Abstract
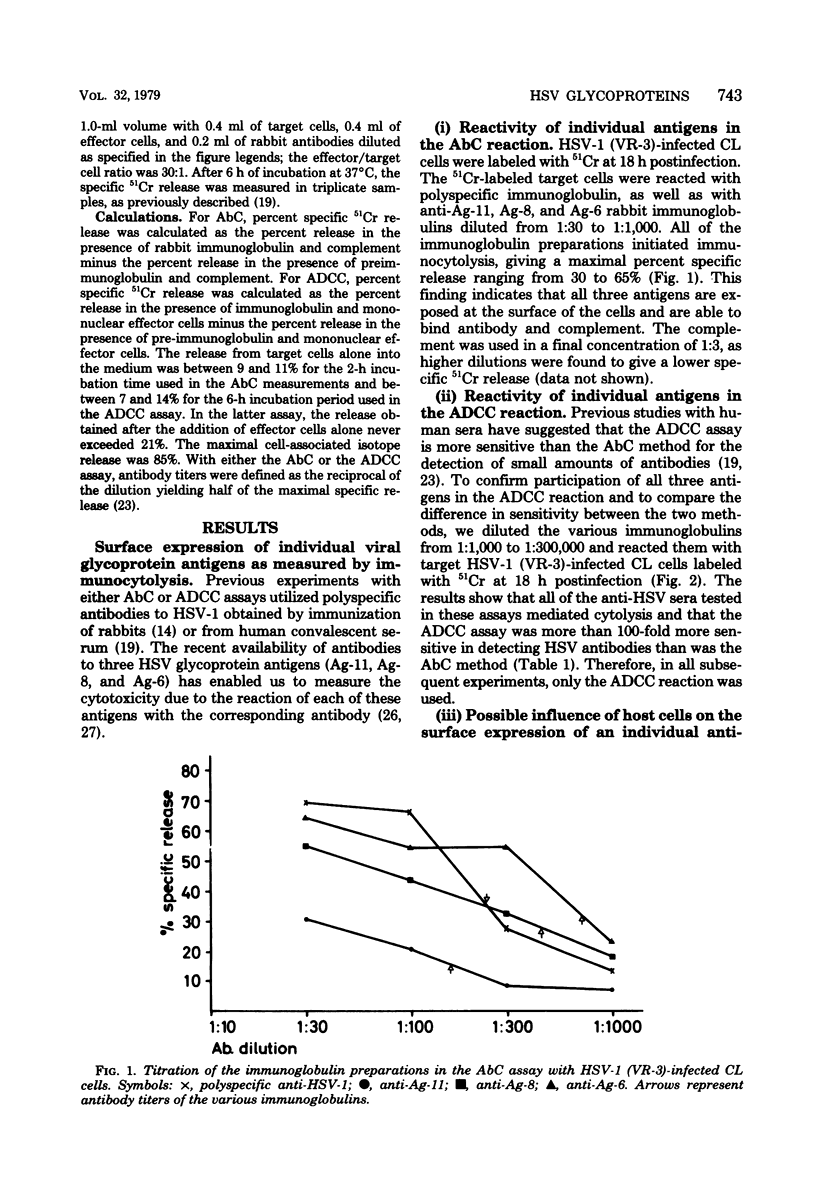
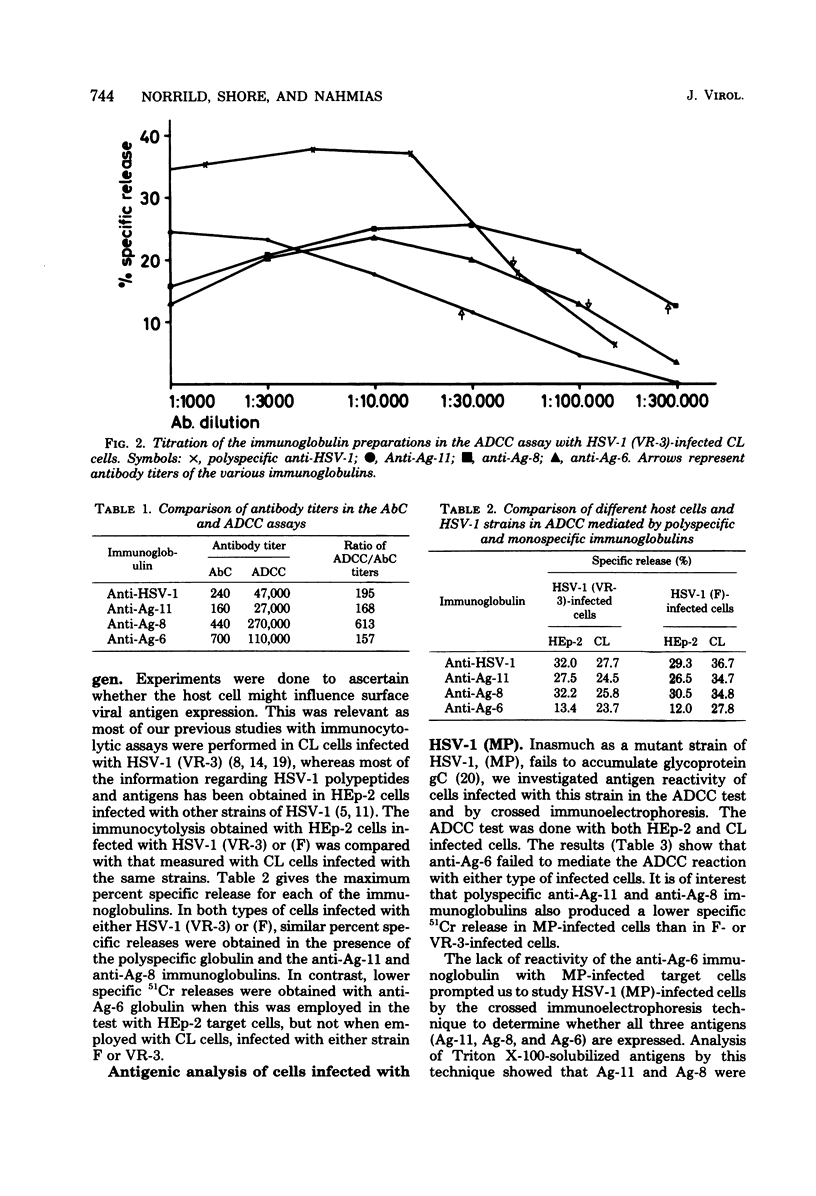
Tissue culture cells infected with herpes simplex type 1 virus express virus-specified glycoprotein antigens on the plasma membrane. Three of these have been previously identified and have been designated as Ag-11, Ag-8, and Ag-6. In the present study, immunoglobulins to each of the antigens were shown to be capable of mediating immunocytolysis in the presence of either complement (antibody-dependent complement-mediated cytotoxicity) or peripheral blood mononuclear cells (antibody-dependent cell-mediated cytotoxicity [ADCC]). Two herpes simplex virus type 1 strains, VR-3 and F, reacted similarly in the ADCC test in the presence of immunoglobulins to Ag-11, Ag-8, and Ag-6 in both infected Chang liver cells and HEp-2 cells. Anti-Ag-6, however, produced a lower ADCC reaction in HEp-2 cells than in Chang liver cells, suggesting differences in the Ag-6 surface expression in, or release from, these cells. Chang liver and HEp-2 cells infected with the MP mutant strain of herpes simplex virus type 1 showed reduced ADCC in the presence of anti-Ag-11 and anti-Ag-8, but no reactivity at all with anti-Ag-6. Crossed immunoelectrophoretic analysis showed that MP-infected cell extracts contain Ag-11 and Ag-8, but lack Ag-6. Polypeptide analysis of herpes simplex virus type 1 strains F, VR-3, and MP showed that Ag-11 consists of the glycoproteins gA and gB, that Ag-8 consists of gD, and that Ag-6 consists of gC. In conclusion, the present study demonstrates that either one of the glycoproteins (gC, gD, and a mixture of gA and gB) can function as a target for immunocytolysis and that the antibody preparation to gC (Ag-6) does not cross-react with any of the other glycoproteins.
Full text
PDF



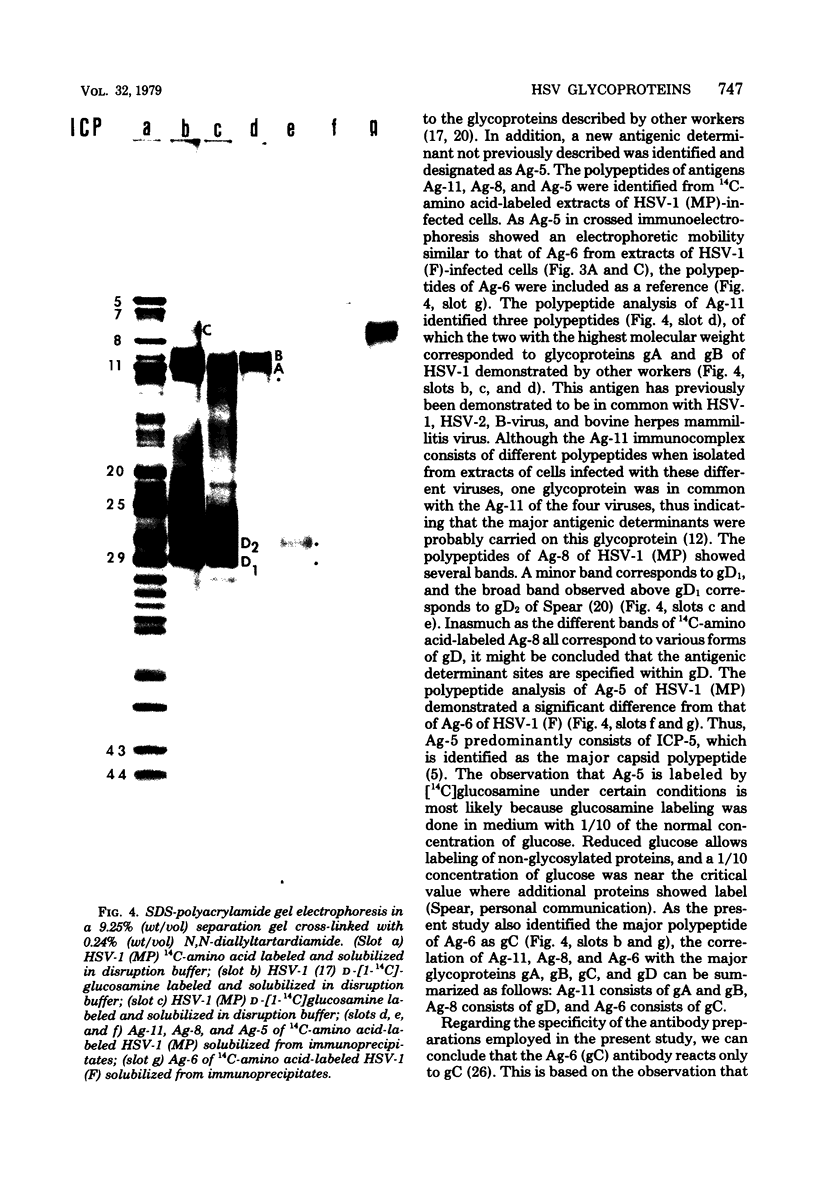

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsen N. H. Intermediate gel in crossed and in fused rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:71–77. doi: 10.1111/j.1365-3083.1973.tb03782.x. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Harris R., Ukaejiofo E. O. Rapid preparation of lymphocytes for tissue-typing. Lancet. 1969 Aug 9;2(7615):327–327. doi: 10.1016/s0140-6736(69)90096-8. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. S., Erickson J. S., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus. X. Proteins excreted by cells infected with herpes simplex virus, types 1 and 2. Virology. 1975 Mar;64(1):132–143. doi: 10.1016/0042-6822(75)90085-9. [DOI] [PubMed] [Google Scholar]
- Kohl S., Starr S. E., oleske J. M., Shore S. L., Ashman R. B., Nahmias A. J. Human monocyte-macrophage-mediated antibody-dependent cytotoxicity to herpes simplex virus-infected cells. J Immunol. 1977 Mar;118(3):729–735. [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Bjerrum O. J., Ludwig H., Vestergaard B. F. Analysis of herpes simplex virus type 1 antigens exposed on the surface of infected tissue culture cells. Virology. 1978 Jun 15;87(2):307–316. doi: 10.1016/0042-6822(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Norrild B., Bjerrum O. J., Vestergaard B. F. Polypeptide analysis of individual immunoprecipitates from crossed immunoelectrophoresis. Anal Biochem. 1977 Aug;81(2):432–441. doi: 10.1016/0003-2697(77)90714-x. [DOI] [PubMed] [Google Scholar]
- Norrild B., Ludwig H., Rott R. Identification of a common antigen of herpes simplex virus bovine herpes mammillitis virus, and B virus. J Virol. 1978 Jun;26(3):712–717. doi: 10.1128/jvi.26.3.712-717.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Vestergaard B. F. Immunoelectrophoretic identification and purification of herpes simplex virus antigens released from infected cells in tissue culture. Intervirology. 1979;11(2):104–110. doi: 10.1159/000149020. [DOI] [PubMed] [Google Scholar]
- Oleske J. M., Ashman R. B., Kohl S., Shore S. L., Starr S. E., Wood P., Nahmias A. J. Human polymorphonuclear leucocytes as mediators of antibody-dependent cellular cytotoxicity to herpes simplex virus-infected cells. Clin Exp Immunol. 1977 Mar;27(3):446–453. [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Spear P. G. Herpesvirus antigens on cell membranes detected by centrifugation of membrane-antibody complexes. Science. 1971 Jan 22;171(3968):298–300. doi: 10.1126/science.171.3968.298. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlehofer J. R., Habermehl K. O., Diefenthal W., Hampl H. Reduction of 51Cr-permeability of tissue culture cells by infection with herpes simplex virus type 1. Intervirology. 1979;11(3):158–166. doi: 10.1159/000149028. [DOI] [PubMed] [Google Scholar]
- Shore S. L., Black C. M., Melewicz F. M., Wood P. A., Nahmias A. J. Antibody-dependent cell-mediated cytotoxicity to target cells infected with type 1 and type 2 herpes simplex virus. J Immunol. 1976 Jan;116(1):194–201. [PubMed] [Google Scholar]
- Spear P. G., Kellejmroian B. Proteins spcified by herpes simplex virus. II. Viral glycoprotins associated with cellular membranes. J Virol. 1970 Feb;5(2):123–131. doi: 10.1128/jvi.5.2.123-131.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subak-Sharpe J. H., Brown S. M., Ritchie D. A., Timbury M. C., Macnab J. C., Marsden H. S., Hay J. Genetic and biochemical studies with herpesvirus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):717–730. doi: 10.1101/sqb.1974.039.01.085. [DOI] [PubMed] [Google Scholar]
- Subramanian T., Rawls W. E. Comparison of antibody-dependent cellular cytotoxicity and complement-dependent antibody lysis of herpes simplex virus-infected cells as methods of detecting antiviral antibodies in human sera. J Clin Microbiol. 1977 Jun;5(6):551–558. doi: 10.1128/jcm.5.6.551-558.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard B. F., Bjerrum O. J., Norrild B., Grauballe P. C. Crossed immunoelectrophoretic studies of the solubility immunogenicity of herpes simplex virus antigens. J Virol. 1977 Oct;24(1):82–90. doi: 10.1128/jvi.24.1.82-90.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard B. F. Crossed immunoelectrophoretic characterization of Herpesvirus hominis type 1 and 2 antigens. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Dec;81(6):808–810. doi: 10.1111/j.1699-0463.1973.tb02282.x. [DOI] [PubMed] [Google Scholar]
- Vestergaard B. F., Norrild B. Crossed immunoelectrophoresis of a herpes simplex virus type 1-specific antigen: immunological and biochemical characterization. J Infect Dis. 1978 Nov;138(5):639–643. doi: 10.1093/infdis/138.5.639. [DOI] [PubMed] [Google Scholar]