Abstract
Background
I-SPY2, a standing, multicenter, adaptive phase 2 neoadjuvant trial ongoing in high-risk clinical stage II/III breast cancer, is designed to evaluate multiple, novel experimental agents added to standard chemotherapy for their ability to improve the rate of pathologic complete response (pCR). Experimental therapies are compared against a common control arm. We report efficacy for the tyrosine kinase inhibitor neratinib.
Methods
Eligible women had ≥2.5 cm stage II/III breast cancer, categorized into 8 biomarker subtypes based on HER2, hormone-receptor status (HR), and MammaPrint. Neratinib was evaluated for 10 signatures (prospectively defined subtype combinations), with primary endpoint pCR. MR volume changes inform likelihood of pCR for each patient prior to surgery. Adaptive assignment to experimental arms within disease subtype was based on current Bayesian probabilities of superiority over control. Accrual to experimental arm stop at any time for futility or graduation within a particular signature based on Bayesian predictive probability of success in a confirmatory trial. The maximum sample size in any experimental arm is 120 patients,
Results
With 115 patients and 78 concurrently randomized controls, neratinib graduated in the HER2+/HR− signature, with mean pCR rate 56% (95% PI: 37 to 73%) vs 33% for controls (11 to 54%). Final predictive probability of success, updated when all pathology data were available, was 79%.
Conclusion
Adaptive, multi-armed trials can efficiently identify responding tumor subtypes. Neratinib added to standard therapy is highly likely to improve pCR rates in HER2+/HR2212; breast cancer. Confirmation in I-SPY 3, a phase 3 neoadjuvant registration trial, is planned.
INTRODUCTION
The treatment of aggressive, locally advanced breast cancers increasingly includes neoadjuvant therapy prior to surgical resection, providing a window of opportunity to learn to better tailor treatments based upon early assessments of the molecular characteristics of the cancer. The existence of a well characterized, surrogate endpoint – pathologic complete response (pCR) assessed at the time of surgery – that is strongly correlated with both event-free and overall survival, makes neoadjuvant therapy an ideal setting for rapid clinical development of targeted therapies. The I-SPY 2 trial provides a standing, or ‘platform’ trial framework to capitalize on this unique opportunity, by employing adaptive randomization for efficient, focused clinical development of paired therapies and biomarkers. The overall trial objective is to reduce the cost, time, and number of patients needed to identify effective drugs to treat aggressive, locally advanced breast cancer.1,2
In I-SPY 2, patients are randomized to one of several experimental arms, each evaluating a novel agent in combination with standard neoadjuvant chemotherapy, compared to a common standard of care control. The adaptive randomization algorithm utilizes both the molecular characteristics of the cancers and incorporates accumulated outcome data to more efficiently identify tumor subtype signatures – combinations of molecular subtypes - in which specific agents are most effective. Agents reaching predefined thresholds of efficacy in one or more specific signatures are said to ‘graduate’.
Here we report the efficacy and safety results from the experimental arm of I-SPY 2 evaluating neratinib, an irreversible pan-ErbB/HER tyrosine kinase inhibitor. I-SPY 2 investigators have also described results of the graduated veliparib/carboplatin arm and MK-2206.3,4 Evaluations of other experimental arms have been completed or are ongoing in I-SPY 2.
Neratinib (HKI-272) is a potent, irreversible small molecule inhibitor of the ErbB/HER kinase family (EGFR/HER2/HER4) that has shown promising activity against HER2+ metastatic breast cancer.5,6 There is also evidence of preclinical activity against HER2-negative tumor cells7,8, suggesting the possibility that pan-ErbB/HER kinase activity against EGFR and possibly HER4 might have activity beyond HER2+ tumors.9 The adaptive randomization approach used in I-SPY2 offers the ability to test this possibility while minimizing exposure of patients with HER2-tumors to treatments that are ineffective. Because neratinib was introduced prior to dual targeting of HER2 becoming standard of care in neoadjuvant treatment, it was tested against, rather than being combined with, trastuzumab. Although HER2-positive patients randomized to the experimental arm did not receive trastuzumab in the neoadjuvant setting, these patients received a full year of adjuvant trastuzumab as dictated by standard of care. Since graduating from I-SPY 2, neratinib has shown benefit as an extended or secondary adjuvant therapy for early stage high risk HER2+ breast cancer, following standard trastuzumab-based adjuvant therapy.10
METHODS
Study Design
I-SPY 2 is an ongoing, multicenter, open-label, adaptive phase 2 ‘platform’ trial with multiple experimental arms that evaluate novel agents combined with standard neoadjuvant therapy in breast cancers at high risk of recurrence.11 Experimental treatments are compared against a common control arm, with the primary endpoint being pathologic complete response (pCR), which is defined as no residual cancer in either the breast or lymph nodes at time of surgery.12
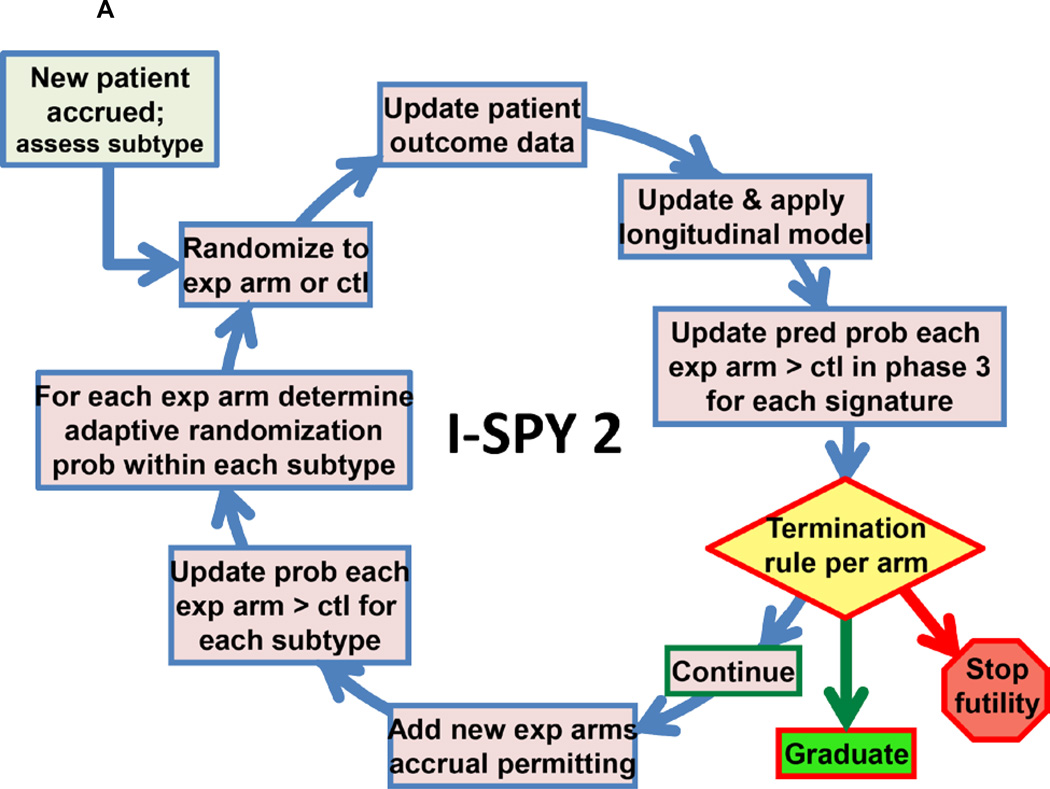
Biomarker assessments (HER2, HR, MammaPrint) performed at baseline are used to classify patients into 2×2×2 = 8 prospectively defined subtypes for randomization purposes. HER2 was assessed by standard IHC and FISH assays, and a microarray-based assay of HER2 expression (TargetPrintTM), previously shown to have high concordance with standard IHC and FISH assay.13 The adaptive randomization algorithm assigns patients with biomarker subtypes to competing drugs/arms based on current Bayesian probabilities of achieving pCR within that subtype vs control with 20% of patients assigned to control. Adaptive randomization speeds the identification of treatments that perform better within specific patient subtypes and helps avoid exposing patients to therapies that are unlikely to benefit them (Figure 1A).1,2
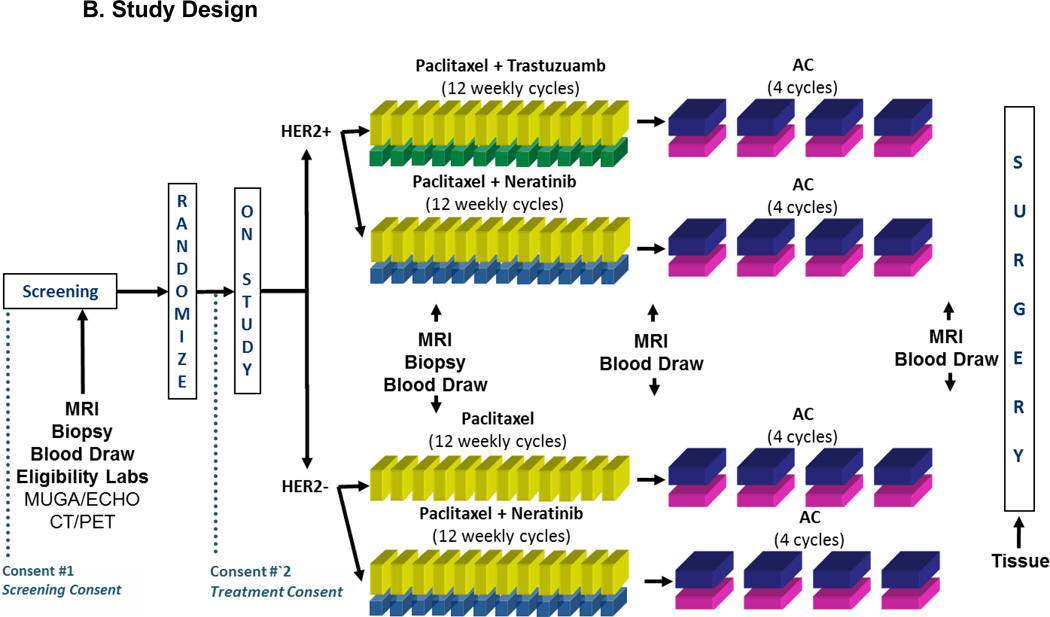
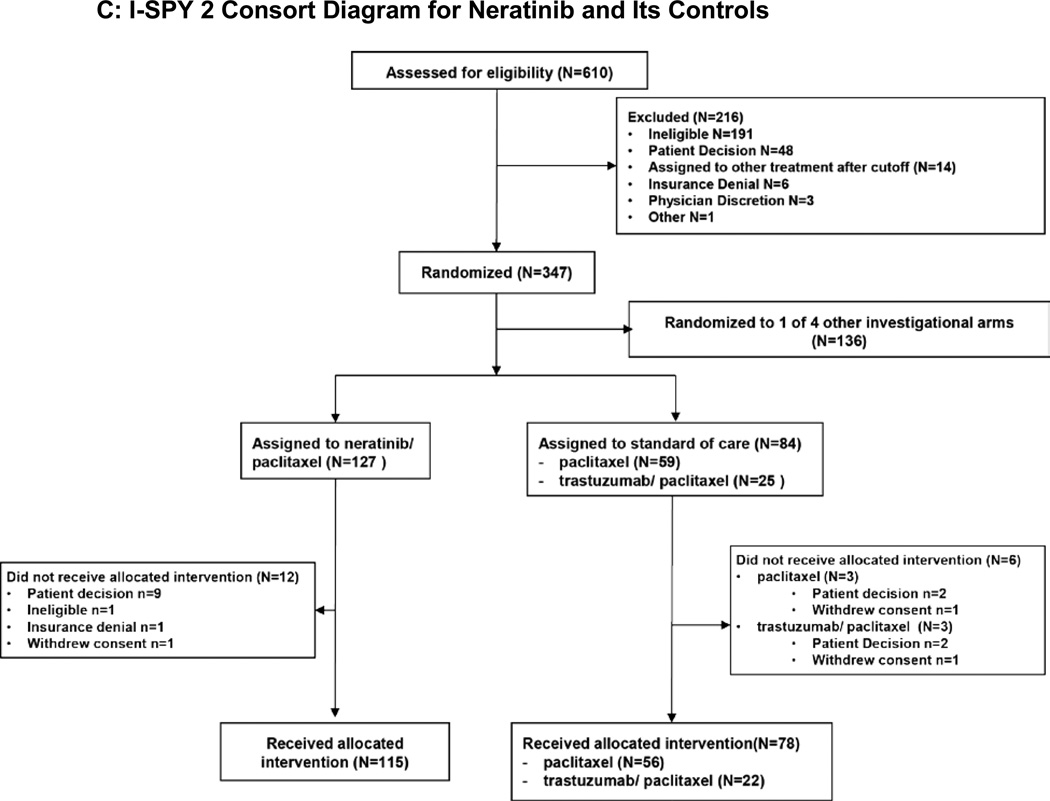
Figure 1.
A. I-SPY 2 Adaptive Design.
B. Study Design.
Study schema for the neratinib experimental arm and control arm in I-SPY2. Following screening, HER2+ patients were randomized to receive either neratinib plus paclitaxel vs. trastuzumab plus paclitaxel. HER2− patients were randomized to receive either neratinib plus paclitaxel vs. paclitaxel alone. HER2+ and HER2− patients then received standard AC treatment to complete their neoadjuvant therapy.
C. I-SPY 2 Consort Diagram for Neratinib and Its Controls.
Did/did not received allotted intervention: Patients were categorized as receiving no experimental therapy or at least 1 dose of experimental therapy.
To assess efficacy, ten clinically relevant biomarker ‘signatures’ were defined in the protocol: All; HR+; HR−; HER2+; HER2−; MP Hi-2; HER2+/HR+; HER2+/HR−; HER2−/HR+; HER2−/HR−. Experimental arms are continually evaluated against control for each of these signatures and “graduate” when and if they demonstrate statistical superiority in pCR rate. Statistical analyses are Bayesian.14
Graduation requires an 85% Bayesian predictive probability of success in a 300-patient equally randomized neoadjuvant phase 3 trial with a traditional statistical design comparing to the same control arm as in I-SPY 2 and primary endpoint pCR (see Supplement).14,1 Predictive probabilities of success are power calculations for a 300-patient trial averaged with respect to the current probability distributions of pCR rates for the experimental arm and control.1,14 The modest proposed size means that graduation occurs only when there is compelling evidence of an arm’s efficacy. Accrual to a graduating arm halts immediately, but all patients on the arm and its concurrent controls must complete surgery before graduation is announced. An experimental arm is dropped for futility if its predictive probability of success in a phase 3 trial <10% for all ten signatures. The maximum total number of patients assigned to any experimental arm is 120.
All participating sites received institutional review board approval. A data safety monitoring board meets monthly.
I-SPY 2 Eligibility and enrollment
I-SPY2 is open to women aged 18 and over, diagnosed with clinical stage II–III disease. Patients must have clinically or radiologically measureable disease in the breast, defined as longest diameter >2.5 cm. If a tumor meets this criteria by clinical exam only, the tumor must be >2 cm by imaging. Participants must have no prior cytotoxic treatment for this malignancy, ECOG performance status of 0–1, and be willing to consent to core biopsy and MRI. Patients with HR+/MP-low tumors are excluded because the potential benefit of chemotherapy is lower in patients with lower proliferative tumors and does not justify the risk of exposure to an investigational agents plus chemotherapy. HER2+ and HR− patients are eligible regardless of MP status.15
All patients provide written, informed consent in order to initiate I-SPY2 screening. If eligible, a second consent is obtained after random assignment and prior to treatment.
Treatment
All participants received standard neoadjuvant therapy consisting of 12 weekly cycles of 80mg IV paclitaxel (T), followed by 4 cycles of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 (AC) IV every 2 to 3 weeks. This report compares patients who were randomized to also receive neratinib (240mg/day) for the first 12 weeks with control patients. Control patients who were HER2+ also received trastuzumab for the first 12 weeks (loading dose 4mg/kg first cycle, followed by maintenance dose of 2mg/kg cycles 2–12) (figure 1B). Subsequent surgery, including sentinel node dissection for node-negative and axillary node dissection for node-positive patients (at diagnosis), was performed according to NCCN and local practice guidelines. Radiation and endocrine adjuvant therapy was recommended following surgery using standard guidelines.
A protocol modification approved in January 2012 added a prophylactic course of loperamide to control diarrhea in patients receiving neratinib, beginning with 4mg on day 1 of neratinib, 2 mg 8 hours later, and then 2 mg twice daily for two weeks. Patients were instructed to take an additional 2 mg immediately after the first unformed stool, then 2 mg every 4 hours until the absence of diarrhea for 12 consecutive hours (maximum 16 × 2 mg pills per day). The frequency of loperamide administration was decreased at patient discretion once the diarrhea was controlled.
Assessments
MRI and core biopsy are performed in all consented participants during screening and repeated 3 weeks after treatment began. MRI imaging is repeated between chemotherapy regimens, and prior to surgery. All surgical specimens are evaluated by pathologists trained to assess residual tumor burden (RCB). Biomarker assessments include the Agendia 70 gene MammaPrint and TargetPrint HER2 gene expression using the Agendia 44K full genome microarray and reverse phase phosphoprotein array. Patients are stratified into MammaPrint High1 (MP1) and MammaPrint High2 (MP2), determined by the predefined median cut-point (−0.154) of I-SPY 1 participants who fit the eligibility criteria for I-SPY 2 (Supplemental figure).16
Statistical Considerations
We report the final Bayesian probability distributions of pCR rates for the neratinib arm and its concurrently randomized controls for each of the 10 signatures by providing the estimated pCR rates (means of the final respective distributions) and 95% probability intervals. These distributions are based on the final observed results within the 8 biomarker subtypes using a covariate-adjusted logistic model where the covariates are HER2, HR, and MP. We do not provide the raw data within the individual biomarker subtypes because our analysis enables greater precision than would any raw-data estimates of pCR rate, whether within subtypes or across subtypes in signatures. Using the final distributions of pCR rates for each of the 10 signatures we give the probabilities that neratinib’s pCR rate is greater than the control rate as well as the respective predictive probabilities of future-trial success.
RESULTS
Patient Population
During the period of March 2010 to January 2013, 127 participants were enrolled and randomized to receive neratinib, of which 12 dropped out prior to receiving treatment, yielding 115 evaluable patients. Of 84 patients concurrently randomized to the control arm, 78 were evaluable for pCR (Figure 1C).
Baseline patient characteristics (Table 1) show that the experimental and control arms were well balanced in their demographics, HR status and clinical presentation. Adaptive randomization resulted in an enriched population of HER2+ participants in the experimental vs. control arm (57% vs. 28%).
Table 1.
Baseline Characteristics
| Neratinib +/− Paclitaxel n=115 |
Paclitaxel +/− trastuzumab n=78 |
|
|---|---|---|
| Median Age range | 51 (24 – 70) | 48 (24–71) |
| Ethnicity, n (%) | ||
| White | 92 (80%) | 62 (79.5%) |
| Asian | 16 (14%) | 11 (14.1%) |
| African/American | 7 (6%) | 5 (6.4%) |
| Menopausal status (%) | ||
| Premenopausal | 56 (49%) | 40 (51%) |
| Perimenopausal- | 4 (3%) | 6 (8%) |
| Postmenopausal | 44 (38%) | 22 (28%) |
| Not Applicable | 11 (10%) | 10 (13%) |
| HR Status (%) | ||
| Positive | 60 (52%) | 43 (55%) |
| Negative | 55 (48%) | 35 (45%) |
| HER2 Status (%) | ||
| Positive (IHC and/or FISH) | 65 (57%) | 22 (28%) |
| Negative | 50 (43%) | 56 (72%) |
| Clinical presentation | ||
| MRI tumor diameter (median, cm) |
3.7 (1.5 – 11.8) | 3.95 (1.2 – 13) |
| Axillary node palpable (%) | 54 (47%) | 36 (46%) |
| Axillary node non-palpable (%) |
61 (53%) | 42 (54%) |
Efficacy
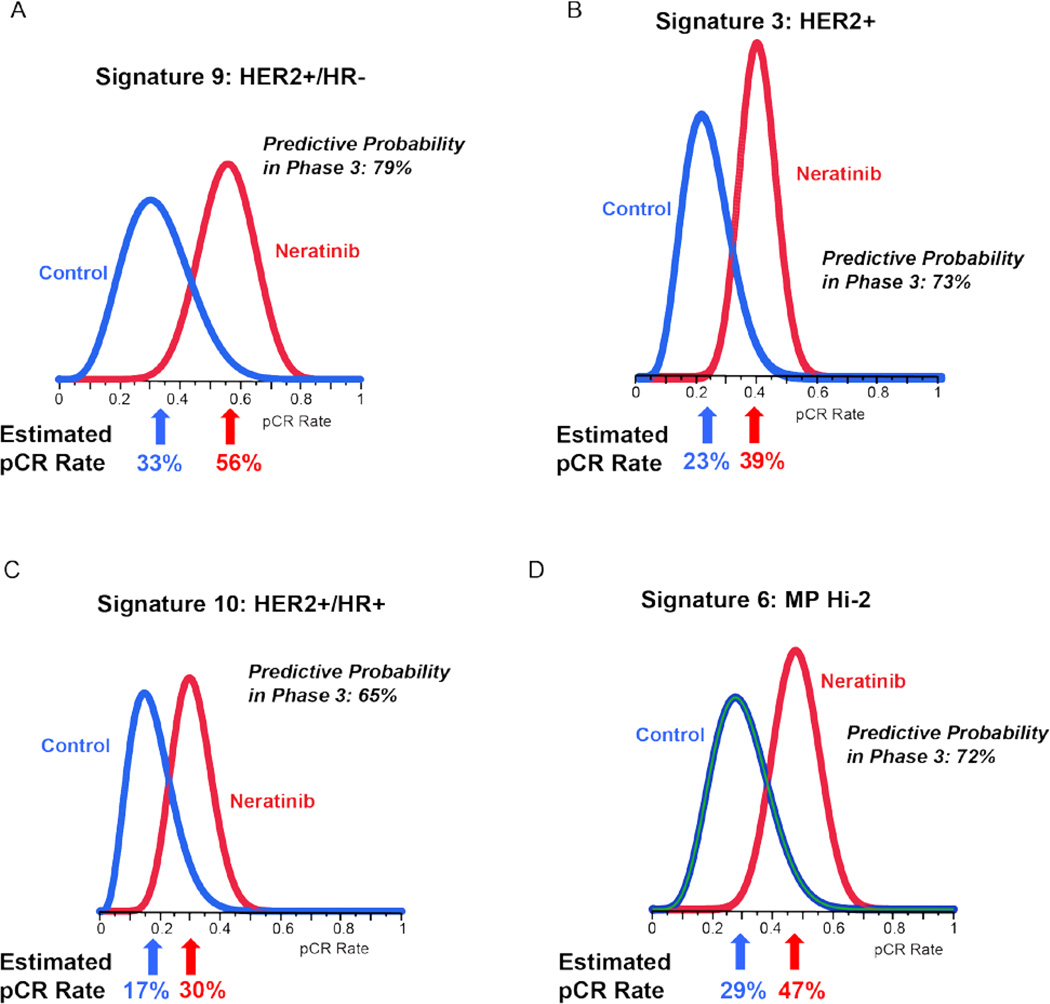
Figure 2 shows the Bayesian posterior probability distributions for 4 of the 10 signatures. Neratinib graduated in the HER2+/HR− signature (Table 2). As shown in Figure 2A, the estimated pCR rate of HER2+/HR− patients in the neratinib arm was 56% (95% PI: 37 to 73%), compared to 33% (95% PI: 11 to 54%) in the (trastuzumab) control arm. The resulting probability that the neratinib arm was superior to the control arm was 95% and the probability of success for neratinib in a phase 3 clinical trial of 300 patients was 79%, as shown in Table 2.
Figure 2. Probability distributions for select signatures.
Histograms showing posterior (final) probability distributions for neratinib and control pCR rates for 4 of the 10 signatures listed in Table 2. Panel 2A is for HER2+/HR−, the graduating signature for neratinib. Estimated pCR Rate is the mean of the respective distribution. Predictive Probability in Phase 3 is a calculation based on the respective pair of histograms and is explained in the text.
Table 2.
Final posterior and predictive probabilities in 10 signatures
|
Signature |
Estimated pCR Rate (95% Probability Interval) |
Probability Neratinib is Superior to Control |
Predictive Probability of Success in Phase 3 |
|
|---|---|---|---|---|
| Neratinib | Control | |||
| ALL | 33% (24%–40%) | 23% (14% – 33%) | 93% | 48% |
| HR+ | 23% (13% – 33%) | 16% (6% – 28%) | 81% | 40% |
| HR− | 44% (30% – 55%) | 31% (17% – 45%) | 92% | 58% |
| HER2+ | 39% (28% – 51%) | 23% (8% – 38%) | 95% | 73% |
| HER2− | 28% (15% – 37%) | 24% (13% – 35%) | 69% | 25% |
| MP2 | 48% (30% – 60%) | 29% (11% – 48%) | 93% | 72% |
| HER2+/HR+ | 30% (18% – 44%) | 17% (3%–32%) | 91% | 65% |
| HER2+/HR− | 56% (37% – 73%) | 33% (11% – 54%) | 95% | 79% |
| HER2−/HR+ | 14% (3% – 25%) | 16% (5% – 27%) | 42% | 14% |
| HER2−/HR− | 38% (22% – 50%) | 31% (15% – 46%) | 77% | 40% |
Although neratinib graduated only in HER2+/HR−, as shown in Table 2, there was additional evidence of superior activity over control in several other signatures. In the HER2+/HR+ participants, estimated pCR rates were 30% vs. 17%, respectively (Figure 2C), with 91% probability of neratinib superiority over control and 65% predicted probability of phase 3 success. Similarly, over all HER2+ patients (regardless of HR status), the neratinib pCR rate outperformed the trastuzumab control by 39% versus 23% (Figure 2B), with 95% probability of superiority for the neratinib arm, and 73% predicted probability of success in a neoadjuvant phase 3 trial.
Patients identified as having the highest scores by the MammaPrint assay (MP2) also appeared to gain some benefit from neratinib over the trastuzumab control, with comparative pCR rates of 48% vs. 29% (Figure 2D), 93% probability of superiority over control treatment, and 72% predicted probability of success in a phase 3 trial. There was very little activity in the HR+HER2− and HR−HER2− patients, especially MP1 (Table 2) and the algorithm stopped assigning neratinib in these subtypes during the course of the trial (Supplemental Table 2).
Safety and Tolerability
The combination of neratinib and paclitaxel in the neoadjuvant setting exhibited safety and toxicity comparable to previous studies in advanced breast cancer.6 Diarrhea was the most common adverse event, and Grade 3–4 diarrhea was noted in 38% of patients in the neratinib arm. Diarrhea was mitigated by dose reductions and/or supportive measures, with further improvements noted after the protocol modification to include prophylactic loperamide (Supplemental Table 3). Several hematologic and gastrointestinal adverse events (summarized in Table 3) were significantly higher in the experimental arm, including Grade 1–2 vomiting (p=0.045), Grade 1–2 and Grade 3–4 diarrhea (p < 0.0001), Grade 1–2 aspartate aminotransferase (AST) increase (p=0.0005) and Grade 1–2 and Grade 3–4 alanine aminotransferase (ALT) increase (p=0.0001 and 0.009 respectively).
Table 3.
Adverse Events and Early Discontinuations
| Neratinib (n=115) | Control (n=78) | |||
|---|---|---|---|---|
| Adverse Events | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 |
| Hematologic, n (%) | ||||
| Febrile neutropenia | 0 (0%) | 7 (6.1%) | 0 (0%) | 5 (6.4%) |
| Neutropenia | 16 (15.7%) | 18 (13.9%) | 8 (10.3%) | 9 (11.5%) |
| Thrombocytopenia | 6 (5.2%) | 1 (0.9%) | 0 (0%) | 2 (2.6%) |
| Anemia | 34 (29.6%) | 3 (2.6%) | 16 (20.5%) | 0 (0%) |
| Gastrointestinal, n (%) | ||||
| Diarrhea | 110 (95.6%) | 44 (38.3%) | 39 (50%) | 3 (3.8%) |
| Nausea | 94 (81.7%) | 3 (2.6%) | 65 (83.3%) | 0 (0%) |
| Vomiting | 46 (40%) | 2 (1.7%) | 20 (25.6%) | 0 (0%) |
| Stomatitis* | 52 (45.2%) | 2 (1.7%) | 31 (39.7%) | 2 (2.6%) |
| Aspartate aminotransferase increased |
30 (26.1%) | 5 (4.3%) | 5 (6.4%) | 1 (1.3%) |
| Alanine aminotransferase increased | 42 (36.5%) | 13 (11.3%) | 9 (11.5%) | 1 (1.3%) |
| Dose Modifications | ||||
| Early discontinuation, n (%)** | ||||
| All | 21 (18.3%) | 3 (3.8%) | ||
| Toxicity | 13 (11.3%) | 1 (1.3%) | ||
| Progression | 6 (5.2%) | 0 (0%) | ||
| Other | 2 (1.7%) | 2 (2.6%) | ||
Stomatitis includes CTCAE terms oral pain, oral hemorrhage, and mucositis oral.
Dose modification is for the taxane phase only and includes patients who went to AC early. This does not include patients who discontinued during AC.
Three serious adverse events - pneumonitis (n=1) and dehydration (n=2) - were reported as probably or definitely attributable to protocol-directed therapy. No patient developed symptomatic congestive heart failure on study. One patient experienced a grade 3–4 decline in the left ventricular ejection fraction.
Dose reductions or holds in the experimental arm occurred in 63.5% of patients for neratinib and 39.1% for paclitaxel. In the control arm, dose reduction or holds for paclitaxel occurred in 11.5% of patients. 11.3% of patients in the experimental group had early discontinuations for toxicity compared to 1.3% of patients in the control arm (see Table 3).
DISCUSSION
In this report, we describe the efficacy, leading to graduation to phase 3, of an experimental arm of I-SPY 2 consisting of paclitaxel plus neratinib followed by adriamycin/cytoxan (TN->AC) for high risk breast cancer characterized by a HER2+/HR− biomarker signature. Within this molecular subtype, neratinib emerged as superior to the current standard of care, trastuzumab, with a high degree of confidence (95% probability), as measured by the mean pCR rate of 55% vs. 33%. In terms of the primary goal of I-SPY 2, which is to facilitate the rapid identification of pairs of agents/biomarker profiles likely to succeed in subsequent phase 3 studies, the neratinib regimen is estimated to have 79% probability of statistical success in a focused neoadjuvant phase 3 study, a result achieved through an experimental arm consisting of a modest 116 participants.
The graduation threshold of 85% defined in the study protocol is reached prior to all patients completing neoadjuvant therapy and reaching primary endpoint assessment (i.e. completed surgery). Once all additional data points (pCR) were accumulated, the probabilities were updated and there was a slight reduction of this probability to 79%. This possibility was anticipated in the I-SPY 2 design and led to our setting a high threshold of 85%.
The finding of the superiority of neratinib over trastuzumab in this subtype is notable given the experience of a number of recent trials seeking to improve upon the efficacy of the current standard of care. Among these are several phase 3 studies of lapatinib, a HER/ErbB tyrosine kinase inhibitor similar to neratinib, which has failed to show superiority over trastuzumab in trials when used in metastatic,17 adjuvant,18 or neoadjuvant settings.19 In the last of these some improvement was noted using a triple combination of lapatinib-trastuzumab-paclitaxel. In the current study, we observed a clear improvement in pCR with neratinib plus paclitaxel compared to trastuzumab plus paclitaxel.
A recent meta-analysis of neoadjuvant trials in HER2+ breast cancer reported an overall pCR rate of 39% for single HER2-targeted agents with anthracycline-taxane based chemotherapy.20 The pCR rate for the HER2+ patients in the trastuzumab plus paclitaxel control arm was 23% overall (Table 2), 33% for HER2+/HR− and 17% for HER2+/HR+. These rates are lower than for trastuzumab-based therapy in previous neoadjuvant trials of HER2+ breast cancer.21 Our study population showed no obvious differences in patient characteristics as compared with other neoadjuvant trials. I-SPY 2 methodology, such as standardized stringent post neoadjuvant tissue analysis,22 may contribute to lower pCR rates.
Toxicity of the neratinib arm was manageable and acceptable. As expected,6,23–25 diarrhea was its most problematic adverse effect, warranting aggressive supportive care. In this regard, an intensive mandatory diarrhea prophylaxis regimen utilizing high-dose loperamide at study initiation and subsequent tapering was evaluated in the NSABP FB-7 Phase 1 trial, which reported frequent diarrhea, limited to Grade 2.25 Prophylactic high-dose loperamide with neratinib is being further evaluated in an ongoing adjuvant trial, NCT02400476.26
Based on I-SPY 2 results and other clinical data, phase 3 testing of neoadjuvant neratinib is moving forward in the successor I-SPY 3 program, aimed at generating accelerated approval following FDA guidance27,28. Although I-SPY 2 results predict a 79% probability of phase 3 success in neoadjuvant treatment for HER2+ HR− patients, a modified design is required to reflect the current standard of dual HER-targeting (pertuzumab-trastuzumab containing regimens) which has already received accelerated approval.29,30 The phase 3 design will test neratinib-pertuzumab-trastuzumab-taxane vs. pertuzumab-trastuzumab-taxane vs. neratinib-trastuzumab-taxane, all followed by AC. The experimental arm will include the graduating HER2+/HR− signature, as well as all other HER2+ patients in order to further refine the HER2+ subtypes that would benefit from combination therapy with neratinib.
Recent debates about neoadjuvant endpoints31 highlight the importance of well-designed prospective trials. The I-SPY 2/3 mechanism has the potential to accelerate drug development via neoadjuvant endpoints and advance a more personalized, biomarker-based approach to the treatment of high-risk breast cancer.
Supplementary Material
Acknowledgments
Funding
We acknowledge the initial support for the I-SPY 2 TRIAL by the following: The Safeway Foundation, Bill Bowes Foundation, Quintiles Transnational Corporation, Johnson & Johnson, Genentech, Amgen, Inc., The San Francisco Foundation, Give Breast Cancer the Boot, Eli Lilly and Company, Pfizer, Inc., Eisai Company Ltd., Side Out Foundation, Harlan Family, The Avon Foundation for Women, Alexandria Real Estate Equities, Inc., and private individuals and family foundations.
Study Sponsor: Quantum Leap Healthcare Collaborative (2013–present); Foundation of the National Institutes of Health (2010–2012).
Study Oversight
The study was designed by the principal investigators and the I-SPY2 investigators. The drug manufacturer supplied the agent that was administered in an outpatient setting. All participating sites received institutional review approval. A data safety monitoring board meets monthly. The FNIH and Quantum Leap are the study sponsors of the I-SPY2 TRIAL which operates as a pre competitive consortia model.
Footnotes
The first author wrote the first draft of the manuscript, edited by the writing team (LE, DB,CY, ML,DY, MP, MB). All authors made the decision to submit the article for publication and vouch for the accuracy and completeness of the reported data. The authors also certify that the study as reported conforms with the protocol (see protocol details at NEJM.org).
The results were presented at the AACR meeting in 2013 by Dr. John Park.
References
- 1.Berry DA. Adaptive clinical trials in oncology. Nat Rev Clin Oncol. 2011;9(4):199–207. doi: 10.1038/nrclinonc.2011.165. [DOI] [PubMed] [Google Scholar]
- 2.Berry DA. The Brave New World of clinical cancer research: Adaptive biomarker-driven trials integrating clinical practice with clinical research. Mol Oncol. 2015;9(5):951–959. doi: 10.1016/j.molonc.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rugo H, Olopade O, DeMichele A, et al. Abstract S5-02: Veliparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: First efficacy results from the I-SPY 2 TRIAL. Cancer Res. 2014;73(24 Supplement) S5–02 – S5–02. [Google Scholar]
- 4.Tripathy D, Chien AJ, Hylton NM, Buxton MB, Ewing CA, Wallace AM, Forero A, Kaplan HG, Nanda R, Albain KS, Moulder SL, Haley BB, DeMichele A, Symmans WF, van ’t Veer L Paoloni MDMI-S 2 TC. Adaptively randomized trial of neoadjuvant chemotherapy with or without the Akt inhibitor MK-2206: Graduation results from the I-SPY 2 Trial. J Clin Oncol. 2015;33(suppl) abstr 524. [Google Scholar]
- 5.Kümler I, Tuxen MK, Nielsen DL. A systematic review of dual targeting in HER2-positive breast cancer. Cancer Treat Rev. 2014;40(2):259–270. doi: 10.1016/j.ctrv.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Chow LW-C, Xu B, Gupta S, et al. Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer. Br J Cancer. 2013;108(10):1985–1993. doi: 10.1038/bjc.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sánchez-Martín M, Pandiella A. Differential action of small molecule HER kinase inhibitors on receptor heterodimerization: therapeutic implications. Int J Cancer. 2012;131(1):244–252. doi: 10.1002/ijc.26358. [DOI] [PubMed] [Google Scholar]
- 8.Mullooly M, Conklin D, McGowan PM, et al. Neratinib to inhibit the growth of triple-negative breast cancer cells. ASCO Meet Abstr. 2015;33(15_suppl):1099. [Google Scholar]
- 9.Feldinger K, Kong A. Profile of neratinib and its potential in the treatment of breast cancer. Breast cancer (Dove Med Press. 2015;7:147–162. doi: 10.2147/BCTT.S54414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan A, Delaloge S, Holmes FA, et al. Neratinib after adjuvant chemotherapy and trastuzumab in HER2-positive early breast cancer: Primary analysis at 2 years of a phase 3, randomized, placebo-controlled trial (ExteNET) ASCO Meet Abstr. 2015;33(15_suppl):508. [Google Scholar]
- 11.Barker aD, Sigman CC, Kelloff GJ, Hylton NM, Berry Da, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86(1):97–100. doi: 10.1038/clpt.2009.68. [DOI] [PubMed] [Google Scholar]
- 12.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 13.Viale G, Slaets L, Bogaerts J, et al. High concordance of protein (by IHC), gene (by FISH; HER2 only), and microarray readout (by TargetPrint) of ER, PgR, and HER2: Results from the EORTC 10041/BIG 03-04 MINDACT trial. Ann Oncol. 2014;25(4):816–823. doi: 10.1093/annonc/mdu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry DA. Bayesian clinical trials. Nat Rev Drug Discov. 2006 Jan;5:27–36. doi: 10.1038/nrd1927. [DOI] [PubMed] [Google Scholar]
- 15.I-SPY 2 TRIAL. Neoadjuvant and Personalized Adaptive Novel Agents to Treat Breast Cancer - Full Text View - ClinicalTrials.gov [Internet] [cited 2015 Oct 23]; Available from: https://clinicaltrials.gov/ct2/show/NCT01042379?term=I-SPY2&rank=1.
- 16.Wolf D, Daemen A, Yau C, et al. Abstract P1-08-01: MammaPrint ultra-high risk score is associated with response to neoadjuvant chemotherapy in the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Cancer Res. 2014;73(24 Supplement) P1–08 – 01–P1 – 08–01. [Google Scholar]
- 17.Gelmon KA, Boyle FM, Kaufman B, et al. Lapatinib or Trastuzumab Plus Taxane Therapy for Human Epidermal Growth Factor Receptor 2-Positive Advanced Breast Cancer: Final Results of NCIC CTG MA.31. J Clin Oncol. 2015;33(14):1574–1583. doi: 10.1200/JCO.2014.56.9590. [DOI] [PubMed] [Google Scholar]
- 18.Piccart-Gebhart MJ. Plenary Session: First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L), or their combination (T+L) in the adjuvant trea. Asco. 2014;32(15_suppl) Abstract #LBA4. [Google Scholar]
- 19.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet (London, England) 2012;379(9816):633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bria E, Carbognin L, Furlanetto J, et al. Impact of neoadjuvant single or dual HER2 inhibition and chemotherapy backbone upon pathological complete response in operable and locally advanced breast cancer: Sensitivity analysis of randomized trials. Cancer Treat Rev. 2014;40:847–856. doi: 10.1016/j.ctrv.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Esserman LJ, Berry Da, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: Results from the I-SPY 1 TRIAL - CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 23.Saura C, Garcia-Saenz JA, Xu B, et al. Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2014;32(32):3626–3633. doi: 10.1200/JCO.2014.56.3809. [DOI] [PubMed] [Google Scholar]
- 24.Gandhi L, Bahleda R, Tolaney SM, et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol. 2014;32(2):68–75. doi: 10.1200/JCO.2012.47.2787. [DOI] [PubMed] [Google Scholar]
- 25.Jankowitz RC, Abraham J, Tan AR, et al. Safety and efficacy of neratinib in combination with weekly paclitaxel and trastuzumab in women with metastatic HER2-positive breast cancer: An NSABP Foundation Research Program phase i study. Cancer Chemother Pharmacol. 2013;72:1205–1212. doi: 10.1007/s00280-013-2262-2. [DOI] [PubMed] [Google Scholar]
- 26.A Study Looking at the Incidence and Severity of Diarrhea in Patients With Early-Stage HER2+ Breast Cancer Treated With Neratinib and Loperamide [Internet] Available from: https://clinicaltrials.gov/ct2/show/NCT02400476.
- 27.Esserman LJ, Woodcock J. Accelerating identification and regulatory approval of investigational cancer drugs. JAMA. 2011;306(23):2608–2609. doi: 10.1001/jama.2011.1837. [DOI] [PubMed] [Google Scholar]
- 28.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366(26):2438–2441. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- 29.Gianni L, Pienkowski T, Im Y-H, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 30.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24(9):2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 31.DeMichele A, Yee D, Berry DA, et al. The Neoadjuvant Model is Still the Future for Drug Development in Breast Cancer. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.