Abstract
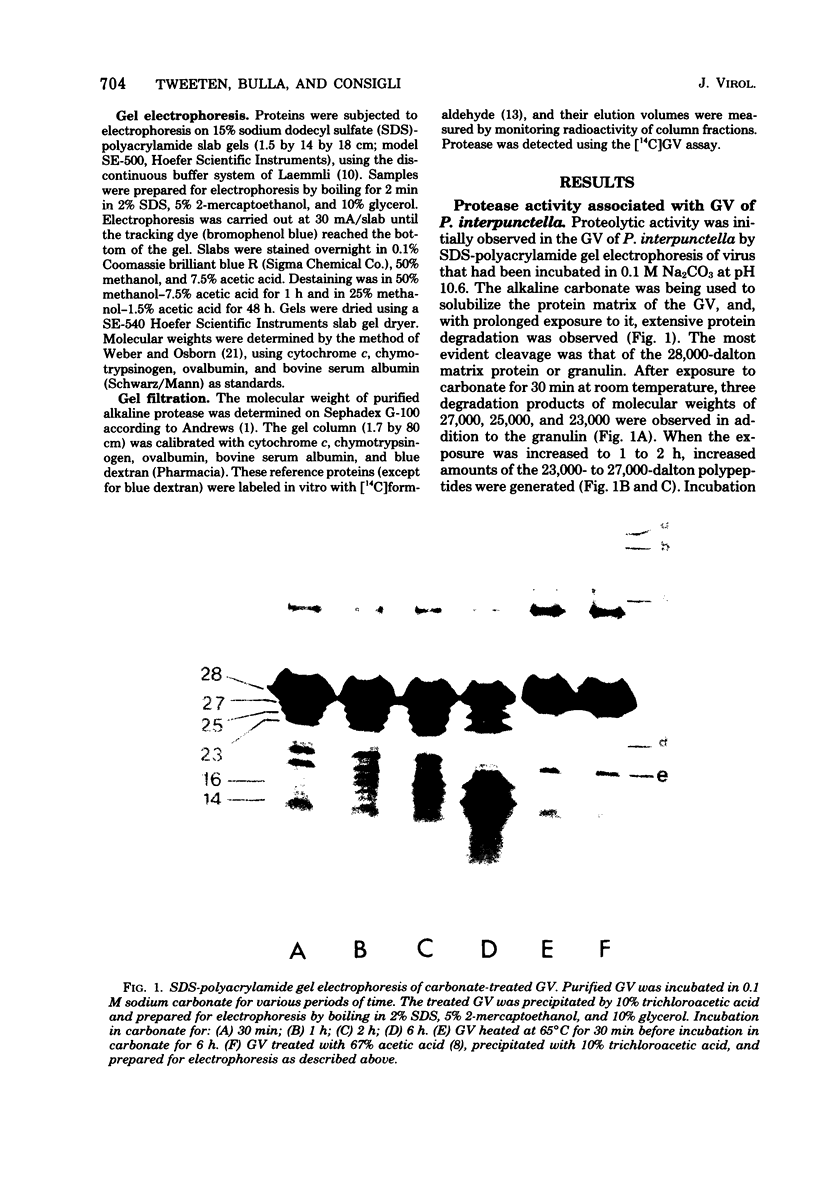
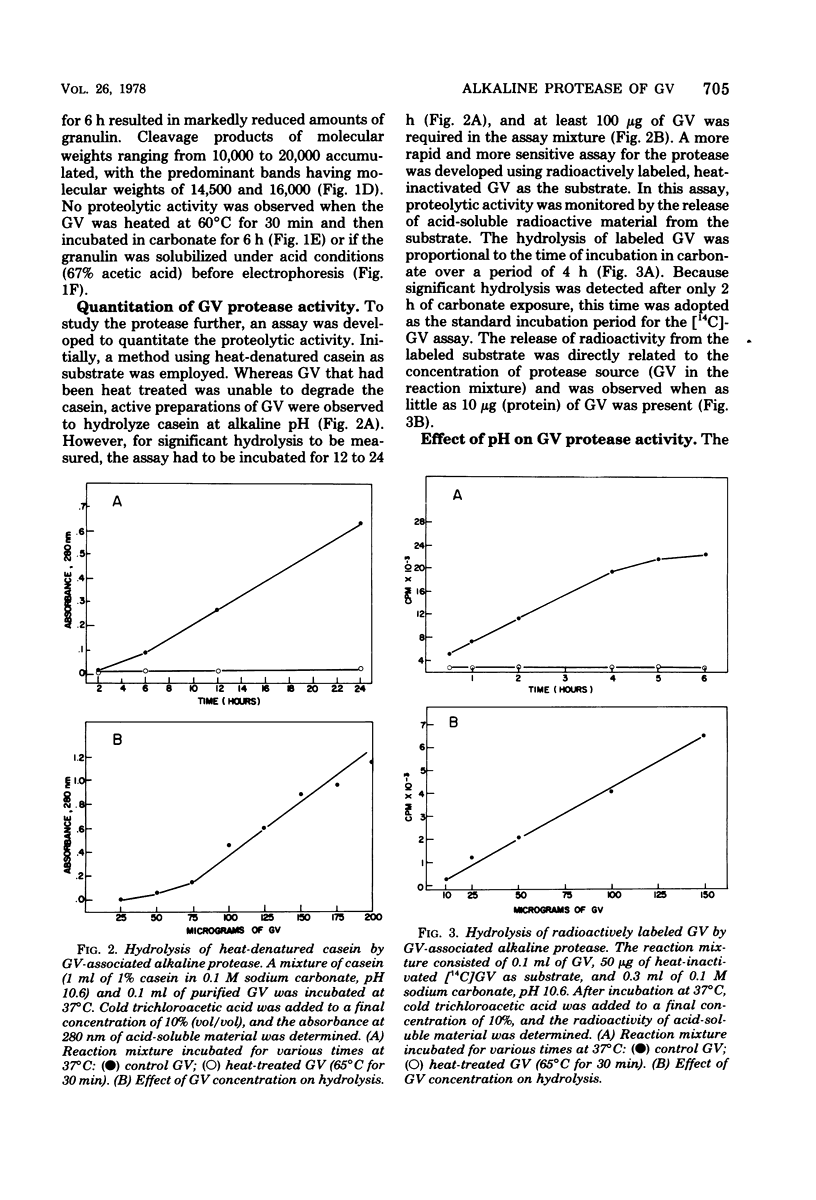
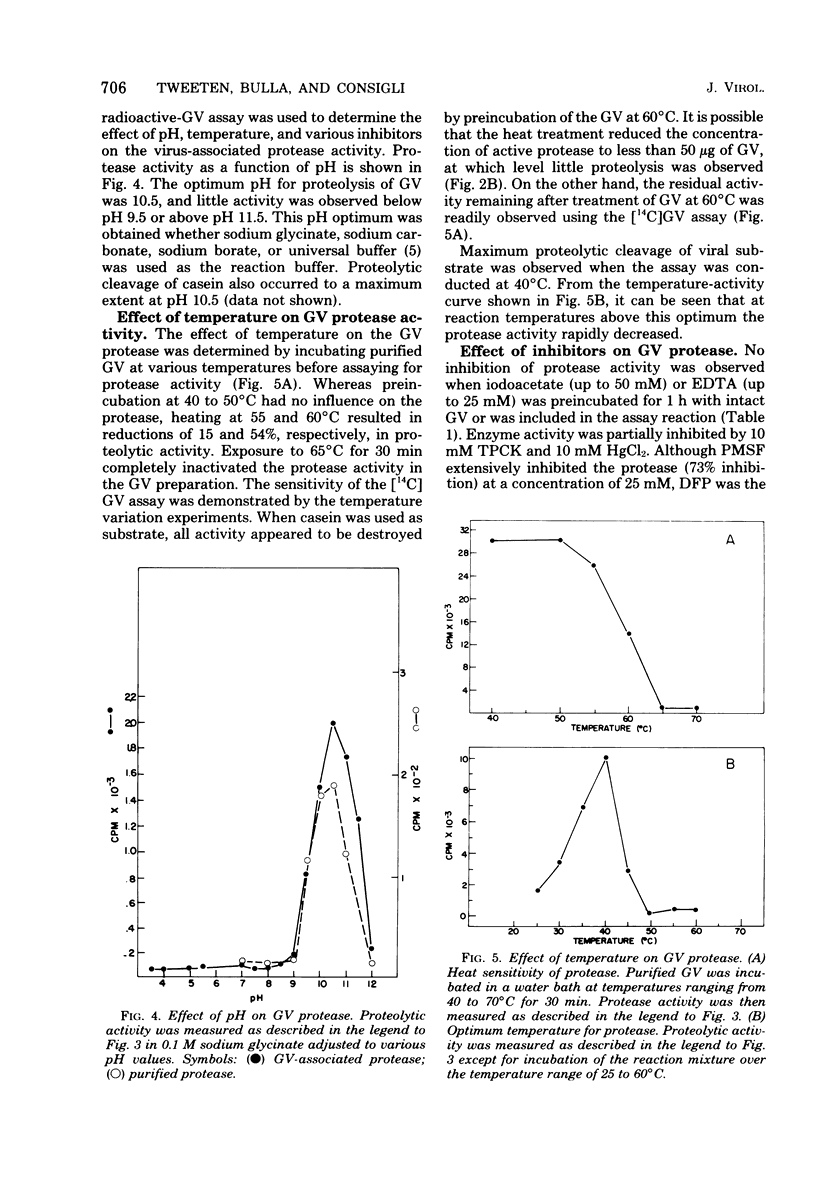
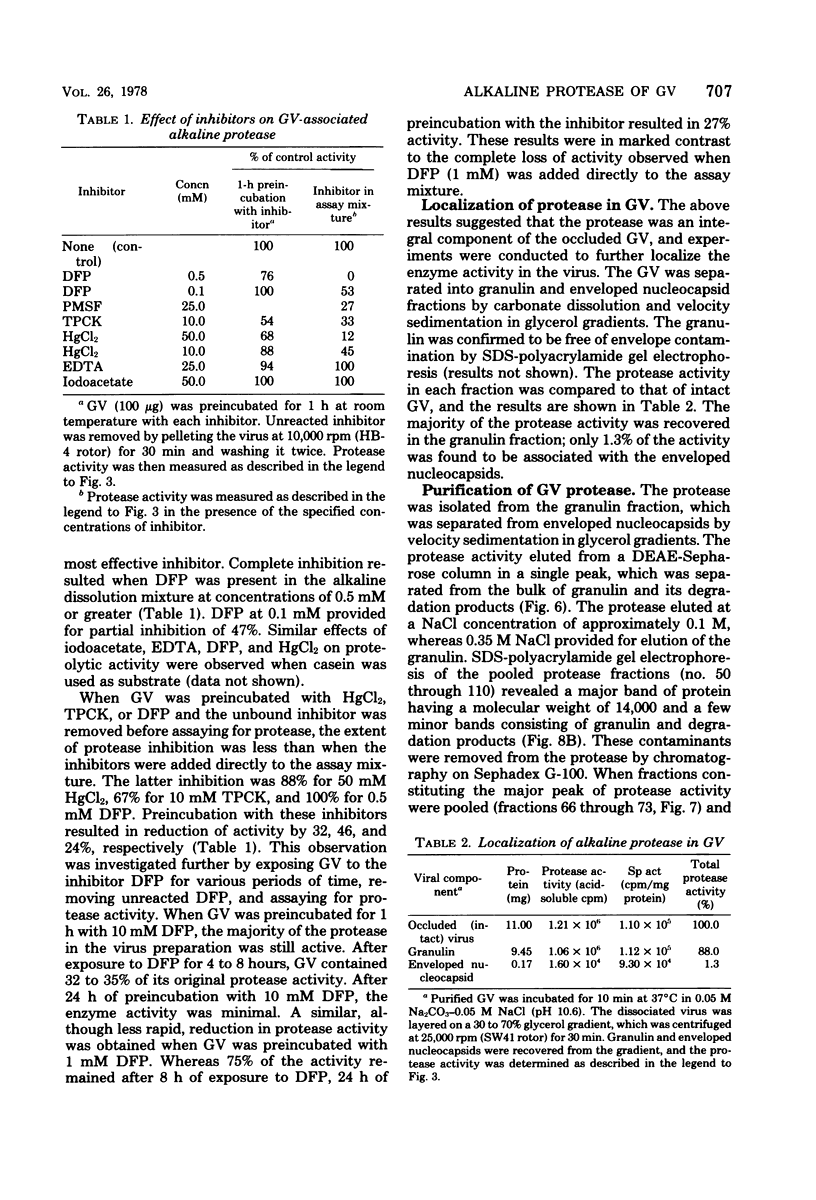
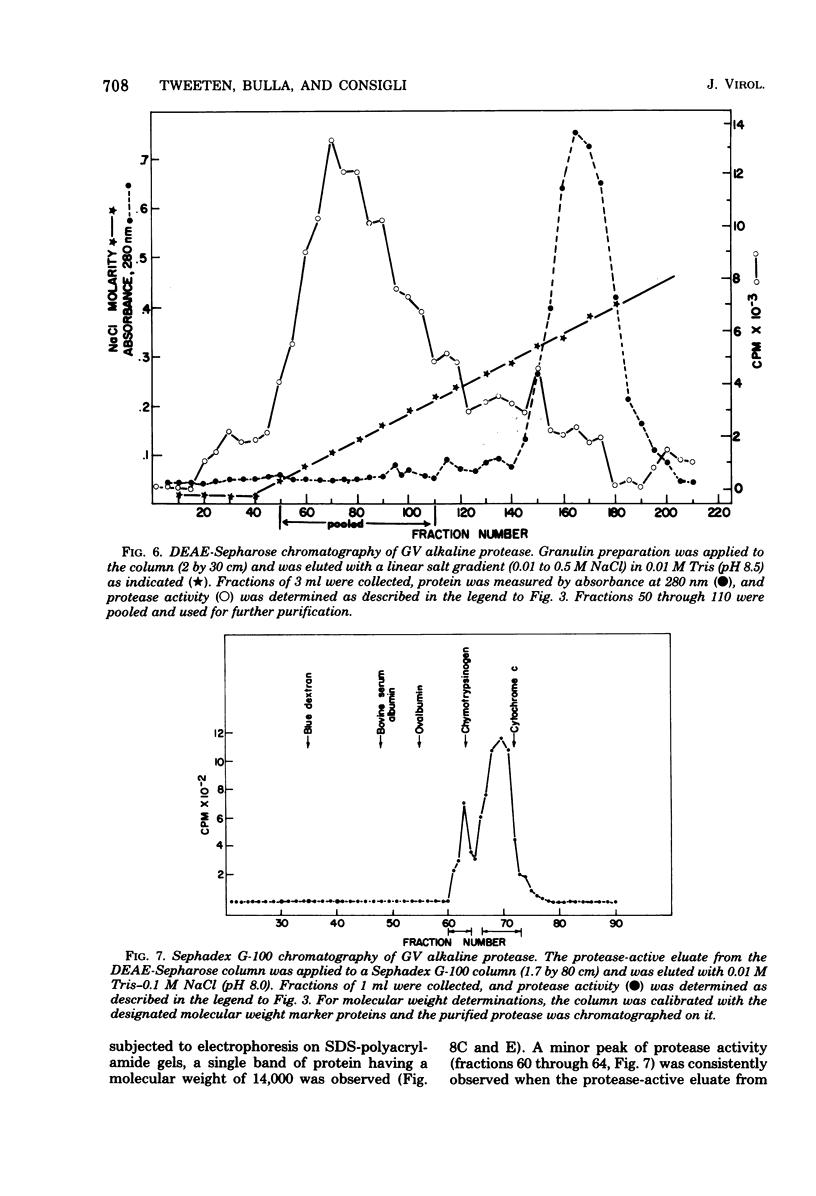
An alkaline protease was found to be associated with the granulosis virus of the Indian meal moth. Plodia interpunctella. The protease was located within the protein matrix of the occluded virus and hydrolyzed the major constituent of this matrix, a 28,000-dalton protein (granulin), to a mixture of polypeptides ranging in molecular weight from 10,000 to 27,000. A rapid, sensitive assay for the protease was developed using radioactively labeled granulosis virus as substrate. With this assay, the proteolytic activity could be detected by measuring the release of acid-soluble peptides from the labeled virus. The protease had a pH optimum of 10.5 and a temperature optimum of 40 degrees C and was inhibited by diisopropyl phosphorofluoridate, phenylmethylsulfonyl fluoride, and L-(1-tosylamido-2-phenyl) ethyl chloromethyl ketone. Purification of the protease from matrix protein was achieved by anion-exchange and gel permeation chromatography. The molecular weight of the isolated protease, determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and gel filtration, was approximately 14,000.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppstein D. A., Thoma J. A., Scott H. A., Young S. Y., 3rd Degradation of matrix protein from a nuclear-polyhedrosis virus of Trichoplusia ni by an endogenous protease. Virology. 1975 Oct;67(2):591–594. doi: 10.1016/0042-6822(75)90459-6. [DOI] [PubMed] [Google Scholar]
- Harrap K. A., Payne C. C., Robertson J. S. The properties of three baculoviruses from closely related hosts. Virology. 1977 Jun 1;79(1):14–31. doi: 10.1016/0042-6822(77)90330-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. In vitro radioisotopic labeling of proteins associated with purified polyoma virions. J Virol. 1974 Dec;14(6):1627–1629. doi: 10.1128/jvi.14.6.1627-1629.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. D., Egawa K. Physical and chemical properties of Trichoplusia ni granulosis virus granulin. J Virol. 1973 Nov;12(5):1092–1103. doi: 10.1128/jvi.12.5.1092-1103.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. D. Electron microscopic observations on granulosis virus entry, uncoating and replication processes during infection of the midgut cells of Trichoplusia ni. J Ultrastruct Res. 1971 Jun;35(5):606–625. doi: 10.1016/s0022-5320(71)80014-x. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Smith G. E. Trichoplusia ni granulosis virus granulin: a phenol-soluble, phosphorylated protein. J Virol. 1975 Nov;16(5):1108–1116. doi: 10.1128/jvi.16.5.1108-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]