Abstract
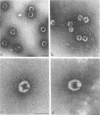
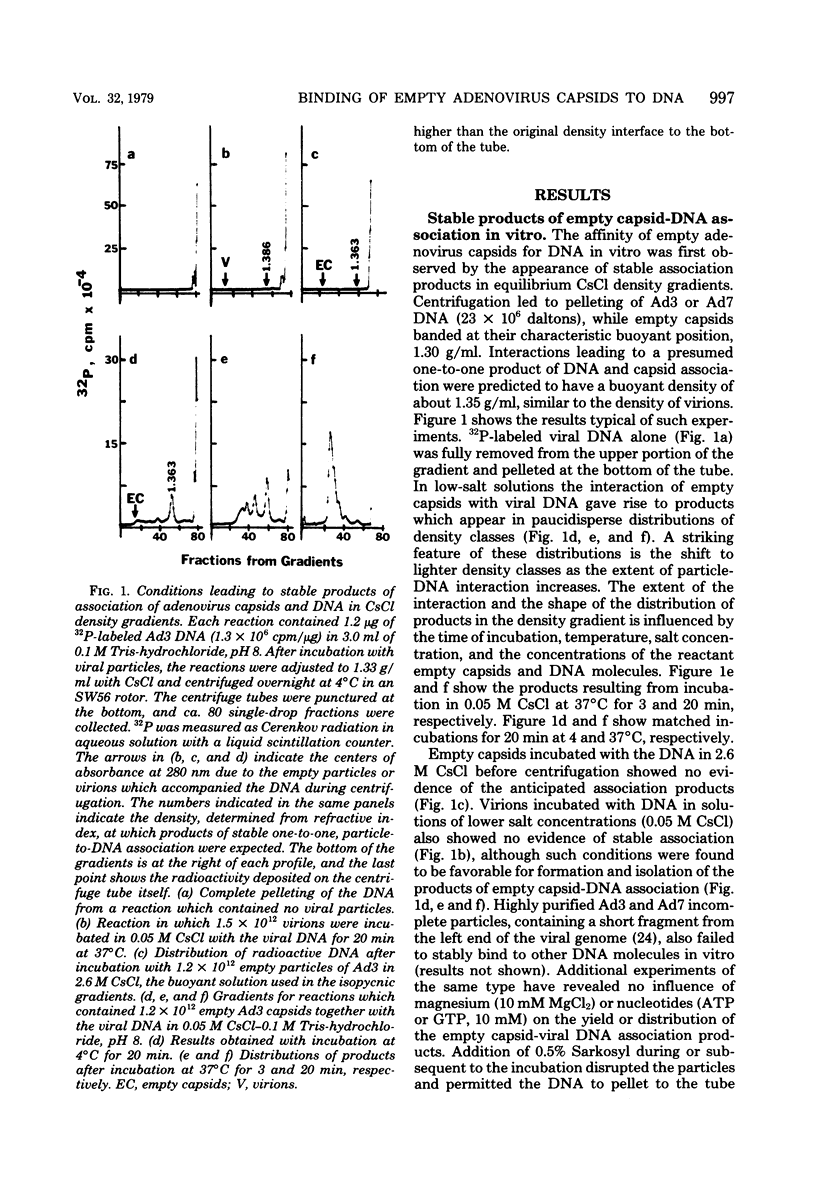
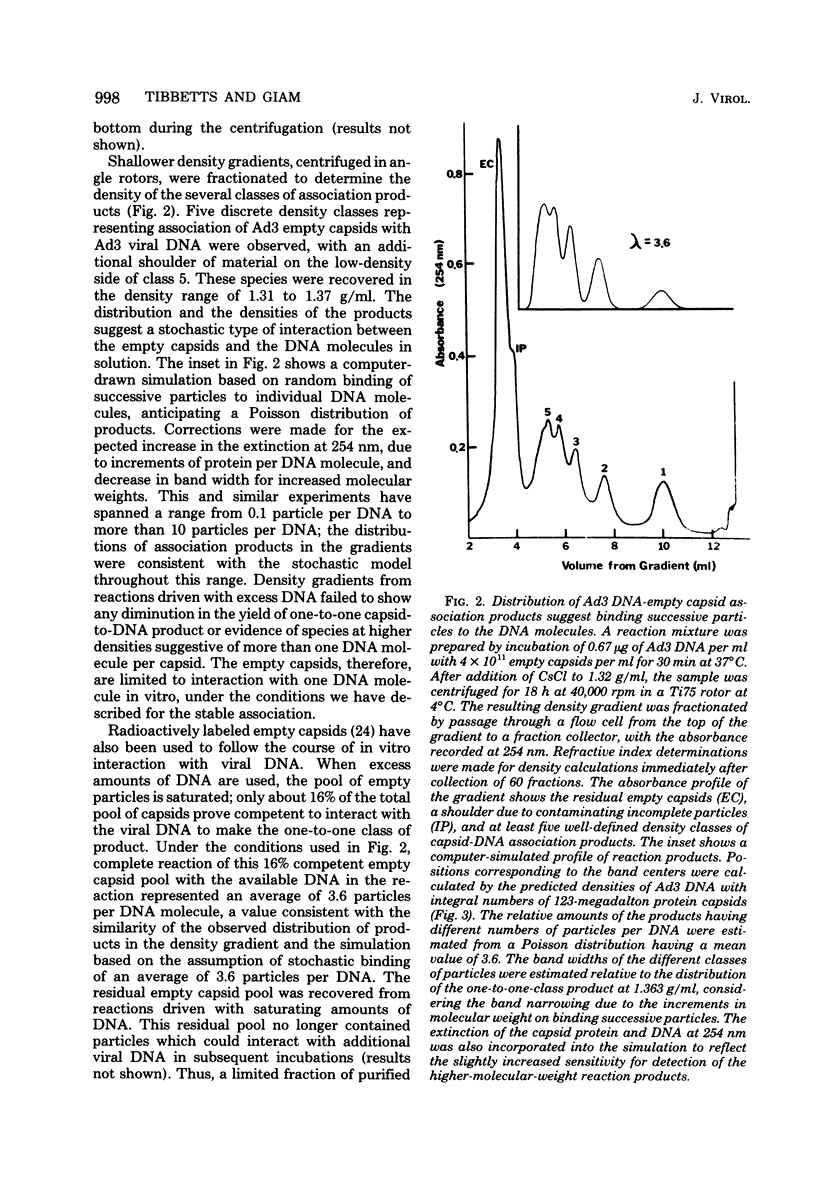
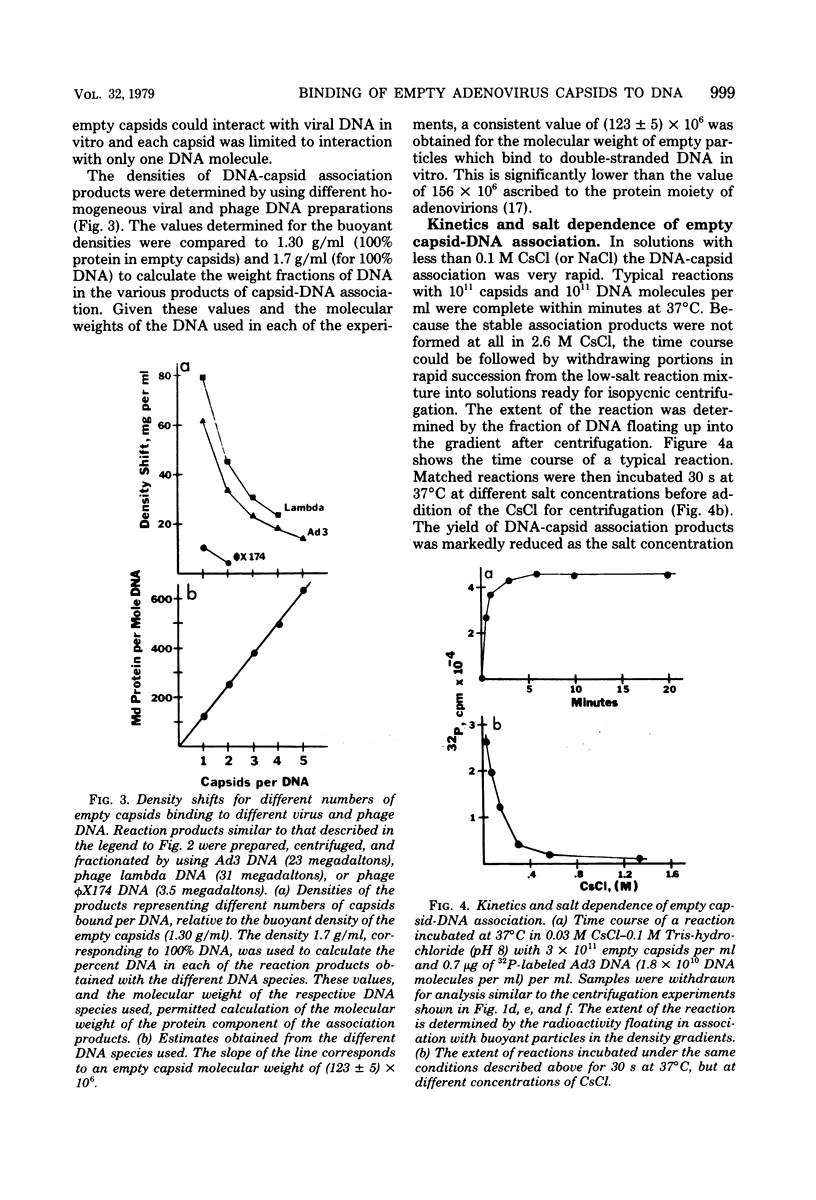
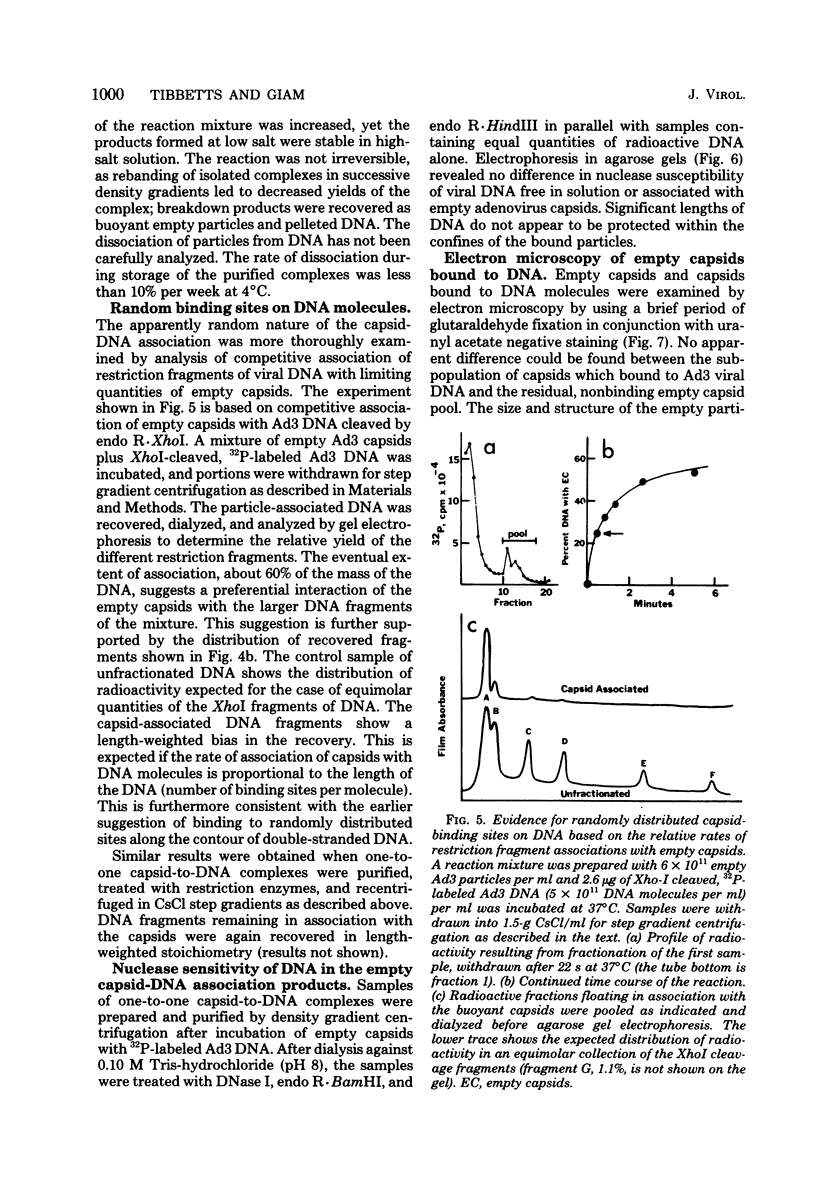
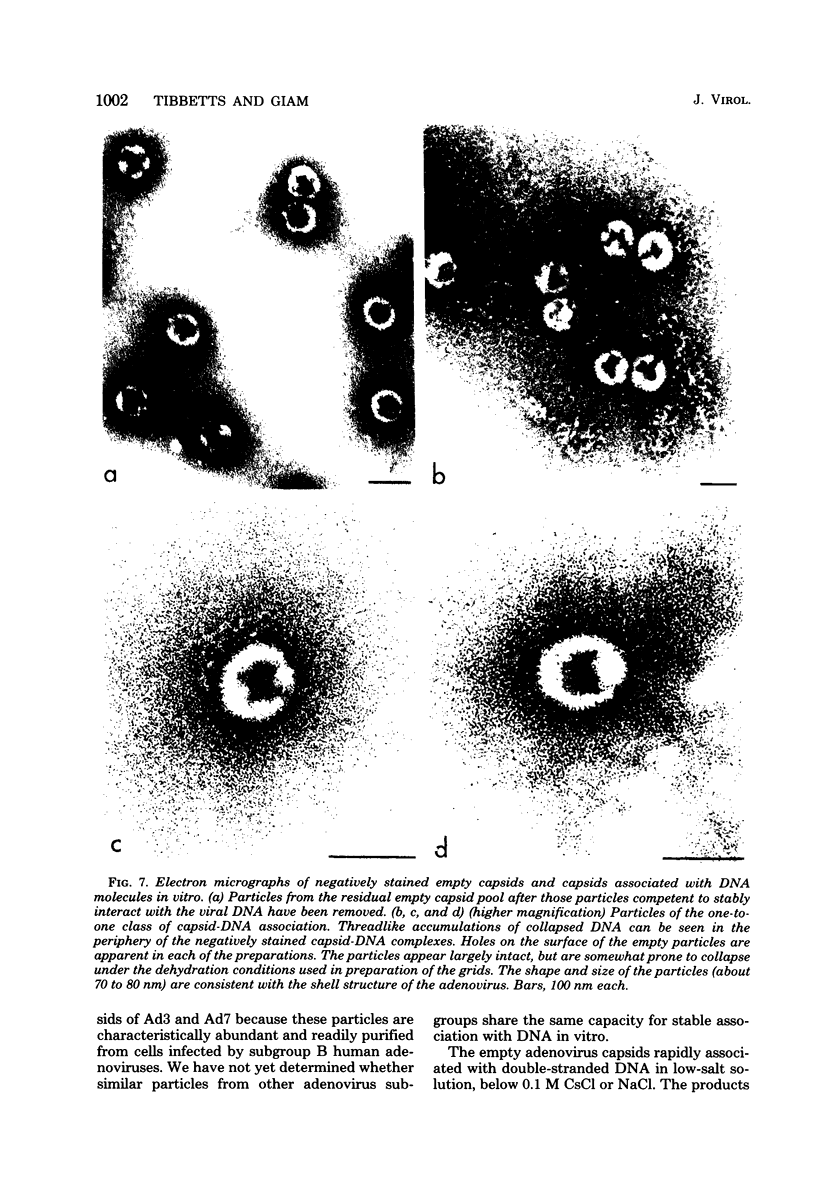
Several lines of evidence suggest that empty adenovirus capsids are preassembled intermediates in the pathway of virion assembly. We have observed that purified empty capsids of subgroup B adenoviruses have a remarkable affinity for DNA in vitro. The products of capsid-DNA association are sufficiently stable, once formed in low-salt solution, to permit purification and characterization in CsCl density gradients. Neither virions nor the DNA-containing incomplete particles of subgroup B adenoviruses can give rise to such in vitro reaction products. The average molecular weight of the empty adenovirus capsids is about 123 X 10(6), consistent with the absence of viral core peptides and a small deficiency of exterior shell polypeptides. Electron microscopy of negatively stained capsids and the capsids bound to DNA reveals a typical adenovirus size and architecture. The particles appear with a surface discontinuity that is presumed to expose the DNA binding site(s). The DNA molecules associated with the empty capsids are susceptible to the actions of DNase and restriction endonucleases. The dependence of rate of capsid-DNA association on DNA length suggests randomly distributed binding sites on the DNA molecules. Although the DNA molecules can successively acquire additional empty capsids, the empty particles themselves are restricted to interactionwith only one DNA molecule. Electron microscopy of the capsid-DNA complexes spread in cytochrome c films shows that the particles are bo-nd along the contour of extended duplex DNA. The amount of DNA within each bound particle appears to be less than 300 base pairs, as estimated by the length of the DNA molecules visible outside of the bound particle. The empty capsid-DNA association product described in this report provides an interesting substrate for further investigation of the DNA packaging process in a defined in vitro system, with extracts or purified components from infected cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aposhian H. V., Thayer R. E., Qasba P. K. Formation of nucleoprotein complexes between polyoma empty capsides and DNA. J Virol. 1975 Mar;15(3):645–653. doi: 10.1128/jvi.15.3.645-653.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black L. W., Silverman D. J. Model for DNA packaging into bacteriophage T4 heads. J Virol. 1978 Nov;28(2):643–655. doi: 10.1128/jvi.28.2.643-655.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner R., Vinograd J. The evaluation of standard sedimentation coefficients of sodium RNA and sodium DNA from sedimentation velocity data in concentrated NaCl and CsCl solutions. Biochim Biophys Acta. 1965 Sep 6;108(1):18–29. doi: 10.1016/0005-2787(65)90104-8. [DOI] [PubMed] [Google Scholar]
- Corden J., Engelking H. M., Pearson G. D. Chromatin-like organization of the adenovirus chromosome. Proc Natl Acad Sci U S A. 1976 Feb;73(2):401–404. doi: 10.1073/pnas.73.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Martin G. R., Torpier G., Boulanger P. A. Adenovirus type 2 assembly analyzed by reversible cross-linking of labile intermediates. J Virol. 1978 May;26(2):357–363. doi: 10.1128/jvi.26.2.357-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Milleville M., Boulanger P. A., Martin G. R. Temperature-sensitive mutant of adenovirus type 2 blocked in virion assembly: accumulation of light intermediate particles. J Virol. 1978 May;26(2):344–356. doi: 10.1128/jvi.26.2.344-356.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Harrison S. C. DNA arrangement in isometric phage heads. Nature. 1977 Aug 18;268(5621):598–602. doi: 10.1038/268598a0. [DOI] [PubMed] [Google Scholar]
- Edvardsson B., Everitt E., Jörnvall H., Prage L., Philipson L. Intermediates in adenovirus assembly. J Virol. 1976 Aug;19(2):533–547. doi: 10.1128/jvi.19.2.533-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsson B., Ustacelebi S., Williams J., Philipson L. Assembly intermediates among adenovirus type 5 temperature-sensitive mutants. J Virol. 1978 Feb;25(2):641–651. doi: 10.1128/jvi.25.2.641-651.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Pettersson U., Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973 Mar;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- Fey G., Hirt B. Fingerprints of polyoma virus proteins and mouse histones. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):235–241. doi: 10.1101/sqb.1974.039.01.030. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M., Maizel J. V., Jr The polypeptides of adenovirus. V. Young virions, structural intermediate between top components and aged virions. Virology. 1974 Feb;57(2):409–424. doi: 10.1016/0042-6822(74)90181-0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Ginsberg H. S. Role of adenovirus structural proteins in the cessation of host-cell biosynthetic functions. J Virol. 1968 May;2(5):430–439. doi: 10.1128/jvi.2.5.430-439.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murialdo H., Becker A. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiol Rev. 1978 Sep;42(3):529–576. doi: 10.1128/mr.42.3.529-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U. Molecular biology of adenoviruses. Virol Monogr. 1975;14:1–115. doi: 10.1007/978-3-7091-8391-5_1. [DOI] [PubMed] [Google Scholar]
- Richards K. E., Williams R. C., Calendar R. Mode of DNA packing within bacteriophage heads. J Mol Biol. 1973 Aug 5;78(2):255–259. doi: 10.1016/0022-2836(73)90114-9. [DOI] [PubMed] [Google Scholar]
- Riemer S. C., Bloomfield V. A. Packaging of DNA in bacteriophage heads: some considerations on energetics. Biopolymers. 1978 Mar;17(3):785–794. doi: 10.1002/bip.1978.360170317. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Maat J., van Ormondt H., Sussenbach J. S. The nucleotide sequence at the termini of adenovirus type 5 DNA. Nucleic Acids Res. 1977 Dec;4(12):4371–4389. doi: 10.1093/nar/4.12.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist B., Everitt E., Philipson L., Hoglund S. Assembly of adenoviruses. J Virol. 1973 Mar;11(3):449–459. doi: 10.1128/jvi.11.3.449-459.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung M. T., Lischwe M. A., Richards J. C., Hosokawa K. Adenovirus chromatin I. Isolation and characterization of the major core protein VII and precursor Pro-VII. J Biol Chem. 1977 Jul 25;252(14):4981–4987. [PubMed] [Google Scholar]
- Tibbetts C., Johansson K., Philipson L. Hydroxyapatite chromatography and formamide denaturation of adenovirus DNA. J Virol. 1973 Aug;12(2):218–225. doi: 10.1128/jvi.12.2.218-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C., Pettersson U. Complementary strand-specific sequences from unique fragments of adenovirus type 2 DNA for hybridization-mapping experiments. J Mol Biol. 1974 Oct 5;88(4):767–784. doi: 10.1016/0022-2836(74)90398-2. [DOI] [PubMed] [Google Scholar]
- Tibbetts C. Physical organization of subgroup B human adenovirus genomes. J Virol. 1977 Nov;24(2):564–579. doi: 10.1128/jvi.24.2.564-579.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C. Viral DNA sequences from incomplete particles of human adenovirus type 7. Cell. 1977 Sep;12(1):243–249. doi: 10.1016/0092-8674(77)90202-1. [DOI] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters W. D., Russell W. C. Studies on the assembly of adenovirus in vitro. J Gen Virol. 1971 Feb;10(2):181–194. doi: 10.1099/0022-1317-10-2-181. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Adenovirus-2 DNA contains an inverted terminal repetition. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3054–3057. doi: 10.1073/pnas.69.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]