. Multiple forms of sarc gene proteins from Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):366–370. doi: 10.1073/pnas.75.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Rubin H. Effects of local cell concentrations upon the growth of chick embryo cells in tissue culture. Exp Cell Res. 1968 Mar;49(3):666–678. doi: 10.1016/0014-4827(68)90213-9. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Wickus G. G., Burge B. W. Enzymatic iodination of Sindbis virus proteins. J Virol. 1973 May;11(5):730–735. doi: 10.1128/jvi.11.5.730-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Khan A. S., Sliski A. H., Essex M. Feline oncornavirus-associated cell membrane antigen: evidence for an immunologically crossreactive feline sarcoma virus-coded protein. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5608–5612. doi: 10.1073/pnas.74.12.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M. The control of cellular morphology in embryonic cells infected with rous sarcoma virus in vitro. Virology. 1960 Feb;10:182–197. doi: 10.1016/0042-6822(60)90038-6. [DOI] [PubMed] [Google Scholar]
- Tung J. S., Yoshiki T., Fleissner E. A core polyprotein of murine leukemia virus on the surface of mouse leukemia cells. Cell. 1976 Dec;9(4 Pt 1):573–578. doi: 10.1016/0092-8674(76)90039-8. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]