Abstract
Objectives:
The objective of this study was to investigate neural effects of acute whole body vibration (WBV) on lower limb muscles regarding corticospinal and spinal excitability.
Methods:
In 44 healthy subjects (16 f/ 28 m), motor evoked potentials (MEP) and H-reflexes in m. soleus (SOL) and gastrocnemius medialis (GM) were elicited before (t1), immediately after (t2), 2 (t3), 4 (t4) and 10 min after (t5) WBV.
Results:
After WBV, MEP amplitudes were significantly increased in SOL (t2+15±30%, t3+22±32%, t4+15±35%, t5+20±30%, P<0.05), but not in GM (t2+32±62%, t3+9±35%, t4+8±36%, t5+22±47%; P=0.07). Contrarily, H-reflexes were significantly reduced in SOL (t2-19±28%, t3-21±22%, t4-20±21%, t5-14±28%, P<0.05) and GM (t2-14±37%, t3-16±25%, t4-18±29%, t5-16±28%, P<0.05).
Conclusions:
A temporary sustained enhancement of corticospinal excitability concomitant with spinal inhibition after WBV points towards persisting neural modulation in the central nervous system. This could indicate greater neural modulation over M1 and descending pathways, while the contribution of spinal pathways is reduced.
Keywords: Supraspinal, Motor Evoked Potential, H-reflex, Neurophysiological Adaptation, Central Nervous System
Introduction
Whole body vibration (WBV) describes a training device through which mechanical oscillations of the support surface are induced passively to the subject. Either in a vertical synchronous, in a side-alternating vertical sinusoidal or in a random manner, vibration is applied with neurophysiological structures being activated[1,2]. The impact of acute exposure to WBV on neuronal control of the skeleton muscle is extensively discussed in literature[1,2]. Various scientific reports state that WBV can immediately have beneficial effects on strength and power[3], motor coordination[4,5] and postural control[6,7] among athletes[8], sedentary populations[9] or patients in rehabilitation[10,11]. The aforementioned improvements were speculated to be associated with enhanced neural excitation, possibly achieved by modulation on the spinal and supraspinal level of the central nervous system (CNS)[12-14]. Whereas spinal modulations refer to involuntary reflex muscle activation, the supraspinal level is considered to involve brain structures for voluntary movement control[15,16]. Based on the evidence of neural modulation following WBV, the application in subjects suffering from disorders of movement control, such as in neurological diseases affecting the CNS, has increasingly been focused on. Investigations clearly point towards neuronal modulation regarding spasticity in patients with spinal cord injuries[17] or cerebral palsy[10]. By improving neuromuscular activation, e.g. through muscle strength[5,6,10], positive effects with regard to gait pattern[6] and gross motor functioning were achieved in several populations with neurological disorders[5,10]. But despite existing research demonstrating the improvement of functional motor control following WBV, further investigations are needed to clarify the underlying neurophysiological modulation during and after WBV.
To date, just one study exists demonstrating supraspinal adaptation during WBV in the m. tibialis anterior (TA)[18] with a recovery to baseline immediately after. On a supraspinal level, sensory input can be integrated in subcortical and cortical areas of the central nervous system[19,20]. Those responses are of a longer duration compared to reflex activity, but comprise planned, situation-adapted and greatly more specific muscle responses. By transcranial magnetic stimulation (TMS), cortical axons in the motor cortex at M1 are depolarized and the induced excitation of neural tissue[16] is projected via corticospinal pathways to the α-motoneurons[21,22]. In the study of Mileva and colleagues (2009)[18], it was shown that WBV during squat exercise compared to no WBV led to a corticospinal facilitation concomitant with intracortical modulation in terms of increased intracortical inhibition and diminished intracortical facilitation[18]. Surprisingly, in m. soleus – the muscle the most proximal and instantaneously affected by the WBV stimulus[23] –, Mileva et al. (2009)[18] could not demonstrate any change in corticospinal excitability (MEP amplitude). Furthermore, for local vibration applied to the tendon or muscle belly, authors demonstrated that vibration induces a succession of stretch reflex responses in the target muscle[16,24]. Meanwhile, WBV can be associated with the activation of more than one muscle group, and it involves sensory as well as motor nerve pathways[18] with a contribution of spinal stretch reflex responses[25]. Despite the difference between both vibration methods regarding sensory integration, they apparently result in similar effects, such as up to a 50% reduction in spinal excitability following vibration[25-27]. In this regard, those excitability changes on a spinal level are predominantly measured by Peripheral Nerve Stimulation (PNS). This is a non-invasive approach, also known as the methodological equivalent to the stretch reflex but bypassing the muscle spindles by electrical stimulation of Ia afferent pathways[28,29]. While several spinal mechanisms have been discussed to be modulated by vibration[30-33], supraspinal contribution to neural adaptation might be assumed after WBV based on the knowledge that the spinal and supraspinal levels of the CNS are connected, for instance by the fusimotor system[34] and corticospinal pathways[12,20,35].
An intact interaction between supraspinal and spinal modulation is indispensable for any kind of movement, such as during locomotion[36] or postural control in everyday life[37]. While vibration is the only treatment so far that is reported to persistently reduce spinal excitability[25-27,32], great possibilities for the application as a training modality could be enabled if an improvement of supraspinal modulation could be recorded at the same time: By enhancing the control of voluntary movement via enhanced cortical control, this training modality might be advantageous for those who have impairments of movement control, such as for patients suffering from neurological disorders[38].
Therefore, despite high quality research investigating the effects of local muscle or tendon vibration[33,39,40], neurophysiological, especially corticospinal, modulation after WBV still needs to be clarified. The existing variability and ambiguity of WBV effects in the current literature may be ascribed to differing research protocols in separate investigations of neurophysiological mechanisms. In connection and expansion to previous investigations demonstrating neural effects during WBV[18], this study aimed to test for acute effects and the respective time course by taking a holistic approach: While the specification of involved neural modulation cannot be determined with the application of the TMS methodology alone[15,20], in the current study, effects were investigated on a supraspinal and spinal level in one study. Based on this basic scientific approach, neurophysiological mechanisms underlying the adaptations to WBV can be narrowed down. It was hypothesized that cortical and subcortical facilitation can be achieved, accompanied by reduced spinal excitability after an acute bout of WBV which points towards greater control of voluntary movement. For the current investigation, the muscles of the lower limb were chosen due to the proximity to the vibration stimulus, the high effect by oscillation transmission as well as the involvement in any kind of everyday movement. Additionally based on previous investigations[23], we assumed to achieve the most noticeable neuromuscular effects with the application of side-alternating, in contrast to vertical vibration.
Methods
Subjects
44 subjects (16 female, 28 male, age 26±3 years, height 175.4±8.8 cm, body mass 70.6±12.3 kg) participated in this study. All participants gave written informed consent to the experimental procedure, which was in accordance with the latest revision of the Declaration of Helsinki and approved by the ethics committee of the University of Freiburg (189/15). In addition to acute injuries of the lower extremity, any positive item of the Transcranial Magnetic Stimulation Adult Safety Screen[41], including pregnancy or previous neurological disorders, were exclusion criteria. Furthermore, subjects were excluded from the study, if they felt any kind of uncomfortableness during the externally induced contraction of the m. triceps surae during TMS or PNS.
Experimental design
A single-group cross-sectional study design was used to evaluate acute WBV-induced effects on corticospinal (protocol 1) and spinal (regarding Ia afferent pathways, protocol 2) excitability in the m. triceps surae. Corticospinal and spinal excitability were recorded at six different time intervals: two times before vibration (t0 and t1), immediately after vibration (t2), and 2 (t3), 4 (t4) and 10 min after vibration (t5). Duration of and time frames between stimulations are illustrated in Figure 1. Recordings were made twice before the WBV intervention (t0 and t1) to evaluate the reproducibility of the assessment and to control for stimulation-induced changes (Figure 1[42]–[44]). Time intervals after WBV were selected according to literature[25] which indicates that a 10 min period after WBV is the relevant period for neuronal adaptation associated with vibration. Additionally, because muscular activation has an impact on evoked potentials of TMS and PNS[28,43,44], muscular background activity and goniometric angles of the ankle and knee joint were recorded to control that body position was set to zero for each subject, to allow that the stretch load on the target muscle was the same throughout the measurement. This was ensured by a standardized setup with sensory feedback of feet- and hip-position prior to each protocol. Protocols and subject distribution to the respective protocols were conducted randomly: (i) For 20 subjects, just protocol 1 was executed. (ii) For 11 subjects, both protocols were executed on two different days in a counterbalanced order. (iii) For 13 subjects, both protocols were measured on the same day in a randomized order (TMS & PNS). The aim of this randomization was to control for any impact of the protocols on each other as well as of the conducting order. Both stimulation approaches differ greatly regarding in- and exclusion criteria as well as regarding inter-individual signal quality of evoked potentials. This is why the amount of subjects varied among both protocols.
Figure 1.
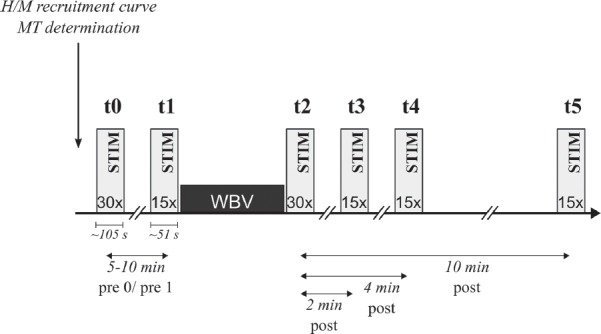
Study design of one protocol. Stimulation (STIM) marks either Peripheral Nerve Stimulation (PNS) or Transcranial Nerve Stimulation (TMS) stimulation before (t0 & t1), directly after (t2) as well as 2 (t3), 4 (t4) and 10 min after 1 min bout of whole body vibration (t5).
Whole body vibration
A side-alternating vertical sinusoidal vibration platform was used according to Ritzmann et al. (2013a)[23] (Galileo Sport, Novotec Medical, Pforzheim, Germany) which generates vibration by platform oscillations along the sagittal axis. The axis of rotation was placed in-between the subjects’ feet, and the feet were placed 17 cm from the axis of rotation resulting in a vibration peak-to-peak displacement of 6 mm between the hallux and the second toe. The vibration frequency was set to 30 Hz[23] resulting in a peak acceleration of 10.9 g (Root Mean Squared Acceleration of 75.4ms-2). Subjects were exposed to a 1 min bout of WBV[25]. Subjects stood freely in an upright position on top of the platform. Vibrations were applied barefooted or with socks, depending on the setup in which skipping was minimized. Weight was evenly distributed on both feet; static body position was maintained in forefoot stance with a knee angle of 5°. Hands were placed on the hip, head and eyes were forward-facing.
Protocol 1 – Transcranial magnetic stimulation
In protocol 1, 44 subjects were tested on corticospinal excitability of the right m. soleus (SOL) and gastrocnemius medialis (GM) by applying noninvasive TMS to M1 of the contralateral motor cortex. With a 90-mm circular coil and a Magstim 200 (Magstim, Dyfed, UK), a magnetic field was produced over the motor cortex, leading to electrical stimulation of neurons. The applied stimuli were transferred by corticospinal pathways and evoked motor evoked potentials (MEP) in the chosen muscles (waveform: monophasic, pulse width: 200 µs). For localization of the hot spot of the m. triceps surae on M1, the coil was positioned 0.5 cm posterior to the vertex and over the midline of the scalp and, subsequently, moved anterior and left from the vertex, while the MEP size of SOL and m. tibialis anterior (TA) were monitored on an oscilloscope. The coil was fixed with a helmet apparatus, as described in previous investigations[45]. The motor threshold (1.0 MT), which was defined as MEP amplitudes larger than 50 mV in three of five consecutive trials[46,47], was determined while standing for SOL for each subject to provide task-specific motor thresholds. TA background EMG was monitored to make sure that there was no antagonistic activation in this muscle. Stimulation intensity during t0-t5 was set to 1.2 MT[43]. If values during MT-determination exceeded the definition as described above, stimuli of suprathreshold values but with constant MEPs over time were chosen to ensure comparability. Magnetic stimulation was triggered to occur every 4 s to avoid effects of fatigue, resulting in 15 MEPs at time points t1, t3-t5. Due to the relevance of those data points, 30 MEPs were evoked for t0 and t2. The main focus was on vibration-induced changes in MEP amplitudes indicating modulations in corticospinal excitability and the cortical silent period (CSP), referring to an interruption of voluntary muscle contraction after stimulation[48] which indicates cortical inhibition including spinal and supraspinal mechanisms[30,48,49].
Protocol 2 – Peripheral nerve stimulation (PNS)
In protocol 2, spinal excitability of SOL and GM were measured in 24 subjects by PNS. With the technique of PNS electrically induced H-reflexes in the m. triceps surae are generated by stimulating the tibial nerve in the popliteal fossa with 1 ms square-wave pulses using an electrical stimulator (Digitimer DS7, Digitimer, Welwyn Garden City, UK). The anode (10x5 cm dispersal pad) was placed below the patella. Stimulations were applied by searching and fixing the perfect spot of the nervus tibialis with a cathode pad (2 cm in diameter)[44]. Prior to measurements, H/M recruitment curves were recorded during upright stance detecting the maximal H-reflex (Hmax) and M-wave (Mmax)[44]. Therefore, intensity of the stimulation current was successively elevated so that H-reflexes and, with further increasing current, M-wave amplitudes were elicited which finally reached a maximum value by means of a plateau (supramaximal). For data collection, the stimulation current was adjusted to elicit SOL H-reflexes with an amplitude of 25% Mmax[50]. In accordance with protocol 1, electrical stimulation was triggered to occur every 4 s resulting in 15 H-reflexes at time points t1, t3-t5 and 30 H-reflexes for t0 and t2. The methodological approach of H-reflex conditioning by TMS[51] was not used in the current study, because of inter- and intraindividual time and amplitude variability of the H-reflex amplitude after WBV[25]. For the coincidence of neuronal volleys, an H-reflex of identical amplitude would be required to be evoked almost simultaneously with the MEP[20].
Data collection for outcome measures
Electromyography (EMG): Bipolar Ag/AgCl surface electrodes (Ambu Blue Sensor P, Ballerup, Denmark; diameter 9 mm, center-to-center distance 34 mm) were placed over the SOL, GM and TA of the right leg according to SENIAM[52]. The longitudinal axes of the electrodes were in line with the direction of the underlying muscle fibers. The reference electrode was placed on the patella. Skin-electrode impedance was kept below 2.5 kΩ by means of shaving, light abrasion, degreasing, and disinfection of the skin[53]. Signals were transmitted to the amplifier (1000x amplified, band-pass filter 10 Hz–1 kHz,) via shielded cables and recorded with 1 kHz.
Prior to the experiment, isometric maximal voluntary contractions (MVC) were performed against manual resistance for the recorded muscles according to Roelants et al. (2006)[54] for normalization. Plantarflexion (SOL and GM) and dorsiflexion (TA) were instructed with the knee either being bent (SOL) or extended during contraction (GM & TA). Subjects’ body position and antagonistic muscle activity were controlled strictly by the principle investigator. With one-minute pauses between trials, contractions were held for 3 s.
Kinematics: Ankle angles (dorsiflexion and plantarflexion) and knee angles (knee flexion and extension) were recorded by electro-goniometers (Biometrics®, Gwent, UK). Those were custom-designed, being composed of a rotary potentiometer as rotation axis (Megatron, Munic, Germany) and two attached movable aluminum endplates (length 10 cm). Goniometers were attached to rotation axes of the right ankle (malleolus lateralis) and knee joint (knee joint cavity), with the movable endplates aligned in the direction of the respective body axis. Thereby, for the ankle, endplates were fixed to the longitudinal axis of the foot or shank, pointing towards the fifth metatarsal and the epicondyle of the femur, respectively. At the knee joint, endplates were in line with the longitudinal axis of the femur and trunk, aligned to the malleolus lateralis and the greater trochanter. Neutral position was set at an angle of 90° between the fifth metatarsal and the fibula for the ankle, with plantarflexion being reflected by an ankle angle greater than 90°. Knee flexion angle was set to zero at full extension between the femur and the fibula. Signals were transmitted to the computer, via shielded cables, recorded with 1 kHz and band-pass filtered (10 Hz to 1 kHz).
Data processing
For both protocols, the amplitude between lowest and highest peak (“peak-to-peak amplitudes”) in MEPs and H-reflexes were determined and averaged for the total amount of stimulations. Each motor output to the respective stimulation was included in the calculation with the exception of those trials when subjects could not accomplish the standardized body position. Time intervals of peak-to-peak calculations were set individually in the graphic output of the EMG sheets of MEP and H-reflex stimulation with the boundaries being defined as the first and third discrepancy when raw EMG values did deviate positively or negatively compared to baseline values. Percentage change at each time point was calculated in relation to t1, being set at 100%. The H-reflex and MEP latencies were defined as the time frame between the stimulation and the onset of the first deviation in EMG activity compared to baseline values (in s); determination was set visually. The CSP was calculated by graphical determination according to Garvey et al. (2001)[49]. Therefore, on- and offset of the silent period were defined and determined as data points falling below or above a defined lower variation limit, respectively. This limit was calculated for each subject individually by subtracting the mean absolute consecutive differences of pre-stimulus EMG multiplied by a predefined factor of 2.66 from pre-stimulus EMG mean values[49]. Presetting for all subjects and conditions was controlled retrospectively by the background EMG of the respective muscle and joint kinematics of the lower limb in a time frame of 100 ms prior to the stimulus. For background activity and MVC, EMG signals were rectified, integrated and averaged (iEMG [mVs]). While for MVC, the time point of the highest EMG integral was evaluated, background activity was normalized to the respective MVC. Angular excursions of the ankle and knee were averaged for each subject (°). In case of changes due to body position, evaluated by iEMG and goniometric data, subjects were excluded from the following statistical analysis.
Statistics
To test for WBV-induced changes in MEP and H-reflex characteristics, such as the amplitude, latency as well as MEP CSP over time (t1-t5), a repeated measures analysis of variance (rmANOVA) was used. The normality of the data was evaluated with the Kolmogorov-Smirnov test; data followed a normal distribution. If the assumption of sphericity assessed by Mauchly’s sphericity test was violated, the Greenhouse-Geisser correction was used. A between-subject factor was included to control for adaptations of MEP and H-reflex amplitudes due to the protocol conducting order and type: The factor “conducting setup” included: (i) conducting of just one protocol, versus (ii) conducting of both protocols on different days, versus (iii) conducting of both protocols on the same day. Level of significance was set to P<0.05. Additionally, changes at post time points (t2-t5) were assessed with one-tailed student’s t-test and corrected for multiple testing by Bonferroni adjustments; the p-value (pi test) of each test was multiplied by the number of post-tests (pi=pi test * n, n=number of tests=4). Statistical significance was reached in cases of pi<0.05 and was marked with a symbol (*). P-values from rmANOVA are marked with capital “P”, those with a lowercased “p” are from corrected t-test calculations.
For MEP and H-reflex characteristics (amplitude, latency as well as MEP CSPSOL), reproducibility tests between both pre-measurements were statistically controlled by intraclass correlation coefficients (ICCs) using a one-way random single measure model with two items as time points (t0-t1). To make sure that particular parameters (ankle and knee joint position, pre-activation in SOL, GM, and TA) did not change over time, test–retest reliability of goniometry and background EMG was provided by calculating ICCs using a one-way random single measure model with six items as pre and post time points (t0-t5). Outcomes were described by Cronbachs α according to Fleiss (1986)[55].
All analyses were executed by using SPSS 23.0 (SPSS, Inc., Chicago, IL, USA). Values are presented as means±standard deviations (M±SD).
Results
Means for MEPs and H-reflexes are displayed in [Tables 1 and 2] and illustrated in Figure 2. Reproducibility tests (t0 vs. t1) revealed excellent values of Cronbachs α ranging between 0.910-0.994 for MEP and H-reflex characteristics. For none of the parameters or the different time points, significant differences could be observed. Cronbach tests also demonstrated high reliability for background EMG and joint kinematics over time points t0-t5 for both protocols (see Tables 1 & 2).
Table 1.
Results of Transcranial Magnetic Stimulation (TMS) before and after WBV. Neuromuscular data are normalized to t1±standard deviations (in %). Joint excursions are demonstrated in (°) with the neutral positions being defined as 90° in the ankle and 0° in the knee joint (extension). Significant differences in pairwise comparison to t1 (p<0.05) are marked with *; significant adaptations over time are demonstrated by rmANOVA with P<0.05 and intraclass correlation coefficients (ICCs) are illustrated in the last column.
| Protocol 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| TMS | t0 | t1 | t2 | t3 | t4 | t5 | rm ANOVA | ICCs (2 items t0-t1) |
| Amplitude MEPSOL | 0.98±0.19 | 1 | 1.15±0.30* | 1.22±0.32* | 1.15±0.35 | 1.20±0.30* | 0.003 | 0.983 |
| Amplitude MEPGM | 1.01±0.19 | 1 | 1.32±0.62 | 1.09±0.35 | 1.08±0.36 | 1.22±0.47 | 0.074 | 0.984 |
| CSPSOL | 1.04±0.18 | 1 | 1.42±0.75 | 1.09±0.44 | 1.18±0.35 | 1.02±0.43 | 0.152 | 0.910 |
| Latency MEPSOL (ms) | 36±5 | 36±5 | 36±5 | 36±5 | 36±5 | 36±5 | 0.881 | 0.977 |
| Latency MEPGM (ms) | 34±4 | 34±5 | 34±5 | 34±4 | 34±4 | 34±4 | 0.910 | 0.963 |
| Background EMG (100ms prior stimulus) | ICCs (6 items t0-t5) | |||||||
| SOL | 0.96±0.13 | 1 | 1.02±0.15 | 1.05±0.20 | 1.04±0.18 | 1.07±0.13 | 0.988 | |
| GM | 1.02±0.14 | 1 | 1.06±0.50 | 0.98±0.23 | 1.02±0.32 | 0.99±0.38 | 0.976 | |
| TA | 1.01±0.06 | 1 | 1.00±0.10 | 1.03±0.15 | 1.02±0.14 | 1.05±0.14 | 0.996 | |
| Goniometry | ICCs (6 items t0-t5) | |||||||
| Ankle Joint angle (°) | 92.45±9.36 | 92.62±9.51 | 93.76±9.16 | 94.46±9.46 | 94.03±9.20 | 93.11±8.93 | 0.996 | |
| Knee Joint angle (°) | -2.06±14.80 | -1.98±14.69 | -0.81±15.09 | -1.05±15.41 | -0.89±15.32 | -1.49±15.45 | 0.997 | |
Table 2.
Results of Peripheral Nerve Stimulation (PNS) before and after WBV. Neuromuscular data are normalized to t1±standard deviations (in %). Joint excursions are demonstrated in (°) with the neutral positions being defined as 90° in the ankle and 0° in the knee joint (extension). Significant differences in pairwise comparison to t1 (p<0.05) are marked with *; significant adaptations over time are demonstrated by rmANOVA with P<0.05 and intraclass correlation coefficients (ICCs) are illustrated in the last column.
| Protocol 2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| PNS | t0 | t1 | t2 | t3 | t4 | t5 | rm ANOVA | ICCs (2 items t0-t1) |
| Amplitude H-reflexSOL | 0.99±0.13 | 1 | 0.81±0.28* | 0.79±0.22* | 0.80±0.21* | 0.86±0.28* | <0.001 | 0.988 |
| Amplitude H-reflexGM | 1.05±0.23 | 1 | 0.86±0.37 | 0.84±0.25* | 0.82±0.29* | 0.84±0.28* | 0.014 | 0.989 |
| Amplitude M-waveSOL | 1.00±0.18 | 1 | 0.91 ±0.21 | 0.92±0.18 | 0.93±0.20 | 0.94±0.22 | 0.153 | 0.994 |
| Amplitude M-waveGM | 1.07±0.26 | 1 | 0.99±0.31 | 1.04±0.39 | 0.99±0.18 | 0.95±0.25 | 0.495 | 0.993 |
| Latency H-reflexSOL (ms) | 34±2 | 34±2 | 35±2* | 34±2 | 34±2 | 34±2 | <0.001 | 0.982 |
| Latency H-reflexGM (ms) | 32±3 | 33±3 | 33±3* | 33±3 | 33 ±3 | 33±3 | 0.407 | 0.988 |
| Background EMG (100ms prior stimulus) | ICC (6 items t0-t5) | |||||||
| SOL | 1.00±0.01 | 1 | 1.01±0.02 | 1.02±0.02 | 1.02±0.03 | 1.03±0.03 | 0.989 | |
| GM | 1.00±0.03 | 1 | 1.01±0.06 | 1.03±0.09 | 1.03±0.10 | 1.02±0.09 | 0.963 | |
| TA | 1.00±0.00 | 1 | 1.01±0.01 | 1.01±0.02 | 1.02±0.02 | 1.02±0.02 | 0.990 | |
| Goniometry | ICCs (6 items t0-t5) | |||||||
| Ankle Joint angle (°) | 89.00±7.31 | 89.09±7.50 | 89.33±7.46 | 89.53±7.95 | 89.47±7.93 | 89.27±7.74 | 0.997 | |
| Knee Joint angle (°) | -9.03±6.47 | -9.36±6.48 | -8.82±5.99 | -8.92±6.50 | -9.02±6.54 | -8.71±6.04 | 0.994 | |
Figure 2.
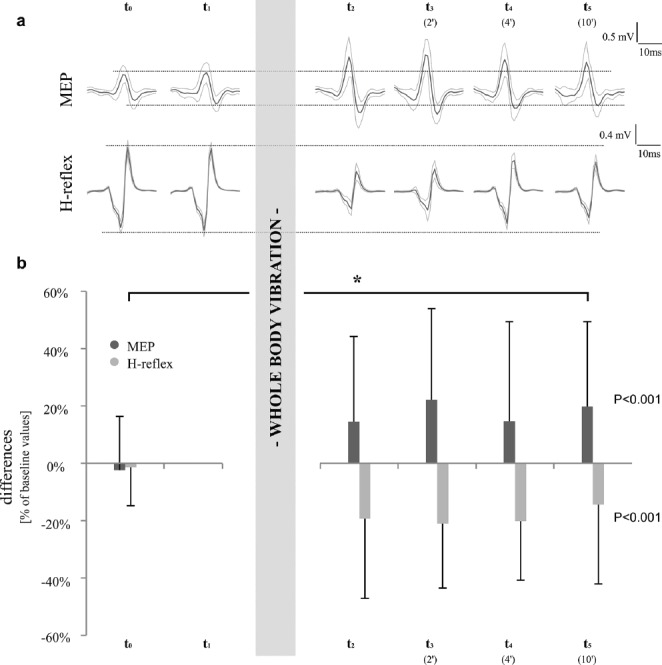
Peak-to-Peak amplitude values of soleus MEP- and H-reflex-stimulation for both protocols for one representative subject (a) and as averaged means±standard deviations (b). Data are presented as differences compared to baseline values (t1), protocol 1 is illustrated in dark columns, protocol 2 in light columns. Significant results (P<0.05) are marked with *.
Protocol 1 – TMS
After data screening, ten subjects had to be excluded from the analysis due to a changed body position. Resultant, a total of 34 subjects were evaluated for protocol 1. After a 1 min bout of WBV, MEPSOL amplitudes significantly increased over time (P<0.05) with mean differences of +15±30% (t2, p=0.02*), +22±32% (t3, p<0.01*), +15±35% (t4, p=0.05) and +20±30% (t5, p<0.01*). In MEPGM, amplitudes were elevated by +32±62% (t2, p=0.07), +9±35% (t3, p=0.58), +8±36% (t4, p=0.71) and +22±47% (t5, p=0.10), but values did not reach statistical significance (P=0.07). No effects were demonstrated for between-subject effects (MEPSOL P=0.215, MEPGM P=0.219). Motor evoked potential latencies in SOL and GM remained unchanged over time (Table 1).
The CSP in SOL did not change significantly over time with values ranging from +42% (t2), +9% (t3) and +18% (t4) to +2% (t5), with P=0.15 compared to baseline values.
Muscle background activity for m. triceps surae as well as ankle and knee joint deflections prior to TMS remained unchanged for all recorded muscles and joints (for details, see Tables 1, Figure 3).
Figure 3.
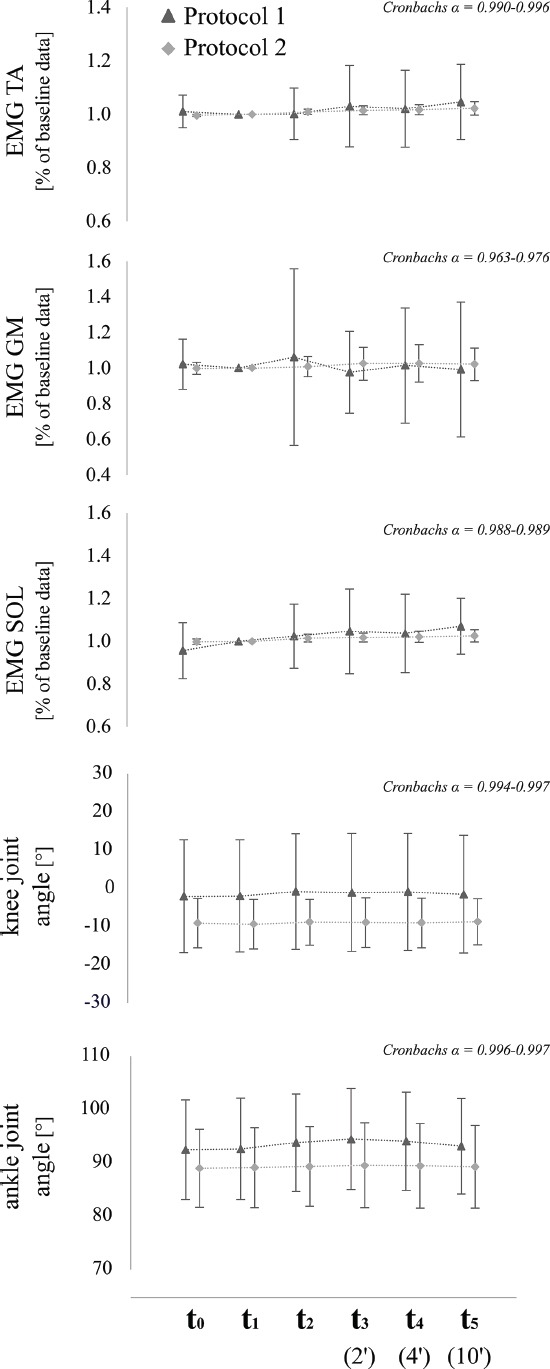
Averaged mean values±standard deviations of EMG (SOL, GM, TA) and kinematic data (ankle & knee joint angles) of both protocols for the respective time point (t0-t5). Values from protocol 1 (MEP) are illustrated with triangles, those from protocol 2 (H-reflex) with squares. ICCs are presented by means of Cronbachs α for each muscle, joint and for both protocols.
Protocol 2 – PNS
After data screening, a total of 24 subjects were included for statistical analysis. Contrary to the MEPs, there was a significant decrease of H-reflexSOL amplitude over time (P<0.05) ranging from -19±28% (t2, p=0.01*) to -21±22% (t3, p<0.01*) and -20±21% (t4, p<0.01*) to -14±28% 10 min post vibration (t5, p=0.05*). A significant reduction (P<0.05) could also be demonstrated in H-reflexGM with similar percentage changes: -14±37% (t2, p=0.14), -16±25% (t3, p=0.01*), -18±29% (t4, p=0.01*) and -16±28% (t5, p=0.02*). Once again, between-subject comparisons were not significant for the conducting setup over time (H-reflexSOL P=0.939, H-reflexGM P=0.832). The H-reflex latency in SOL changed significantly with an increase of 0.5±0.7 ms from t1 to t2 (P<0.001); but remained unchanged over time in GM (Table 2).
Muscle background activity for m. triceps surae as well as ankle and knee joint deflections prior to PNS remained unchanged for all recorded muscles and joints (for details, see Table 2, Figure 3).
Discussion
The objective of the current study was to investigate the acute effects on supraspinal and spinal excitability and to evaluate the time course of central modulation following WBV. The main findings were: (1) that MEP amplitudes were increased immediately after WBV and remained elevated for 10 min, and (2) H-reflex amplitudes were reduced after WBV which persisted for the analyzed period of 10 min as well. This investigation demonstrates for the first time that a short intervention of side-alternating WBV (1 min, 30 Hz) is already sufficient to elicit facilitating effects on neuronal circuits, concomitant to a suppression of spinal Ia afferent reflex responses during upright stance conditions. Those neural modulations can be associated with a shift in motor command from reflex-associated (spinal) to cortical and subcortical control centers (supraspinal), persisting for a time course of 10 min as minimum.
Functional importance of corticospinal modulation
This is the first study that demonstrates a temporary sustained enhancement of corticospinal input after WBV without any changes in MEP latency. Effects amounted from 8 to 32% in the m. triceps surae, the muscle that is instantaneously affected by vibration oscillations due to the proximity to the device[56,57], pointing towards a facilitation of neuronal transmission to lower limb muscles. With the application of TMS only, the origin of central modulation can be manifold, but, by forestalling results from protocol 2, the combination of both protocols allows further clarification that facilitation likely occurs on a supraspinal level. The persisting adaptation of corticospinal and spinal excitability in lower limb muscles over a time course of 10 min after WBV exposure is in expansion to previous study results, in which WBV during exercise led to a modulation of corticospinal pathways and, additionally, of intracortical circuitries for TA activity[18]. Since WBV – in contrast to local vibration – simultaneously activates synergistic and antagonistic muscle groups of the lower leg[58], it is argued that WBV-induced neural adaptations are likely to occur in more than one muscle. Current results even point towards an ongoing modulation of the corticospinal pathways which, in particular, may be highly relevant regarding the benefits for functional mobility. The contributions of corticospinal pathways and subcortical structures have been associated to be of importance in postural control[35,59,60], as well as during voluntary tasks such as locomotion[61,62]. For instance, greater postural instability is associated with larger MEP amplitudes and corticospinal facilitation[59]. Through WBV, high accelerations cause perturbations[18], and consequently, postural stability is impeded so that greater cortical input is required to control body posture. Additionally, cortical contribution has been documented to be associated with force generating capacity[63,64], including explosive power in the stretch-shortening-cycle[45,65] and in the execution of precision tasks[66].
Functional importance of spinal modulation
A diminished H-reflex excitability – opposed to the increased MEP amplitudes – demonstrates that Ia afferent pathways of spinal circuits can be excluded in any of the time points as being involved in central facilitation[39,44]. The H-reflex allows the assessment of monosynaptic reflex activity in the spinal cord[67], estimating α-motoneuron excitability when presynaptic inhibition remains constant[29]. Previous studies demonstrated an H-reflex inhibition after WBV[25-27,32] diminishment persisted only up to 30 s[26], 36 s[27], 1 min[32] or 5 min[25] while reflex amplitudes gradually recovered to baseline values. In expansion, our study revealed that inhibition effects amounted from -16 to -21% and neural adaptation was sustained for a period of 10 min; thus, sensory input from muscle spindles remained inhibited for a 10 min gap for triceps surae. The surprising vibration paradox[68] is that the CNS seems to continuously suppress WBV-induced muscle contractions via the I afferent reflex circuitry that makes vibration training so particular compared to other exercise interventions[53,69]. In fact, experiments revealed a succession of stretch reflexes during vibration to be the major source of muscle activity[53]. H-reflex inhibition is attributed to be important regarding practical application such as for movement control. For instance, the suppression of reflex activity is assumed to be related to uncontrollable oscillations of the ankle joint during posturally demanding tasks[70]. This is why it can functionally be associated with improved postural control during stance and balance tasks[20,28,70], enhanced motor coordination as well as improved task-specific modulation during the step cycle, as demonstrated in patients with neurological disorders[71]. Thus, our results may explain the advantage of a WBV-intervention, in particular when the suppression of the Ia afferent reflex loop is beneficial to execute precise movements during everyday locomotion.
Central modulation on a supraspinal level
Merging both protocols, opposed modulations clearly point towards cortical and/or subcortical facilitation for 10 min following one single bout of WBV. This is in accordance with previous studies which used local vibration[40] or WBV[18]. Vibration-induced MEP facilitation has been reported to demonstrate corticospinal excitability[16,18,22,40] and may have a variety of origins along cortical[18,20], subcortical[59] and spinal neurons over corticospinal pathways[18,72,73]. Because diminished H-reflexes provide evidence that Ia afferent pathways do not appear to be involved in MEP facilitation, subcortical or other cortical contributions are the most likely mechanisms to account for the current effects. Based on previous investigations[18,40], intracortical mechanisms can be assumed to be unlikely to lead to the MEP facilitation as demonstrated in the current study as well. However, even though insignificant, a clear tendency towards a vibration-induced prolongation in CSP could be observed in the current study. Methodologically, lowering the amount of subjects in the first protocol might have been the reason for the statistical insignificance. Nonetheless, in neurological disorders, spasticity is assumed to be correlated with a shorter CSP[74], whereas a prolongation of CSP could be achieved through local vibration of antagonistic muscles in this patient group[30,75]. This may be of considerable functional relevance, because we concluded that an enhancement of inhibition in neuronal circuitries during and after WBV may have an impact on spasticity management.
Concerning diminished excitability on a spinal level, several mechanisms may be discussed. Due to constant values for M-waves (Table 2), central instead of peripheral mechanisms should be considered concerning H-reflex reduction after vibration: While presynaptic and reciprocal inhibition have been discussed elsewhere[25,27,32], post-activation depression, which has been described to last for a short time of 10 s after vibration only[50], is not likely to be causal for the current persistent modulations. Additionally, H-reflex latencies were just slightly prolonged after WBV (<2%) so that changes in transmission velocity cannot be assumed to have a great influence on current central modulations as well. Further research is needed to specify the issue of the underlying mechanisms to WBV. Nonetheless, despite vibration-induced activation of lower limb muscles, methodological side effects could be ruled out due to consistent values over time in background EMG and joints angles in the present investigation (Figure 3). Thus, neuromuscular mechanisms may be the predominant cause for the observed modulation, which was also reflected by muscle-specific adaptations concerning the triceps surae muscle. Even though the H-reflex size of both heads, SOL and GM, was modulated after WBV, there were greater adaptations for SOL[22]. Modulation due to differing properties of the one-articular SOL with slow-twitch fibers, versus the two-articular GM with mainly fast-twitch fibers, may be considered[76]. Vibration evidently affects the muscle spindles; thus, the most probable explanation for the current results is the greater density of muscle spindles in the SOL[77].
Prospective
Current results establish a great opportunity for future research. First, from a methodological point of view, assessments with a (focal) figure-of-eight coil could minimize the need for intensive data screening and subject exclusion prior to the evaluation of results. Second, independent of the respective methods and underlying mechanisms, increased cortical or subcortical modulation may be associated with greater functional ability[35,60,62] that may explain the acute WBV-induced performance benefits in sedentary people, patients or athletes reported in the literature[8-11,78]. The linkage of corticospinal projections during force-related adaptations of motor control, in combination with possible benefits resulting from reflex inhibition, may point towards greater voluntary muscle control. By enhancing this voluntary control, the execution of fine motor movements is considerably enhanced[12], and this 10 min time frame can be used for targeted voluntary motor training. Specifically, patients with deficient spasticity-related control of the locomotor apparatus might take advantage of improved training of voluntary motor function[38]. Therefore, neuronal modulation during[30] as well improved dexterity after local vibration[75] points towards improved spasticity management. All of those results clearly imply improved functional ability, thus encouraging the application of movement therapy in neurological disorders immediately following vibration as has already been proposed by previous researchers[10,17,27].
Conclusion
To conclude, the current investigations demonstrated acute neural modulation following WBV persisting for a time course of 10 min as minimum. Because of diminished spinal excitability it can be assumed that the underlying mechanisms of MEP facilitation are probably located on a corticospinal level. This indicates greater voluntary movement control and might be beneficial for patients suffering from neurological disorders: Due to the persistency of effects, the implementation of WBV might be beneficial for instance prior to voluntary movement training to enhance targeted variables. On the basis of these results, more investigations are needed to localize the cause of the observed corticospinal increase of excitation and to clarify if those underlying mechanisms may be applicable during movement tasks.
Acknowledgements
The authors want to thank all subjects participating in the current study and to everyone who contributed to making this investigation possible. The article processing charge was funded by the German Research Foundation (DFG) and the University of Freiburg in the funding program Open Access Publishing.
Footnotes
The authors have no conflict of interest.
Edited by: D. Cochrane
References
- 1.Cochrane DJ. Vibration exercise: the potential benefits. Int J Sports Med. 2011;32(2):75–99. doi: 10.1055/s-0030-1268010. [DOI] [PubMed] [Google Scholar]
- 2.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010;108(5):877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 3.Roelants M, Delecluse C, Verschueren SM. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J Am Geriatr Soc. 2004;52(6):901–08. doi: 10.1111/j.1532-5415.2004.52256.x. [DOI] [PubMed] [Google Scholar]
- 4.Ness LL, Field-Fote EC. Whole-body vibration improves walking function in individuals with spinal cord injury: a pilot study. Gait Posture. 2009;30(4):436–40. doi: 10.1016/j.gaitpost.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stark C, Nikopoulou-Smyrni P, Stabrey A, Semler O, Schoenau E. Effect of a new physiotherapy concept on bone mineral density, muscle force and gross motor function in children with bilateral cerebral palsy. J Musculoskelet Neuronal Interact. 2010;10(2):151–58. [PubMed] [Google Scholar]
- 6.Lee B-K, Chon S-C. Effect of whole body vibration training on mobility in children with cerebral palsy: a randomized controlled experimenter-blinded study. Clin Rehabil. 2013;27(7):599–607. doi: 10.1177/0269215512470673. [DOI] [PubMed] [Google Scholar]
- 7.Orr R. The effect of whole body vibration exposure on balance and functional mobility in older adults: a systematic review and meta-analysis. Maturitas. 2015;80(4):342–58. doi: 10.1016/j.maturitas.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Fort A, Romero D, Bagur C, Guerra M. Effects of whole-body vibration training on explosive strength and postural control in young female athletes. J Strength Cond Res. 2012;26(4):926–36. doi: 10.1519/JSC.0b013e31822e02a5. [DOI] [PubMed] [Google Scholar]
- 9.Gojanovic B, Feihl F, Gremion G, Waeber B. Physiological response to whole-body vibration in athletes and sedentary subjects. Physiol Res. 2014;63(6):779–92. doi: 10.33549/physiolres.932628. [DOI] [PubMed] [Google Scholar]
- 10.Ahlborg L, Andersson C, Julin P. Whole-body vibration training compared with resistance training: effect on spasticity, muscle strength and motor performance in adults with cerebral palsy. J Rehabil Med. 2006;38(5):302–08. doi: 10.1080/16501970600680262. [DOI] [PubMed] [Google Scholar]
- 11.Tankisheva E, Bogaerts A, Boonen S, Feys H, Verschueren S. Effects of intensive whole-body vibration training on muscle strength and balance in adults with chronic stroke: a randomized controlled pilot study. Arch Phys Med Rehabil. 2014;95(3):439–46. doi: 10.1016/j.apmr.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31(1):3–7. doi: 10.1097/00003677-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Cochrane DJ, Stannard SR. Acute whole body vibration training increases vertical jump and flexibility performance in elite female field hockey players. Br J Sports Med. 2005;39(11):860–65. doi: 10.1136/bjsm.2005.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittweger J, Mutschelknauss M, Felsenberg D. Acute changes in neuromuscular excitability after exhaustive whole body vibration exercise as compared to exhaustion by squatting exercise. Clin Physiol Funct Imaging. 2003;23(2):81–86. doi: 10.1046/j.1475-097x.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- 15.Petersen NT, Pyndt HS, Nielsen JB. Investigating human motor control by transcranial magnetic stimulation. Exp Brain Res. 2003;152(1):1–16. doi: 10.1007/s00221-003-1537-y. [DOI] [PubMed] [Google Scholar]
- 16.Pierrot-Desseilligny E, Burke DC. The circuitry of the human spinal cord: Its role in motor control and movement disorders. Cambridge, UK, New York: Cambridge University Press; 2005. 1 online resource (xxii, 642) [Google Scholar]
- 17.Ness LL, Field-Fote EC. Effect of whole-body vibration on quadriceps spasticity in individuals with spastic hypertonia due to spinal cord injury. Restor Neurol Neurosci. 2009;27(6):621–31. doi: 10.3233/RNN-2009-0487. [DOI] [PubMed] [Google Scholar]
- 18.Mileva KN, Bowtell JL, Kossev AR. Effects of low-frequency whole-body vibration on motor-evoked potentials in healthy men. Exp Physiol. 2009;94(1):103–16. doi: 10.1113/expphysiol.2008.042689. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs JV, Horak FB. Cortical control of postural responses. Journal of neural transmission (Vienna, Austria 1996) 2007;114(10):1339–48. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taube W, Gruber M, Gollhofer A. Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiol (Oxf) 2008;193(2):101–16. doi: 10.1111/j.1748-1716.2008.01850.x. [DOI] [PubMed] [Google Scholar]
- 21.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–07. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 22.Lapole T, Temesi J, Gimenez P, Arnal PJ, Millet GY, Petitjean M. Achilles tendon vibration-induced changes in plantar flexor corticospinal excitability. Exp Brain Res. 2015;233(2):441–48. doi: 10.1007/s00221-014-4125-4. [DOI] [PubMed] [Google Scholar]
- 23.Ritzmann R, Gollhofer A, Kramer A. The influence of vibration type, frequency, body position and additional load on the neuromuscular activity during whole body vibration. Eur J Appl Physiol. 2013;113(1):1–11. doi: 10.1007/s00421-012-2402-0. [DOI] [PubMed] [Google Scholar]
- 24.Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res. 1989;76(1):213–22. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- 25.Ritzmann R, Kramer A, Gollhofer A, Taube W. The effect of whole body vibration on the H-reflex, the stretch reflex, and the short-latency response during hopping. Scand J Med Sci Sports. 2013;23(3):331–39. doi: 10.1111/j.1600-0838.2011.01388.x. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong WJ, Nestle HN, Grinnell DC, Cole LD, Van Gilder, Erica L, Warren GS, et al. The acute effect of whole-body vibration on the hoffmann reflex. J Strength Cond Res. 2008;22(2):471–76. doi: 10.1519/JSC.0b013e3181660605. [DOI] [PubMed] [Google Scholar]
- 27.Sayenko DG, Masani K, Alizadeh-Meghrazi M, Popovic MR, Craven BC. Acute effects of whole body vibration during passive standing on soleus H-reflex in subjects with and without spinal cord injury. Neurosci Lett. 2010;482(1):66–70. doi: 10.1016/j.neulet.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y-S, Zhou S. Soleus H-reflex and its relation to static postural control. Gait Posture. 2011;33(2):169–78. doi: 10.1016/j.gaitpost.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Palmieri RM, Ingersoll CD, Hoffman MA. The hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004;39(3):268–77. [PMC free article] [PubMed] [Google Scholar]
- 30.Binder C, Kaya AE, Liepert J. Vibration prolongs the cortical silent period in an antagonistic muscle. Muscle Nerve. 2009;39(6):776–80. doi: 10.1002/mus.21240. [DOI] [PubMed] [Google Scholar]
- 31.Cody FW, Plant T. Vibration-evoked reciprocal inhibition between human wrist muscles. Exp Brain Res. 1989;78(3):613–23. doi: 10.1007/BF00230249. [DOI] [PubMed] [Google Scholar]
- 32.Kipp K, Johnson ST, Doeringer JR, Hoffman MA. Spinal reflex excitability and homosynaptic depression after a bout of whole-body vibration. Muscle Nerve. 2011;43(2):259–62. doi: 10.1002/mus.21844. [DOI] [PubMed] [Google Scholar]
- 33.Lapole T, Deroussen F, Pérot C, Petitjean M. Acute effects of Achilles tendon vibration on soleus and tibialis anterior spinal and cortical excitability. Appl Physiol Nutr Metab. 2012;37(4):657–63. doi: 10.1139/h2012-032. [DOI] [PubMed] [Google Scholar]
- 34.Pearson KG, Gordon JE. Spinal Reflexes. In: Kandel ER, editor. Principles of neural science. 5th ed. New York: McGraw-Hill; 2013. pp. 790–811. [Google Scholar]
- 35.Taube W, Schubert M, Gruber M, Beck S, Faist M, Gollhofer A. Direct corticospinal pathways contribute to neuromuscular control of perturbed stance. J Appl Physiol 1985. 2006;101(2):420–29. doi: 10.1152/japplphysiol.01447.2005. [DOI] [PubMed] [Google Scholar]
- 36.Grey MJ, Bouyer L, Nielsen JB. Neural control of walking. In: Gollhofer A, Taube W, Nielsen JB, editors. Routledge handbook of motor control and motor learning. Routledge international handbooks. Abingdon, Oxon, New York: Routledge; 2012. pp. 197–212. [Google Scholar]
- 37.Taube W, Gollhofer A. Postural control and balance training. In: Gollhofer A, Taube W, Nielsen JB, editors. Routledge handbook of motor control and motor learning. Routledge international handbooks. Abingdon, Oxon, New York: Routledge; 2012. pp. 252–280. [Google Scholar]
- 38.Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007;6(8):725–33. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- 39.Baudry S, Duchateau J. Independent modulation of corticospinal and group I afferents pathways during upright standing. Neuroscience. 2014;275:162–69. doi: 10.1016/j.neuroscience.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 40.Lapole T, Temesi J, Arnal PJ, Gimenez P, Petitjean M, Millet GY. Modulation of soleus corticospinal excitability during Achilles tendon vibration. Exp Brain Res. 2015;233(9):2655–62. doi: 10.1007/s00221-015-4336-3. [DOI] [PubMed] [Google Scholar]
- 41.Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2001;112(4):720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 42.Rossi-Durand C, Jones KE, Adams S, Bawa P. Comparison of the depression of H-reflexes following previous activation in upper and lower limb muscles in human subjects. Exp Brain Res. 1999;126(1):117–27. doi: 10.1007/s002210050721. [DOI] [PubMed] [Google Scholar]
- 43.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zehr EP. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002;86(6):455–68. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]
- 45.Taube W, Leukel C, Schubert M, Gruber M, Rantalainen T, Gollhofer A. Differential modulation of spinal and corticospinal excitability during drop jumps. J Neurophysiol. 2008;99(3):1243–52. doi: 10.1152/jn.01118.2007. [DOI] [PubMed] [Google Scholar]
- 46.Leukel C, Taube W, Rittweger J, Gollhofer A, Ducos M, Weber T, et al. Changes in corticospinal transmission following 8weeks of ankle joint immobilization. Clin Neurophysiol. 2015;126(1):131–39. doi: 10.1016/j.clinph.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Taube W, Leukel C, Nielsen JB, Lundbye-Jensen J. Repetitive activation of the corticospinal pathway by means of rTMS may reduce the efficiency of corticomotoneuronal synapses. Cereb Cortex. 2015;25(6):1629–37. doi: 10.1093/cercor/bht359. [DOI] [PubMed] [Google Scholar]
- 48.Wolters A, Ziemann U, Benecke R. The cortical silent period. In: Wassermann EM, Epstein CM, Ziemann U, editors. The Oxford handbook of transcranial stimulation. Oxford handbooks series. Oxford, New York: Oxford University Press; 2008. pp. 91–102. [Google Scholar]
- 49.Garvey MA, Ziemann U, Becker DA, Barker CA, Bartko JJ. New graphical method to measure silent periods evoked by transcranial magnetic stimulation. Clin Neurophysiol. 2001;112(8):1451–60. doi: 10.1016/s1388-2457(01)00581-8. [DOI] [PubMed] [Google Scholar]
- 50.Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res. 1989;78(1):28–32. doi: 10.1007/BF00230683. [DOI] [PubMed] [Google Scholar]
- 51.Nielsen J, Petersen N, Deuschl G, Ballegaard M. Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. J Physiol. 1993;471:223–43. doi: 10.1113/jphysiol.1993.sp019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–74. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 53.Ritzmann R, Kramer A, Gruber M, Gollhofer A, Taube W. EMG activity during whole body vibration: motion artifacts or stretch reflexes? Eur J Appl Physiol. 2010;110(1):143–51. doi: 10.1007/s00421-010-1483-x. [DOI] [PubMed] [Google Scholar]
- 54.Roelants M, Verschueren Sabine MP, Delecluse C, Levin O, Stijnen V. Whole-body-vibration-induced increase in leg muscle activity during different squat exercises. J Strength Cond Res. 2006;20(1):124–29. doi: 10.1519/R-16674.1. [DOI] [PubMed] [Google Scholar]
- 55.Fleiss JL. Applied probability and statistics. New York: Wiley; 1986. The design and analysis of clinical experiments. Wiley series in probability and mathematical statistics; p. xiv. 432. [Google Scholar]
- 56.Bressel E, Smith G, Branscomb J. Transmission of whole body vibration in children while standing. Clin Biomech (Bristol, Avon) 2010;25(2):181–86. doi: 10.1016/j.clinbiomech.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 57.Pel J J M, Bagheri J, van Dam L M, van den Berg-Emons H J G, Horemans H L D, Stam HJ, et al. Platform accelerations of three different whole-body vibration devices and the transmission of vertical vibrations to the lower limbs. Med Eng Phys. 2009;31(8):937–44. doi: 10.1016/j.medengphy.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Tankisheva E, Jonkers I, Boonen S, Delecluse C, van Lenthe, Harry Druyts G, Hans LJ, et al. Transmission of whole-body vibration and its effect on muscle activation. J Strength Cond Res. 2013;27(9):2533–41. doi: 10.1519/JSC.0b013e31827f1225. [DOI] [PubMed] [Google Scholar]
- 59.Solopova IA, Kazennikov OV, Deniskina NB, Levik YS, Ivanenko YP. Postural instability enhances motor responses to transcranial magnetic stimulation in humans. Neurosci Lett. 2003;337(1):25–28. doi: 10.1016/s0304-3940(02)01297-1. [DOI] [PubMed] [Google Scholar]
- 60.Tokuno CD, Taube W, Cresswell AG. An enhanced level of motor cortical excitability during the control of human standing. Acta Physiol (Oxf) 2009;195(3):385–95. doi: 10.1111/j.1748-1716.2008.01898.x. [DOI] [PubMed] [Google Scholar]
- 61.Lavoie BA, Cody FW, Capaday C. Cortical control of human soleus muscle during volitional and postural activities studied using focal magnetic stimulation. Exp Brain Res. 1995;103(1):97–107. doi: 10.1007/BF00241968. [DOI] [PubMed] [Google Scholar]
- 62.Schubert M, Curt A, Jensen L, Dietz V. Corticospinal input in human gait: modulation of magnetically evoked motor responses. Exp Brain Res. 1997;115(2):234–46. doi: 10.1007/pl00005693. [DOI] [PubMed] [Google Scholar]
- 63.Morita H, Olivier E, Baumgarten J, Petersen NT, Christensen LO, Nielsen JB. Differential changes in corticospinal and Ia input to tibialis anterior and soleus motor neurones during voluntary contraction in man. Acta Physiol Scand. 2000;170(1):65–76. doi: 10.1046/j.1365-201x.2000.00762.x. [DOI] [PubMed] [Google Scholar]
- 64.Schubert M, Beck S, Taube W, Amtage F, Faist M, Gruber M. Balance training and ballistic strength training are associated with task-specific corticospinal adaptations. Eur J Neurosci. 2008;27(8):2007–18. doi: 10.1111/j.1460-9568.2008.06186.x. [DOI] [PubMed] [Google Scholar]
- 65.Zuur AT, Lundbye-Jensen J, Leukel C, Taube W, Grey MJ, Gollhofer A, et al. Contribution of afferent feedback and descending drive to human hopping. J Physiol. 2010;588(Pt 5):799–807. doi: 10.1113/jphysiol.2009.182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pearce AJ, Kidgell DJ. Corticomotor excitability during precision motor tasks. J Sci Med Sport. 2009;12(2):280–83. doi: 10.1016/j.jsams.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Granit R. The functional role of the muscle spindles--facts and hypotheses. Brain. 1975;98(4):531–56. doi: 10.1093/brain/98.4.531. [DOI] [PubMed] [Google Scholar]
- 68.Desmedt JE, Godaux E. Mechanism of the vibration paradox: excitatory and inhibitory effects of tendon vibration on single soleus muscle motor units in man. J Physiol. 1978;285:197–207. doi: 10.1113/jphysiol.1978.sp012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pollock RD, Woledge RC, Martin FC, Di Newham J. Effects of whole body vibration on motor unit recruitment and threshold. J Appl Physiol 1985. 2012;112(3):388–95. doi: 10.1152/japplphysiol.01223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keller M, Pfusterschmied J, Buchecker M, Müller E, Taube W. Improved postural control after slackline training is accompanied by reduced H-reflexes. Scand J Med Sci Sports. 2012;22(4):471–77. doi: 10.1111/j.1600-0838.2010.01268.x. [DOI] [PubMed] [Google Scholar]
- 71.Hodapp M, Vry J, Mall V, Faist M. Changes in soleus H-reflex modulation after treadmill training in children with cerebral palsy. Brain. 2009;132(Pt.1):37–44. doi: 10.1093/brain/awn287. doi: 10.1093/brain/awn287. [DOI] [PubMed] [Google Scholar]
- 72.Bestmann S, Krakauer JW. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp Brain Res. 2015;233(3):679–89. doi: 10.1007/s00221-014-4183-7. [DOI] [PubMed] [Google Scholar]
- 73.Iglesias C, Nielsen JB, Marchand-Pauvert V. Corticospinal inhibition of transmission in propriospinal-like neurones during human walking. Eur J Neurosci. 2008;28(7):1351–61. doi: 10.1111/j.1460-9568.2008.06414.x. [DOI] [PubMed] [Google Scholar]
- 74.Catano A, Houa M, Noël P. Magnetic transcranial stimulation: clinical interest of the silent period in acute and chronic stages of stroke. Electroencephalogr Clin Neurophysiol. 1997;105(4):290–96. doi: 10.1016/s0924-980x(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 75.Liepert J, Binder C. Vibration-induced effects in stroke patients with spastic hemiparesis - a pilot study. Restor Neurol Neurosci. 2010;28(6):729–35. doi: 10.3233/RNN-2010-0541. [DOI] [PubMed] [Google Scholar]
- 76.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18(1):111–29. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 77.Tucker KJ, Türker KS. Muscle spindle feedback differs between the soleus and gastrocnemius in humans. Somatosens Mot Res. 2004;21(3-4):189–97. doi: 10.1080/08990220400012489. [DOI] [PubMed] [Google Scholar]
- 78.Bosco C, Colli R, Introini E, Cardinale M, Tsarpela O, Madella A, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19(2):183–87. doi: 10.1046/j.1365-2281.1999.00155.x. [DOI] [PubMed] [Google Scholar]


