Abstract
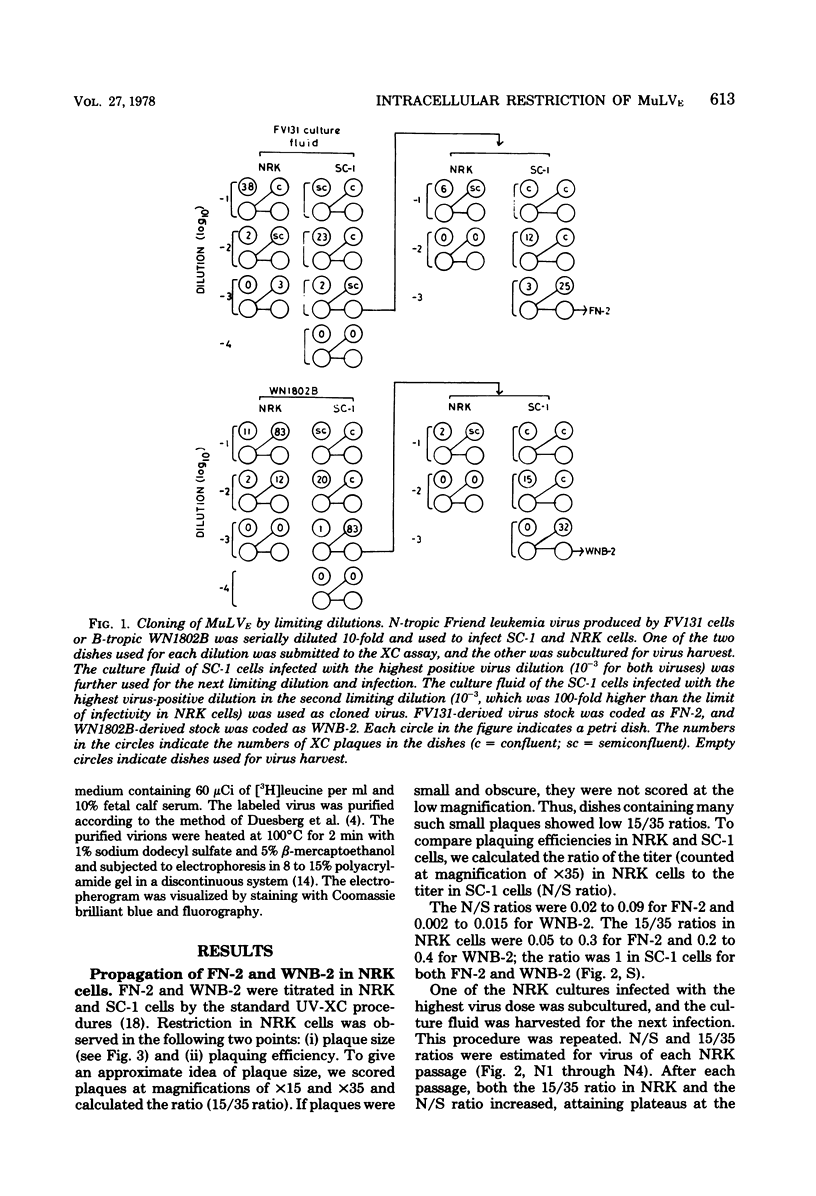
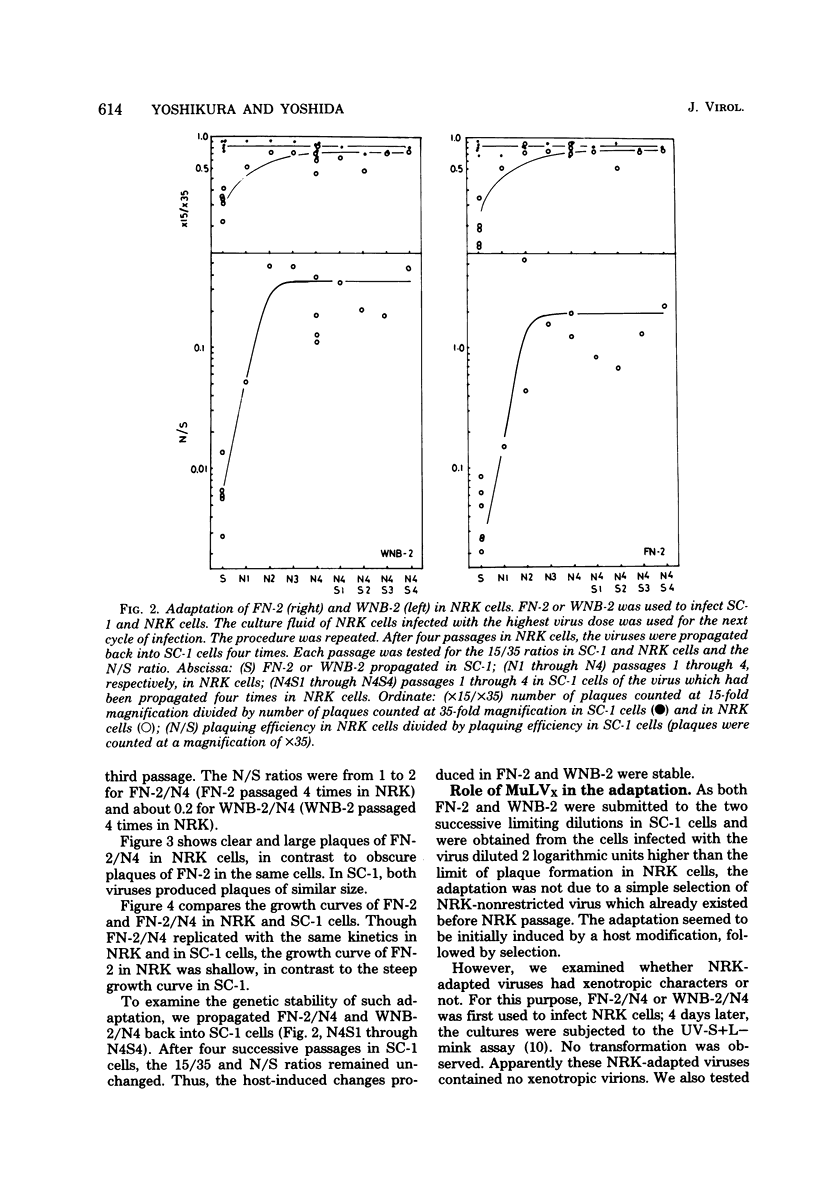
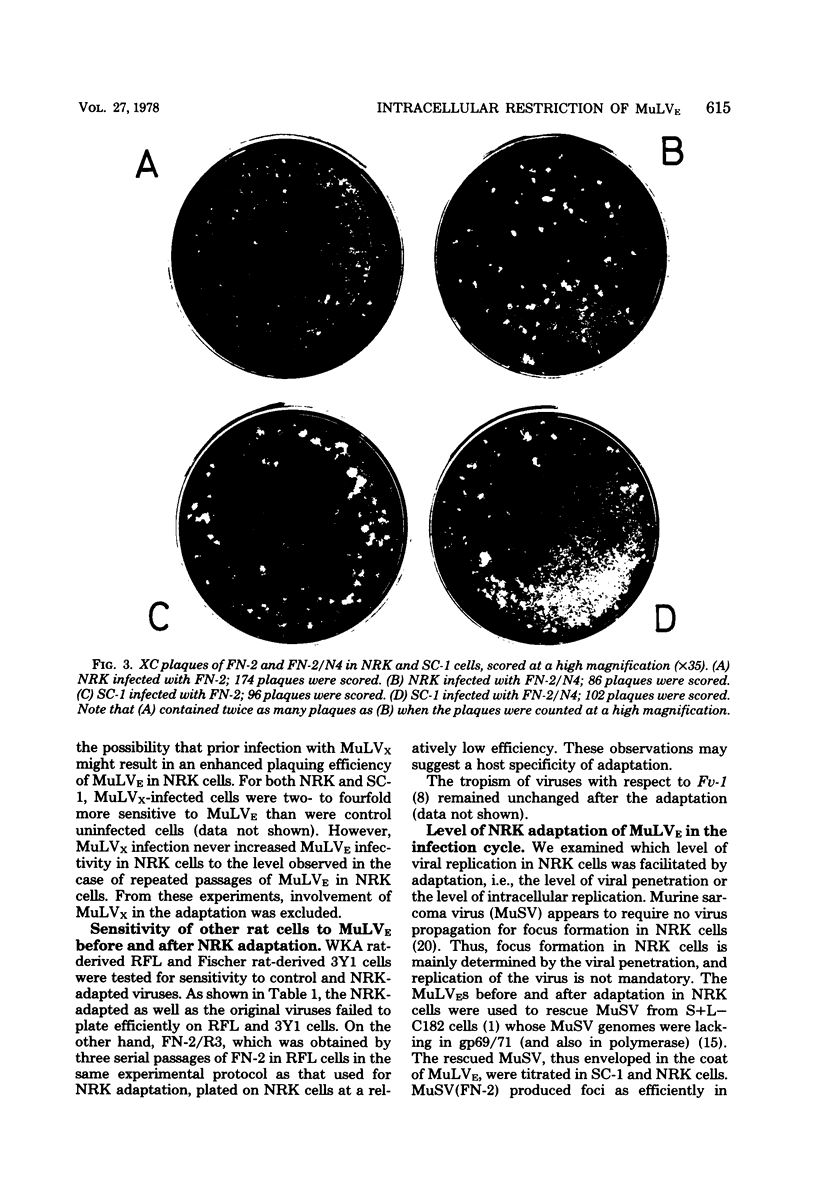
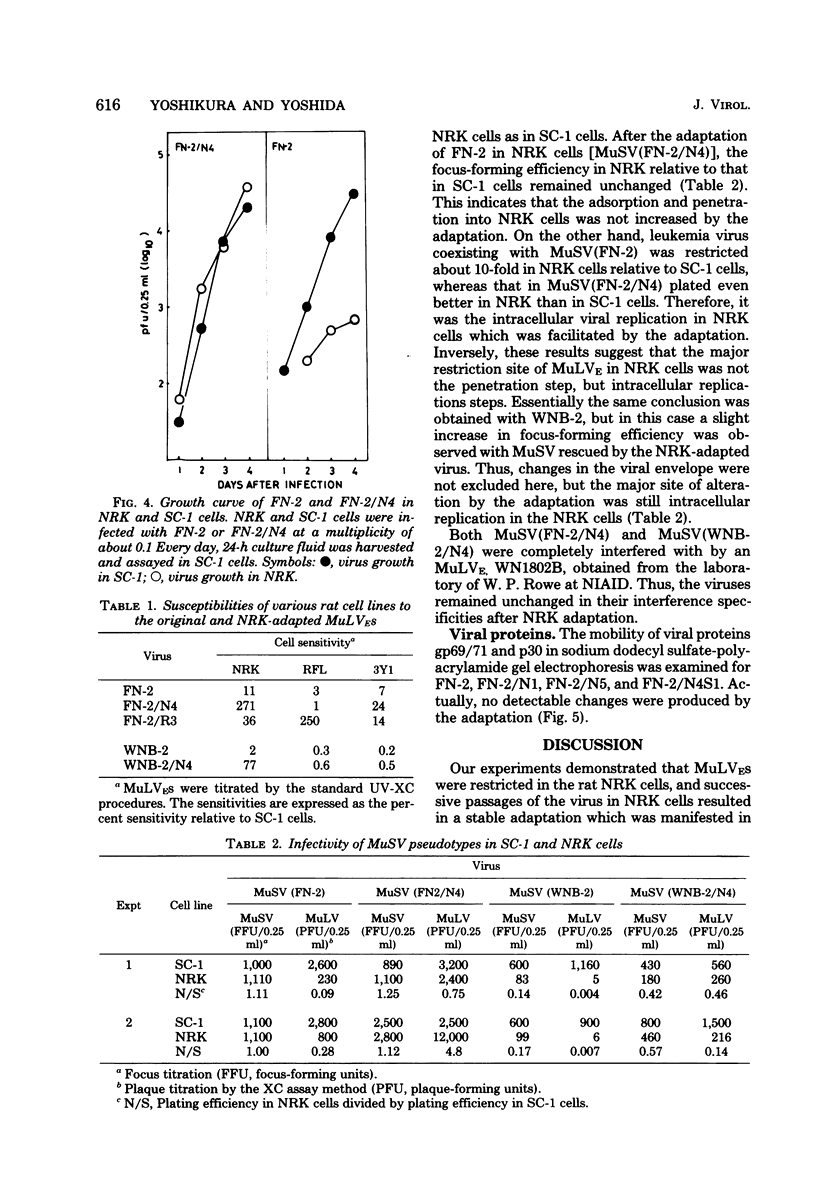
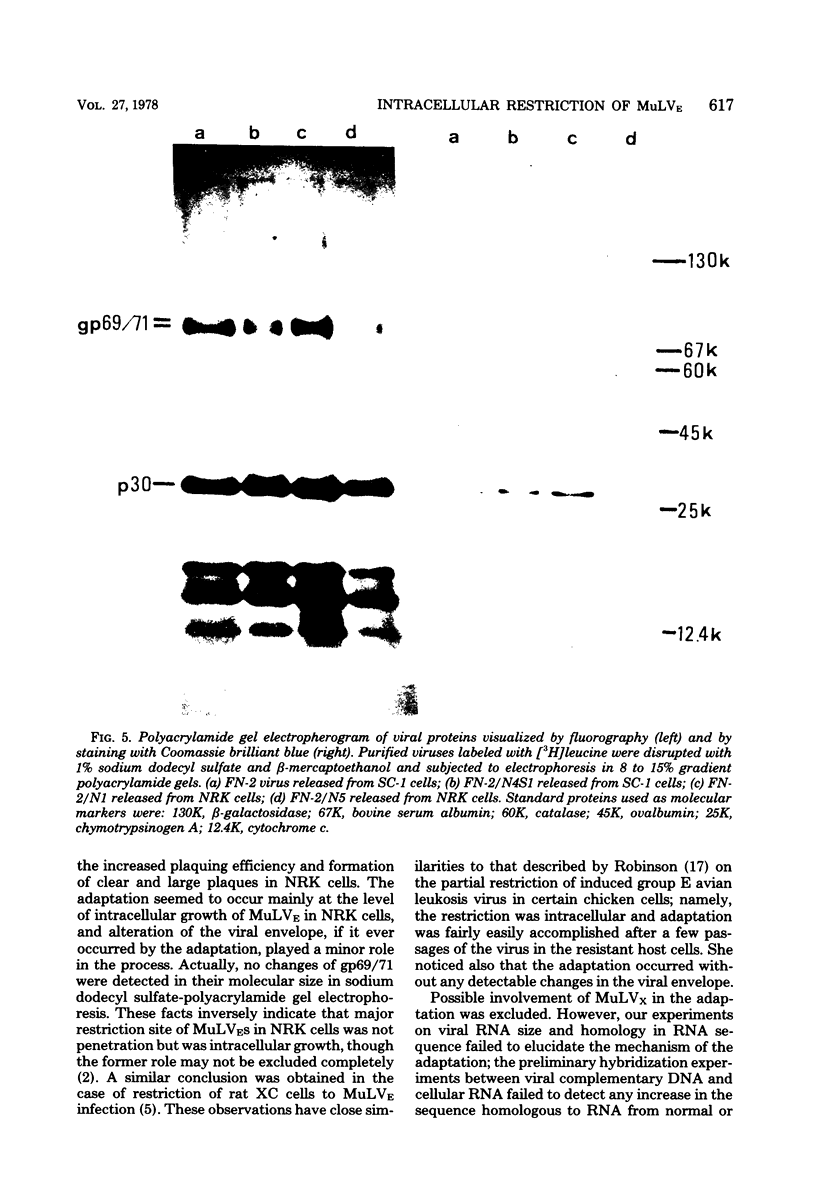
Ecotropic murine leukemia viruses, both N-tropic FN-2 (purified helper component of Friend leukemia virus) and B-tropic WNB-2 (purified WN1802B BALB/c-derived endogenous virus), were partially restricted in rat NRK cells. In NRK cells, they produced obscure small plaques at reduced efficiencies relative to their plaque-producing efficiencies in mouse SC-1 cells (10-fold for FN-2 and 100-fold for WNB-2). After three or four passages in NRK cells, the plaquing efficiencies of the viruses in NRK cells increased to levels close to their efficiencies in mouse cells, and the plaques in NRK cells became larger and clearer. The adaptation was more complete with FN-2 than with WNB-2. The adaptation was not due to simple selection of a virus in the FN-2 stock, but was host induced, as the viruses had been submitted to successive limiting dilutions in SC-1 cells before propagation in NRK cells. Possible commitment of xenotropic virus in the adaptation was excluded. The change was stable, even if the adapted viruses were propagated back into SC-1 cells. The NRK-adapted viruses were restricted in other rat cell lines of different origins, and the virus adapted in another rat cell line, RFL, was still restricted in NRK cells. The adaptation was mainly brought about by increased viral growth within the rat cells and not by an increased efficiency of viral penetration into the rat cells. This inversely suggests that the restriction of the ecotropic murine leukemia viruses in NRK cells was a mainly intracellular event. The mobilities of gp69/71 and p30 in sodium dodecyl sulfatepolyacrylamide gel electrophoresis remained unchanged after adaptation of FN-2 in NRK cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Besmer P., Baltimore D. Mechanism of restriction of ecotropic and xenotropic murine leukemia viruses and formation of pseudotypes between the two viruses. J Virol. 1977 Mar;21(3):965–973. doi: 10.1128/jvi.21.3.965-973.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson H. L., Robinson W. S., Huebner R. J., Turner H. C. Proteins of Rous sarcoma virus. Virology. 1968 Sep;36(1):73–86. doi: 10.1016/0042-6822(68)90118-9. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Bedigian H. G., Meier H. Steroid hormones increase the growth of MuLV in rat tumor XC cells. Virology. 1977 Dec;83(2):462–466. doi: 10.1016/0042-6822(77)90196-9. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Schindler J., Hynes R. Six-NB-tropic murine leukemia viruses derived from a B-tropic virus of BALB/c have altered p30. J Virol. 1977 Jan;21(1):309–318. doi: 10.1128/jvi.21.1.309-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto A., Hartley J. W., Rowe W. P. Detection and quantitation of phenotypically mixed viruses: mixing of ecotropic and xenotropic murine leukemia viruses. Virology. 1977 Sep;81(2):263–269. doi: 10.1016/0042-6822(77)90143-x. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A., Summers J. Rat cell line 3y1 and its virogenic polyoma- and sv40- transformed derivatives. Int J Cancer. 1975 Apr 15;15(4):694–706. doi: 10.1002/ijc.2910150419. [DOI] [PubMed] [Google Scholar]
- Klement V., Rowe W. P., Hartley J. W., Pugh W. E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci U S A. 1969 Jul;63(3):753–758. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M. Titration of murine leukemia viruses with rat cell line RFL. J Virol. 1977 Aug;23(2):436–438. doi: 10.1128/jvi.23.2.436-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Howk R. S., Anisowicz A., Scolnick E. M. Deletion mapping of moloney type C virus: polypeptide and nucleic acid expression in different transforming virus isolates. J Virol. 1976 May;18(2):491–503. doi: 10.1128/jvi.18.2.491-503.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles P. T. An in vitro focus-induction assay for xenotropic murine leukemia virus, feline leukemia virus C, and the feline--primate viruses RD-114/CCC/M-7. Virology. 1975 Sep;67(1):288–291. doi: 10.1016/0042-6822(75)90427-4. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. IV. Dose-response relationships in Fv-1-sensitive and resistant cell cultures. Virology. 1975 Jun;65(2):333–342. doi: 10.1016/0042-6822(75)90039-2. [DOI] [PubMed] [Google Scholar]
- Robinson H. L. Intracellular restriction on the growth of induced subgroup E avian type C viruses in chicken cells. J Virol. 1976 Jun;18(3):856–866. doi: 10.1128/jvi.18.3.856-866.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Williams D., Maryak J., Vass W., Goldberg R. J., Parks W. P. Type C particle-positive and type C particle-negative rat cell lines: characterization of the coding capacity of endogenous sarcoma virus-specific RNA. J Virol. 1976 Dec;20(3):570–582. doi: 10.1128/jvi.20.3.570-582.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers K., Kit S. Clonal isolation of murine sarcoma virus (MSV): characterization of virus produced from transformed cells. Virology. 1971 Dec;46(3):774–785. doi: 10.1016/0042-6822(71)90079-1. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Envelope classification of avian RNA tumor viruses. Bibl Haematol. 1970;(36):153–167. doi: 10.1159/000391704. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Hirokawa Y., Yamada M., Sugano H. Production of friend leukemia virus in a mouse lung cell line. Jpn J Med Sci Biol. 1967 Jun;20(3):225–236. doi: 10.7883/yoken1952.20.225. [DOI] [PubMed] [Google Scholar]