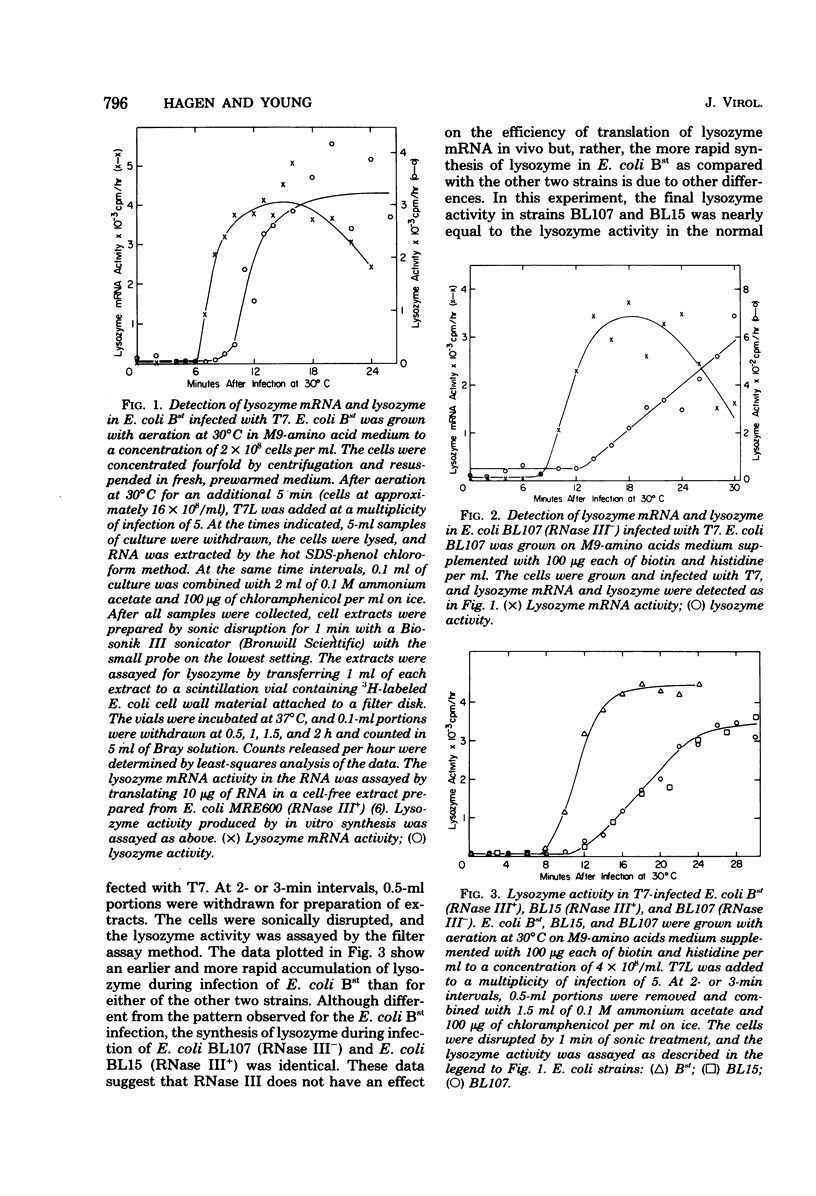
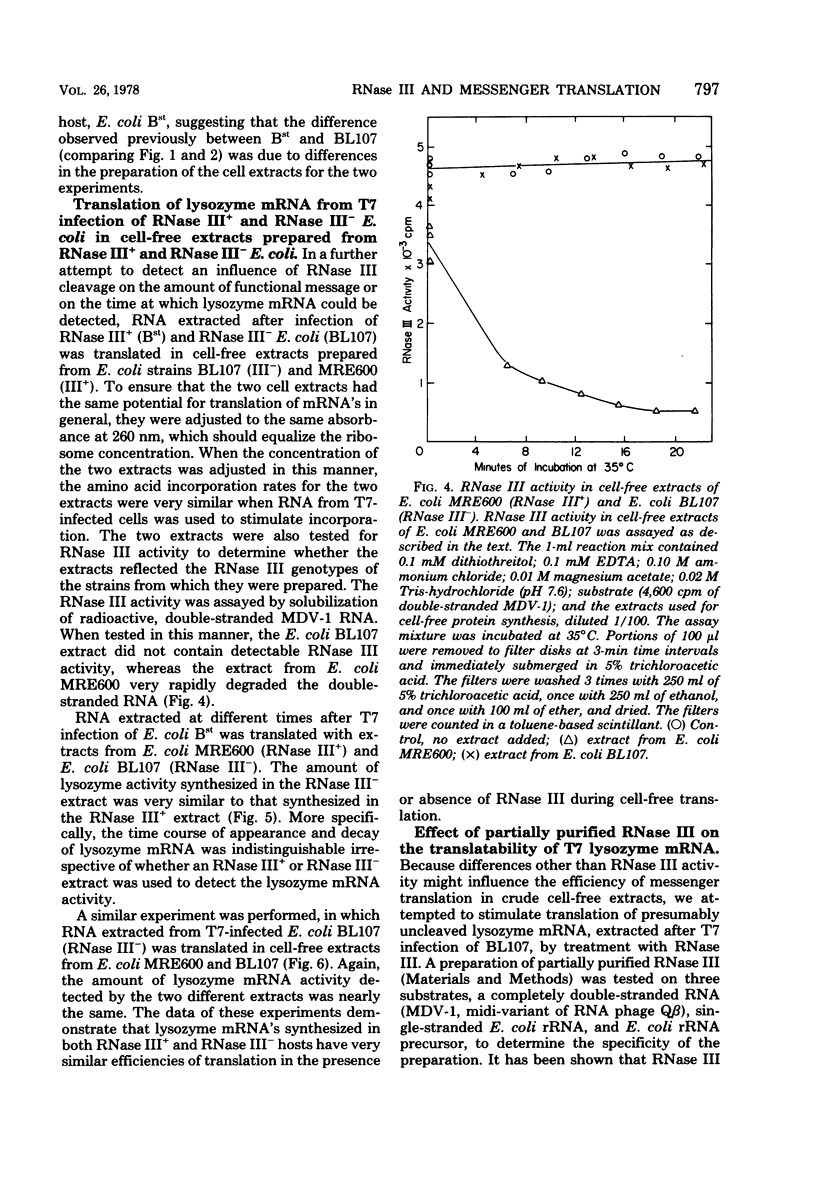
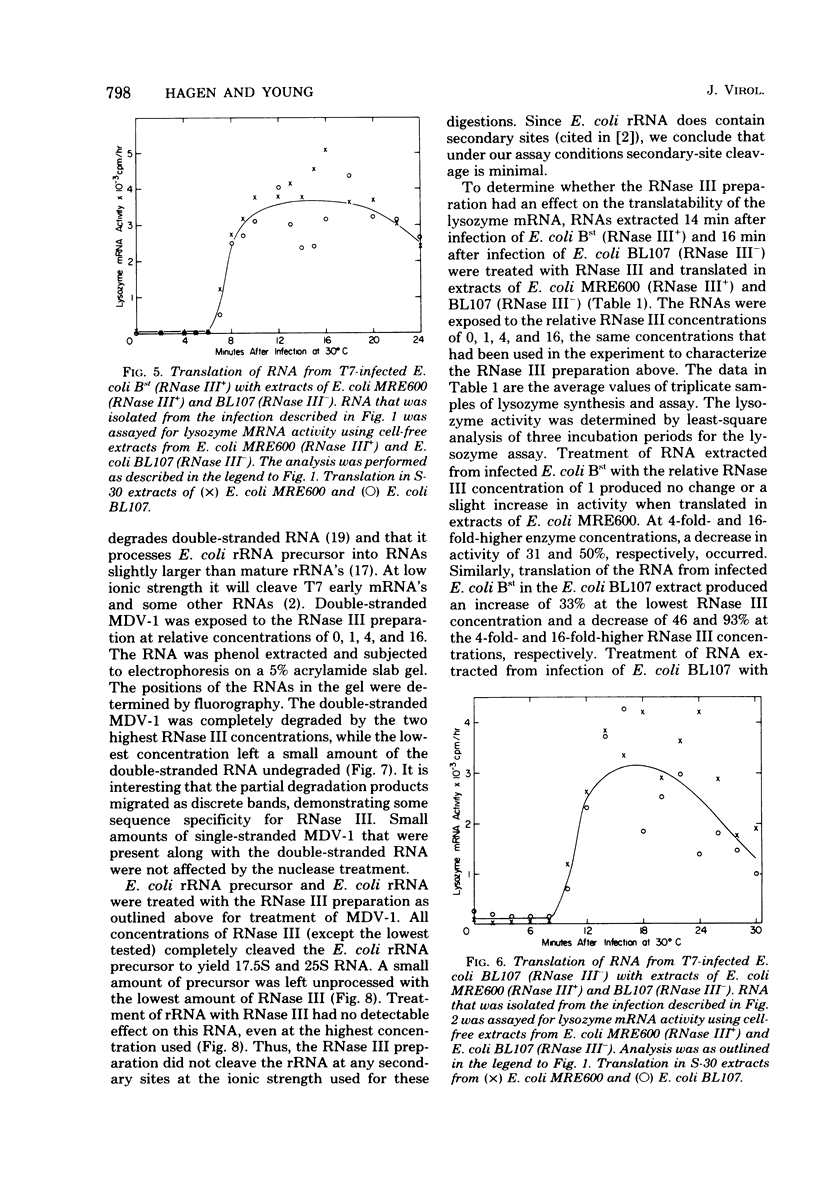
Abstract
RNase III had no positive effect on the translation of bacteriophage T7 lysozyme mRNA in vivo or in vitro. The time of appearance and quanity of lysozyme in T7-infected E. coli BL107, an RNase III- strain, and T7-infected E. coli BL15, a nearly isogenic RNase III+ strain, were indistinguishable. Nearly identical patterns of lysozyme mRNA activity were obtained when RNA extracted at different times after infection of RNase III+ and RNase III- hosts was translated in cell-free extracts of E. coli containing or lacking RNase III. Exposure of RNA extracted from T7-infected E. coli BL107 (RNase III-) to purified RNase III did not increase the lysozyme mRNA activity of this RNA. The only result that implied that RNase III has a differential effect on the translatability of the lysozyme mRNA was the translation of fractionaed RNA from T7-infected E. coli BL107. Translation of the smallest and largest lysozyme messages, 0.33 x 10(6) and 4 x 10(6) to 5 x 10(6) daltons, was the most inefficient in RNase III- cell-free extracts as compared to RNase III+ cell-free translation. The translation of the most abundant, medium-sized lysozyme mRNA between 0.9 x 10(6) and 1.5 x 10(6) daltons was the least affected by the absence of RNase III. The existence of a lag between the appearance of lysozyme mRNA and the appearance of lysozyme in T7 infection was confirmed. In these studies a very rapid method of RNA extraction was used, eliminating the possibility of continued RNA transcription during cell collection and RNA extraction. With this method of analysis, the length of the lag period was established at about 3 min. The possibility that RNase III is the controlling element of the lag period was eliminated by these investigations.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crouch R. J. Ribonuclease 3 does not degrade deoxyribonucleic acid-ribonucleic acid hybrids. J Biol Chem. 1974 Feb 25;249(4):1314–1316. [PubMed] [Google Scholar]
- Dunn J. J. RNase III cleavage of single-stranded RNA. Effect of ionic strength on the fideltiy of cleavage. J Biol Chem. 1976 Jun 25;251(12):3807–3814. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Effect of RNAase III, cleavage on translation of bacteriophage T7 messenger RNAs. J Mol Biol. 1975 Dec 15;99(3):487–499. doi: 10.1016/s0022-2836(75)80140-9. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Hagen F. S., Young E. T. Effect of RNase III on the size of bacteriophage T7 lysozyme mRNA. J Virol. 1978 Jun;26(3):783–792. doi: 10.1128/jvi.26.3.783-792.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen F. S., Young E. T. Preparative polyacrylamide gel electrophoresis of ribonucleic acid. Identification of multiple molecular species of bacteriophage T7 lysozyme messenger ribonucleic acid. Biochemistry. 1974 Jul 30;13(16):3394–3400. doi: 10.1021/bi00713a033. [DOI] [PubMed] [Google Scholar]
- Hagen F., Young E. T. Regulation of synthesis of bacteriophage T7 lysozyme mRNA. Virology. 1973 Sep;55(1):231–241. doi: 10.1016/s0042-6822(73)81026-8. [DOI] [PubMed] [Google Scholar]
- Hercules K., Schweiger M., Sauerbier W. Cleavage by RNase 3 converts T3 and T7 early precursor RNA into translatable message. Proc Natl Acad Sci U S A. 1974 Mar;71(3):840–844. doi: 10.1073/pnas.71.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J. E., Ko G., Young E. T. Comparative analysis of the in vivo and in vitro expression of bacteriophage T7 messenger RNAs during infection of Escherichia coli. J Mol Biol. 1975 Jun 5;94(4):539–554. doi: 10.1016/0022-2836(75)90320-4. [DOI] [PubMed] [Google Scholar]
- Lacroute F., Stent G. S. Peptide chain growth of -galactosidase in Escherichia coli. J Mol Biol. 1968 Jul 14;35(1):165–173. doi: 10.1016/s0022-2836(68)80044-0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
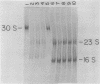
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R., Spiegelman S. Complete nucleotide sequence of a replicating RNA molecule. Science. 1973 Jun 1;180(4089):916–927. doi: 10.1126/science.180.4089.916. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dunn J. J. Ribonucleic acid processing activity of Escherichia coli ribonuclease III. J Biol Chem. 1975 Apr 25;250(8):3050–3056. [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Sakiyama S., Buchanan J. M. In vitro synthesis of deoxynucleotide kinase programmed by bacteriophage "T4-RNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1376–1380. doi: 10.1073/pnas.68.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]