Abstract
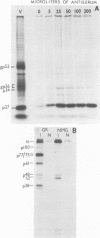
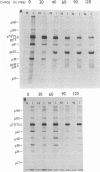
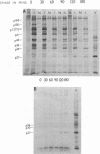
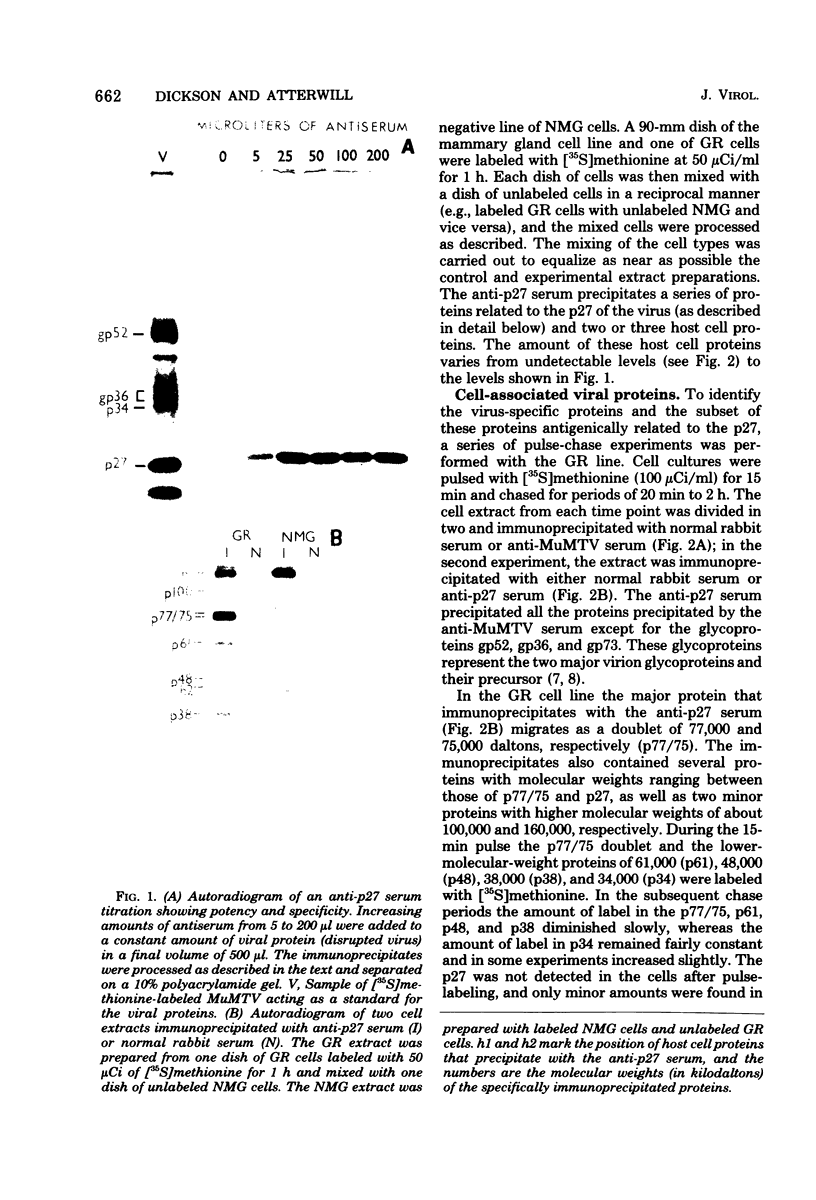
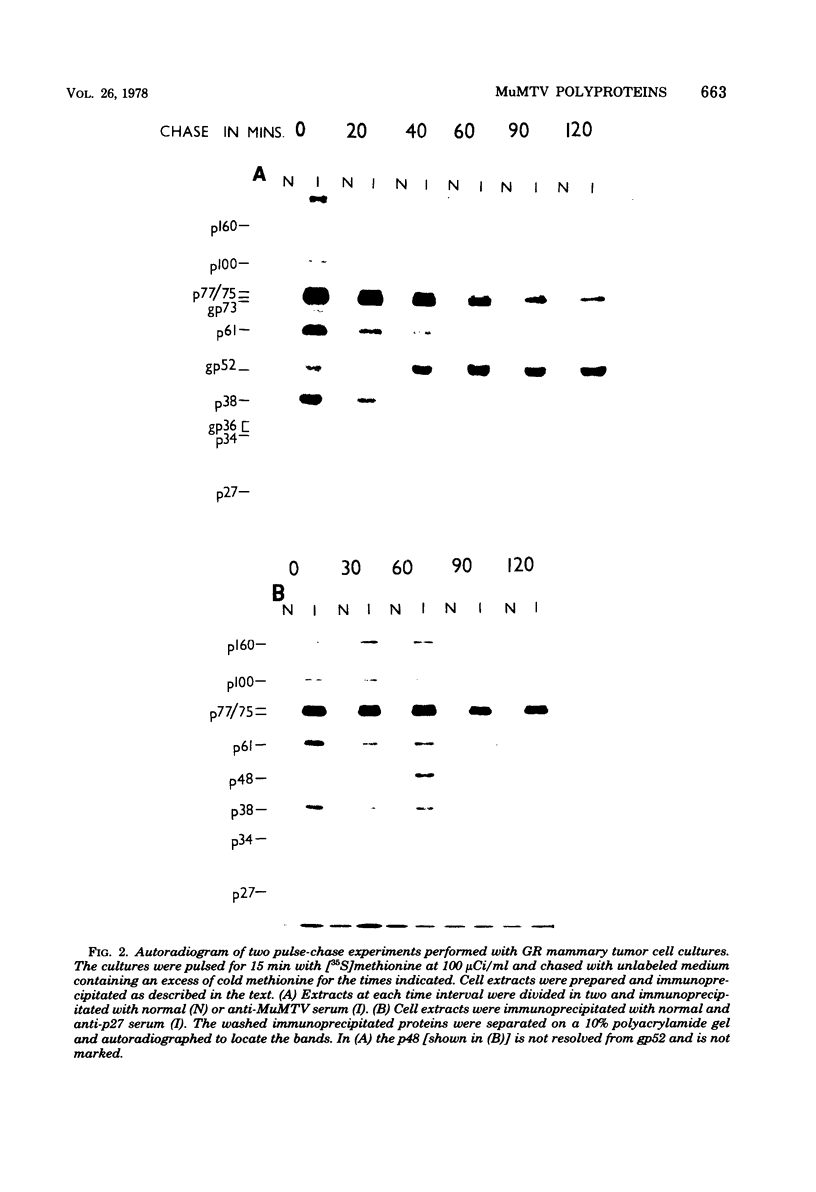
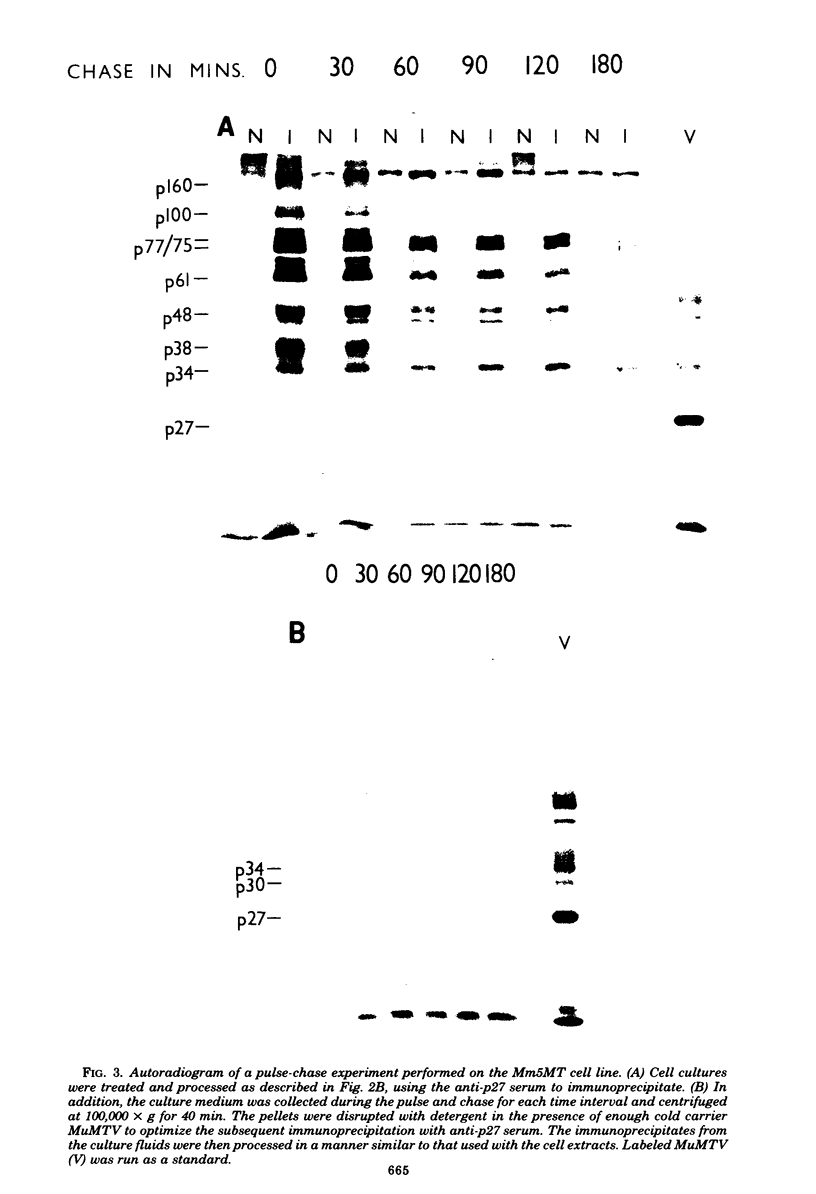
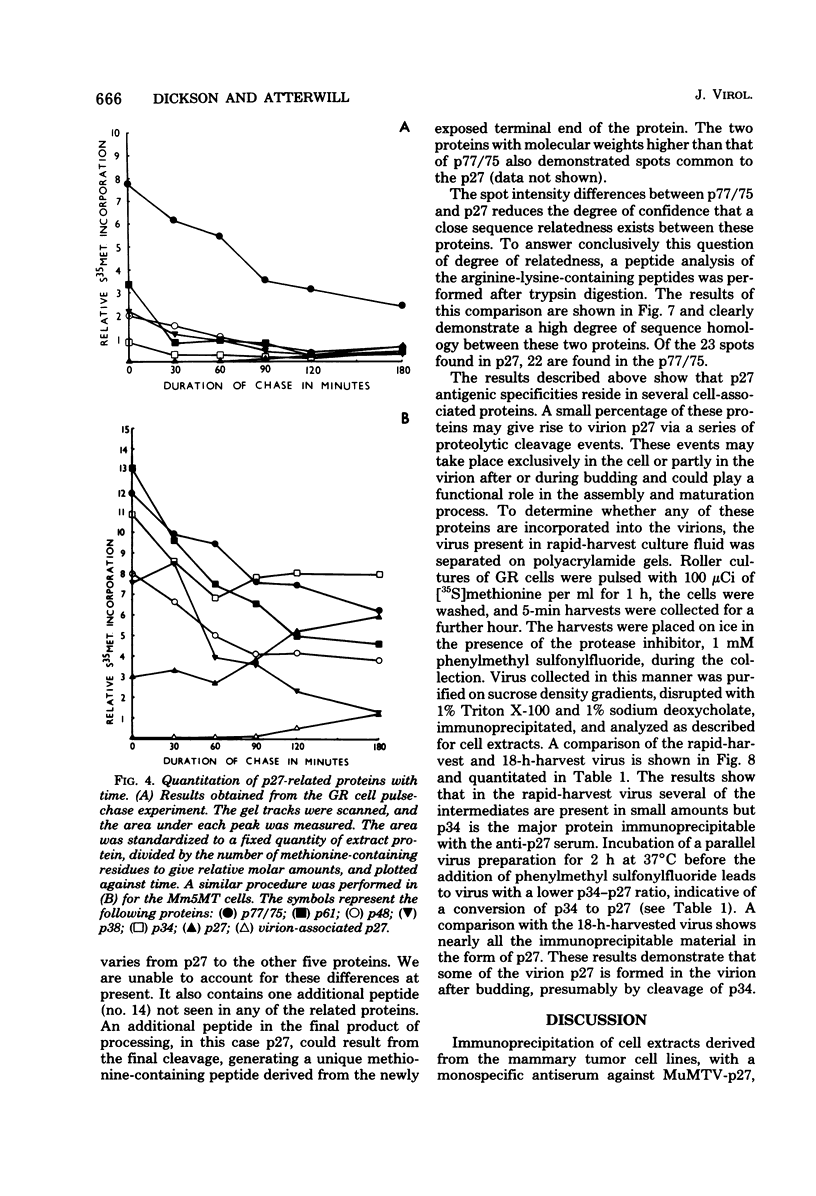
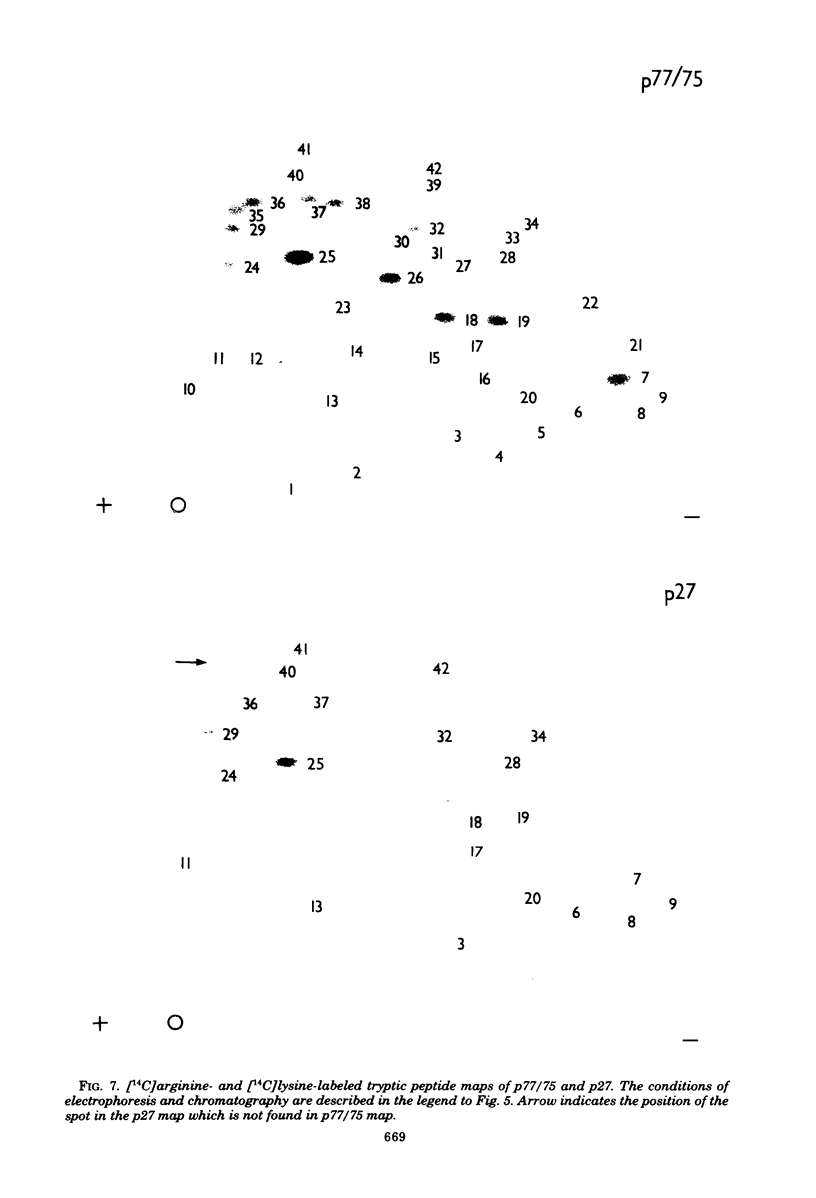
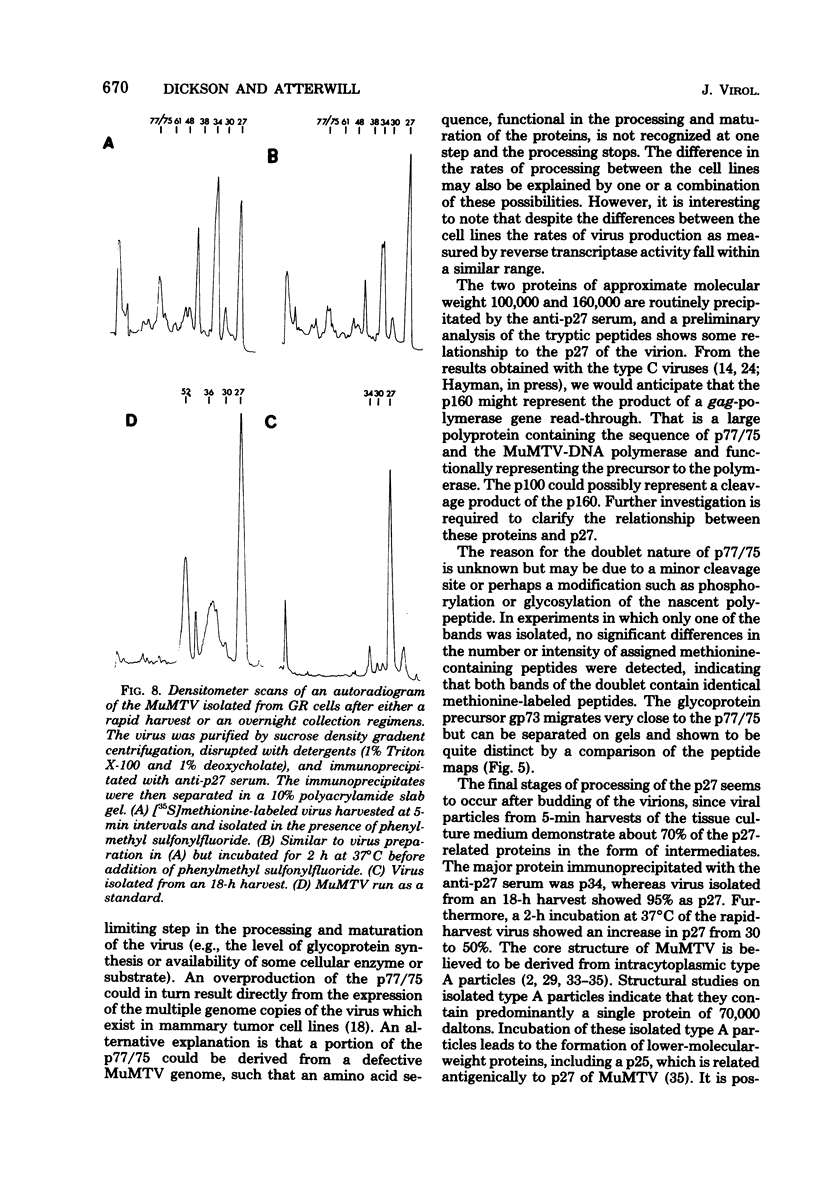
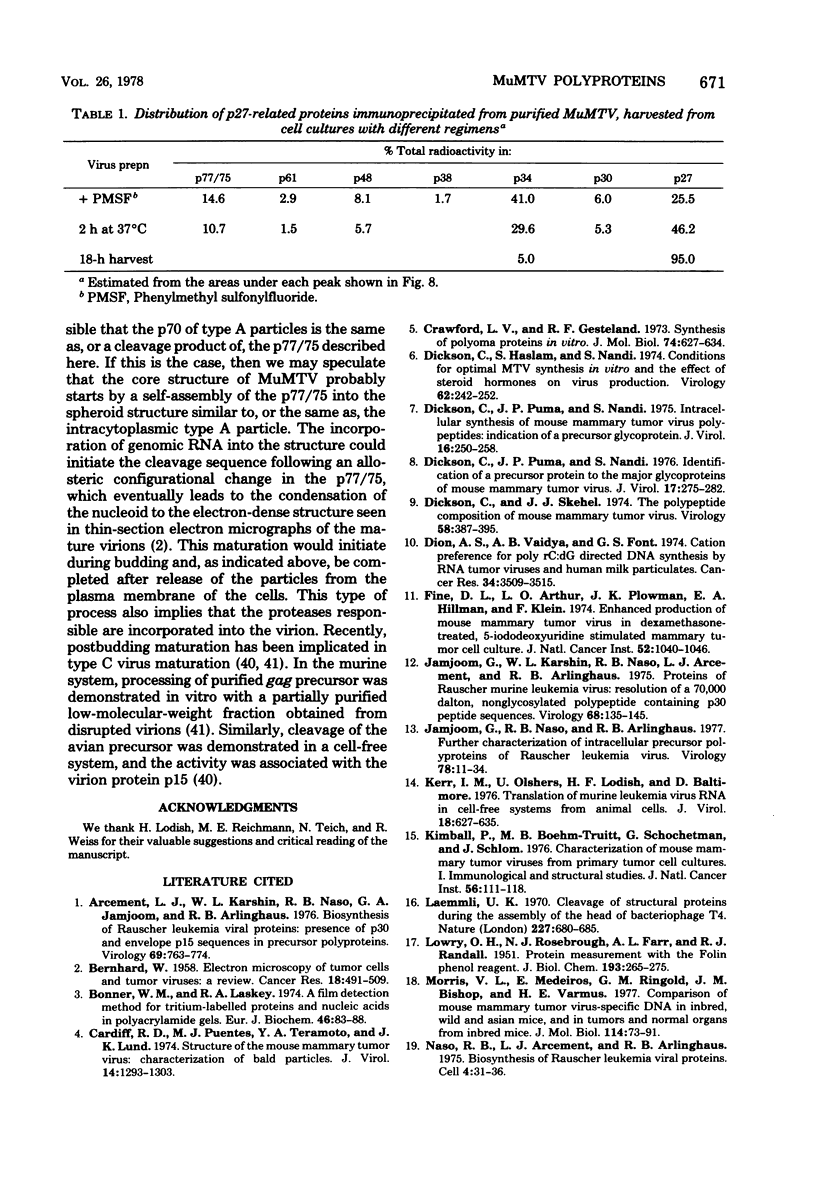
The mouse mammary tumor virus (MuMTV) contains several low-molecular-weight proteins which, together with the genomic RNA, constitute the core structure of the virion. The most abundant protein in the core is the 27,000-dalton protein (p27), and, by analogy to the type C viruses, this protein probably forms the core shell. In mouse mammary tumor cell lines (GR and Mm5MT) producing MuMTV the major p57 antigenic specificity resides in a large protein, which migrates in polyacrylamide gels as a doublet of 77,000 and 75,000 daltons (p 77/75). A series of lower-molecular-weight proteins, p61, p48, p38, and p34, is also present in small amounts and is probably derived by proteolytic cleavage of the p 77/75. These proteins have been identified by immunoprecipitation with monospecific antiserum, and their sequence relatedness to p27 has been determined by an analysis of the peptides after trypsin digestion. After a 15-min pulse with [35S]-methionine, all of the p27-related proteins in these cell lines were labelled and, during a subsequent chase, progressively disappeared. The p27 was labeled poorly during the pulse, but the amount of label in this protein increased during the chase. A quantitation of these experiments suggested that the majority of the p27-related proteins were quite rapidly turned over in these cell lines. Hence, if p27 is derived by a progressive proteolytic cleavage mechanism, then the process is inefficient in the GR cells and only moderately efficient in the Mm5MT cells. When MuMTV was isolated from the culture medium of these cells harvested at 5-min intervals, the major p27-related protein was p34. The p27 accounted for only 29% of the anti-p27 serum immunoprecipitable proteins compared to 95% in virus isolated from an 18-h harvest. Incubation of the rapid-harvest virus at 37 degrees C for 2 h resulted in some conversion of p34 to p27. These results suggest that some of the p27 in MuMTV is formed in the virions by proteolytic cleavage of p34.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcement L. J., Karshin W. L., Naso R. B., Jamjoom G., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins: presence of p30 and envelope p15 sequences in precursor polypeptides. Virology. 1976 Feb;69(2):763–774. doi: 10.1016/0042-6822(76)90504-3. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D., Puentes M. J., Teramoto Y. A., Lund J. K. Structure of the mouse mammary tumor virus: characterization of bald particles. J Virol. 1974 Nov;14(5):1293–1303. doi: 10.1128/jvi.14.5.1293-1303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Gesteland R. F. Synthesis of polyoma proteins in vitro. J Mol Biol. 1973 Mar 15;74(4):627–634. doi: 10.1016/0022-2836(73)90053-3. [DOI] [PubMed] [Google Scholar]
- Dickson C., Haslam S., Nandi S. Conditions for optimal MTV synthesis in vitro and the effect of steroid hormones on virus production. Virology. 1974 Nov;62(1):242–252. doi: 10.1016/0042-6822(74)90319-5. [DOI] [PubMed] [Google Scholar]
- Dickson C., Puma J. P., Nandi S. Identification of a precursor protein to the major glycoproteins of mouse mammary tumor virus. J Virol. 1975 Jan;17(1):275–282. doi: 10.1128/jvi.17.1.275-282.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Puma J. P., Nandi S. Intracellular synthesis of mouse mammary tumor virus polypeptides: indication of a precursor glycoprotein. J Virol. 1975 Aug;16(2):250–258. doi: 10.1128/jvi.16.2.250-258.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Skehel J. J. The polypeptide composition of mouse mammary tumor virus. Virology. 1974 Apr;58(2):387–395. doi: 10.1016/0042-6822(74)90074-9. [DOI] [PubMed] [Google Scholar]
- Dion A. S., Vaidya A. B., Fout G. S. Cation preferences for poly(rC)-oligo(dG)-directed DNA synthesis by RNA tumor viruses and human milk particulates. Cancer Res. 1974 Dec;34(12):3509–3515. [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Further characterization of intracellular precursor polyproteins of Rauscher leukemia virus. Virology. 1977 May 1;78(1):11–34. doi: 10.1016/0042-6822(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Jamjoom G., Karshin W. L., Naso R. B., Arcement L. J., Arlinghaus R. B. Proteins of Rauscher murine leukemia virus: resolution of a 70,000-dalton, Nonglycosylated polypeptide containing p30 peptide sequences. Virology. 1975 Nov;68(1):135–145. doi: 10.1016/0042-6822(75)90155-5. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Olshevsky U., Lodish H. F., Baltimore D. Translation of murine leukemia virus RNA in cell-free systems from animal cells. J Virol. 1976 May;18(2):627–635. doi: 10.1128/jvi.18.2.627-635.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball P. C., Boehm-Truitt M., Schochetman G., Schlom J. Characterization of mouse mammary tumor viruses from primary tumor cell cultures.I. Immunologic and structural studies. J Natl Cancer Inst. 1976 Jan;56(1):111–117. doi: 10.1093/jnci/56.1.111. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins. Cell. 1975 Jan;4(1):31–36. doi: 10.1016/0092-8674(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Wood G., Saunders T. E., Arlinghaus R. B. The cell-free translation of Rauscher leukemia virus RNA into high molecular weight polypeptides. Biochim Biophys Acta. 1975 Mar 10;383(2):195–206. doi: 10.1016/0005-2787(75)90261-0. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Hackett A. J. Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst. 1974 Jul;53(1):261–269. doi: 10.1093/jnci/53.1.261. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Howk R. S., Scolnick E. M., Oroszlan S., Gilden R. V. Immunochemical characterization of two major polypeptides from murine mammary tumor virus. J Virol. 1974 Jun;13(6):1200–1210. doi: 10.1128/jvi.13.6.1200-1210.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Martin G. S., Smith A. E. Cell-free translation of virion RNA from nondefective and transformation-defective Rous sarcoma viruses. J Virol. 1976 Sep;19(3):950–967. doi: 10.1128/jvi.19.3.950-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Salden M. H., Selten-Versteegen A. M., Bloemendal H. Translation of Rauscher murine leukemia viral RNA. A model for the function of virus-specific messenger. Biochem Biophys Res Commun. 1976 Sep 20;72(2):610–618. doi: 10.1016/s0006-291x(76)80084-8. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Strand M., August J. T. High molecular weight precursor polypeptides to structural proteins of Rauscher murine leukemia virus. J Mol Biol. 1976 Nov 15;107(4):459–477. doi: 10.1016/s0022-2836(76)80078-2. [DOI] [PubMed] [Google Scholar]
- Smith G. H., Wivel N. A. Intracytoplasmic A particles: mouse mammary tumor virus nucleoprotein cores? J Virol. 1973 Apr;11(4):575–584. doi: 10.1128/jvi.11.4.575-584.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H. Precursor-product relationship between nonglycosylated polypeptides of A and B particles of mouse mammary tumor virus. Virology. 1977 Feb;76(2):835–850. doi: 10.1016/0042-6822(77)90263-x. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Properties of a P70 proteolytic factor of murine leukemia viruses. Cell. 1977 Nov;12(3):709–719. doi: 10.1016/0092-8674(77)90271-9. [DOI] [PubMed] [Google Scholar]
- Zaanie D., Gielkens A. L., Dekker-michielsen M. J., Bloemers H. P. Virus-specific precursor polypeptides in cells infected with Rauscher leukemia virus. Virology. 1975 Oct;67(2):544–552. doi: 10.1016/0042-6822(75)90454-7. [DOI] [PubMed] [Google Scholar]