Abstract
Animal models of relapse reveal that the motivation to seek drug is regulated by enduring morphological and physiological changes in the nucleus accumbens, as well as transient synaptic potentiation in the accumbens core (NAcore) that parallels drug-seeking behavior. The current study sought to examine the link between the behavioral and synaptic consequences of cue-induced cocaine seeking by optically silencing glutamatergic afferents to the NAcore from the prelimbic cortex (PL). Adeno-associated virus coding for the inhibitory opsin archaerhodopsin was microinjected into PL, and optical fibers were targeted to NAcore. Animals were trained to self-administer cocaine followed by extinction training, and then underwent cue-induced reinstatement in the presence or absence of 15 min of optically-induced inhibition of PL fibers in NAcore. Inhibiting the PL-to-NAcore projection blocked reinstated behavior and was paralleled by decreased dendritic spine head diameter and AMPA/NMDA ratio relative to sham-laser control rats. Interestingly, while spine density was elevated after extinction training, no further effects were observed by cued reinstatement or optical inhibition. These findings validate the critical role for PL afferents to the NAcore in simultaneously regulating both reinstated behavior and the associated transient synaptic potentiation.
The vulnerability to relapse is linked to enduring adaptations in brain circuits regulating motivated behavior (Koob and Volkow 2010). Pathological long-lasting adaptations in synaptic plasticity are thought to be responsible for the propensity of addicts to relapse after extended periods of abstinence (Robinson and Kolb 2004; Russo et al. 2010). The nucleus accumbens is a critical point of convergence for computing motivationally-relevant information, and considerable evidence shows drug-induced structural and functional changes in glutamatergic synapses on accumbens medium spiny neurons (MSNs) (Luscher and Malenka 2011; Smith et al. 2013; Wolf 2010). Specifically, after withdrawal from cocaine or nicotine, but not heroin, there is a marked and enduring synaptic potentiation at MSN excitatory synapses.
In addition to enduring, constitutive changes in accumbens synapses induced by repeated drug use, profound transient synaptic potentiation (t-SP) occurs in MSNs in the core subcompartment of the accumbens (NAcore) when cocaine-, heroin- or nicotine-seeking behavior is reinstated by conditioned cues, context or a noncontingent drug injection (Gipson et al. 2013a; Gipson et al. 2013b; Shen et al. 2011; Shen et al. 2014a; Stankeviciute et al. 2013). Thus, cue-reinstated behavior initiates a rapid, transient increase in spine head diameter in the NAcore, and this morphological potentiation in MSN dendrites is paralleled by an increase in AMPA glutamate receptor currents, as estimated the AMPA/NMDA ratio. The reinstatement of cocaine-seeking requires neuronal activity in the prelimbic cortex (PL) and NAcore (Fuchs et al. 2007; McGinty et al. 2010)(Cornish and Kalivas, 2000; McFarland et al., 2003; Kalivas et al., 2005), and recent optogenetic data specifically supports a role for the PL-to-NAcore projection in cocaine-induced reinstatement (Stefanik et al. 2013b). However, it is not known if PL afferents mediate cue-induced reinstatement and the accompanying t-SP produced in NAcore MSNs. We used an inhibitory optogenetic strategy to show that PL axons and terminals in NAcore mediate both cued reinstatement of cocaine seeking and t-SP in NAcore MSNs.
MATERIALS AND METHODS
Animal housing and surgery
All methods used were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Medical University of South Carolina’s Institutional Animal Care and Use Committee. Male Sprague Dawley rats (250–300g, Charles River Laboratories) were individually housed under temperature- and humidity-controlled conditions with a 12 hour reverse light/dark cycle (lights on at 6:00 P.M.). Rats were fed ad libitum until 7 days post-surgery, after which food was restricted to 20 g of chow pellets per day.
Following one week of handling and acclimation, rats underwent surgery for injection of AAV, implantation of fiber optics, and implantation of indwelling jugular catheters. Animals were anesthetized with ketamine HCl (87.5 mg/kg, i.m.) and xylazine (5 mg/kg, i.m.). Ketorolac (3mg/kg, i.p.) was administered before surgery to provide analgesia. Intra-jugular catheters were implanted as previously described (LaLumiere et al. 2012). Catheters were flushed daily with cefazolin (0.2 mL of 0.1 g/mL) and heparin (0.2 mL of 100 IU) for one week, then daily with heparin for the remainder of the experiment to maintain catheter patency.
For virus injections, 0.7 µl of virus (rAAV2-CAG-ArchT-GFP or rAAV2-CMV-GFP, ~1012 viral particles/ml) was delivered bilaterally through 33 gauge needles (0.14 µL/min for 5 minutes). Needles were left in place for 10 minutes following injection to allow for virus diffusion away from injection site. Virus injection into PL was made at the following stereotaxic coordinates relative to Bregma: PL: - 3.1 mm anteroposterior, +2.0 mm mediolateral, −4.0 mm dorsoventral (12° angle). An optic fiber (Precision Fiber) was implanted bilaterally 0.5mm dorsal to the NAcore at +1.5 mm anteroposterior, + 3.0 mm mediolateral, −6.0 dorsoventral (10° angle). Fibers were secured to the skull using small screws and dental acrylic and animals recovered for one week before behavioral testing.
Self-administration, extinction and reinstatement procedures
Self-administration, extinction, and reinstatement procedures occurred in standard operant chambers equipped with two retractable levers, a house light, cue light, and 2900 Hz tone generator (Med Associates). Before cocaine self-administration training, animals were food deprived for 24 hours and then underwent a single 15 hour food training session in which presses on the active lever resulted in the delivery of a single food pellet (45mg, Noyes) on a fixed-ratio 1 (FR1) schedule of reinforcement. Following food training, animals were restricted to 20g of food per day, given immediately after the behavioral session, for the remainder of the experiment. One day later, animals began 2-hour sessions cocaine self-administration on an FR1 schedule with a 20 second time out. Each active lever press resulted in a 0.05 ml infusion of 0.20 mg cocaine (~15–20mg/kg per animal total over 2-hour session, dissolved in 0.9% sterile saline, NIDA) and the drug-paired cues (concurrent illumination of the stimulus light above the active lever and tone) for 5 seconds. Active lever presses made during the time out were counted but did not result in drug delivery and inactive lever presses were of no consequence. Rats underwent self-administration 6 days/week for at least 2 weeks (minimum of 12 days), until they met maintenance criteria of ≥10 infusions of cocaine over 10 days, as well as discrimination between active and inactive levers (>75% lever presses on active lever). A total of 2 rats not reaching these criteria after 4 weeks were excluded from the study. A subset of animals served as yoked-saline controls.
Following successful acquisition and maintenance of cocaine self-administration, extinction training (2 hours/day) began. During extinction, presses on the previously active lever were recorded but no longer produced drug or presentation of the drug-paired cues. All rats underwent at least 10 days of extinction, until active lever pressing fell to <30% of the average responding during self-administration. Animals were habituated to the fiber optic leashes for ≥3 sessions of both self-administration and extinction.
During the reinstatement sessions, active lever presses produced the light/tone drug-paired cues that had been presented during self-administration, but no drug was delivered. Animals used for dendritic spine or electrophysiological quantification were removed from the boxes and promptly sacrificed after 15 minutes of the reinstatement session. All animals used in 2-hour behavioral tests underwent two reinstatement sessions, counterbalanced with respect to whether illumination was given for the first 15 minutes.
Optical inhibition
Optical inhibition for behavioral experiments was delivered as previously described (Stefanik et al. 2013a; Stefanik et al. 2013b). Briefly, chronically implantable optical fibers were housed inside stainless steel ferrules (for construction details, see (Sparta et al. 2012)) and implanted bilaterally ~0.5mm dorsal to the site intended to receive light. To permit bilateral inhibition, the single end of 2×1 fiber splitter (Precision Fiber) was connected via FC/PC connection to a rotating optical commutator, which was then attached via a fiber to a laser (diode-pumped solid-state, 200mW, 561nm multimode FC/PC fiber coupler connection, OEM Laser Systems). The two split ends of the fiber splitter were threaded through a metal leash, and epoxied into stainless steel ferrules which were then connected to the animal’s head via ceramic sleeves. Light output was measured with an optical power meter and adjusted to ~7 mW of 561 nm light. Based on in vivo measurements, of light output in mammalian brain tissue, these parameters would be expected to provide sufficient light to at least 0.4mm3 of tissue (Yizhar et al. 2011). Light was applied continuously for the first 15 minutes of the reinstatement session. This method of delivery has previously been shown to inhibit neuronal firing without significantly desensitizing the opsin (Huff et al. 2013; Stefanik et al. 2013a; Stefanik et al. 2013b; Tsunematsu et al. 2013).
Immunohistochemistry and imaging
For immunohistochemistry and imaging, animals were anesthetized with pentobarbital (100 mg/ml, i.p.) and then transcardially perfused with phosphate- buffered saline (PBS, pH 7.4) followed by PBS containing 4% (w/v) paraformaldehyde. Brains were post-fixed for 24 hours at room temperature in the perfusion solution. Coronal sections (75 µm thick) were incubated for 60 minutes in 1% hydrogen peroxide, rinsed 3 times in PBS, and then incubated overnight in PBS containing 0.25% triton-X, 0.01% sodium azide, and anti-GFP (rabbit, 1:50,000, Abcam) antibody. Sections were then rinsed once in PBS and incubated for 30 minutes in PBS containing the biotinylated secondary antibody (donkey, 1:1,000, Jackson Immunoresearch) for 30 minutes, rinsed 4 times in PBS, and incubated for 1 hour in an ABC Kit (Vector Labs). Sections were then rinsed once in PBS and incubated in PBS with 0.05% diaminobenzidine with 0.05% hydrogen peroxide for ~5 minutes. Slices were then mounted and ArchT expression was visualized on a light microscope.
Measurement of Spine Morphology
Rats were deeply anesthetized with ketamine HCl (87.5 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.). Transcardial perfusions with phosphate buffered saline (PBS) followed by 1.5% paraformaldehyde (PFA) in PBS. Brains were removed and post-fixed in the same fixative for 30 minutes, then coronally sectioned at 200 µm in PBS on a vibratome.
Diolistic labeling
Tungsten particles (1.3 µm diameter, Bio-Rad) were coated with the lipophilic carbocyanine dye DiI (Invitrogen). DiI-coated particles were delivered diolistically into the tissue at 80 PSI using a Helios Gene Gun system (Bio-Rad) fitted with a polycarbonate filter with a 3.0 µm pore size (BD Biosciences). DiI was allowed to diffuse along neuron axons and dendrites in PBS for 24 hours at 4° C, and then fixed again in a 4% PFA for 1 hour at room temperature. After a brief PBS wash, tissue was mounted onto slides in aqueous medium Prolong Gold (Invitrogen).
Confocal imaging and 3D reconstruction
All dendritic spine quantification procedures were similar to those previously described by our laboratory (Shen et al. 2008; Shen et al. 2009). Briefly, images of DiI-labeled sections were taken on a confocal microscope (LEICA) using a Helium/Neon 543 nm laser. Images of labeled neurons and segments were acquired via optical sectioning using a 63x oil immersion objective with pixel size 0.01 µm in the XY-plane and 0.13 µm intervals along the Z-axis. Segments meeting criteria were randomly selected and during imaging, each segment file was coded so that subsequent analyses would be blind. Images were deconvolved prior to analysis and a 3D perspective was rendered by the Surpass module of the Imaris software package (Bitplane). Only spines on dendrites beginning >75 µm and ending <200 µm distal to the soma and after the first branch point were quantified on cells localized to the NAcore. The length of quantified segments was 45–55 µm. Five to twelve neurons were analyzed from each animal, one segment from each neuron, and the minimum spine head diameter was set at 0.15 µm.
Electrophysiological measurements
Slice preparation for electrophysiology
Rats were anesthetized with ketamine HCl (100 mg/kg Ketaset, Fort Dodge Animal Health, Iowa) and decapitated. The brain was removed from the skull and 220µm thick coronal NAc sections were obtained using a vibratome (VT1200S Leica vibratome; Leica Microsystems, Wetzlar, Germany). Slices were immediately placed into a vial containing artificial cerebrospinal fluid (aCSF) (in mM: 126 NaCl, 1.4 NaH2PO4, 25 NaHCO3, 11 glucose, 1.2 MgCl2, 2.4 CaCl2, 2.5 KCl, 2.0 NaPyruvate, 0.4 ascorbic acid, bubbled with 95% O2 and 5% CO2) and a mixture of 5 mM kynurenic acid and 50 µM D-(-)-2-Amino-5-phosphonopentanoic acid (D-AP5). Slices were incubated at room temperature until recording.
In vitro whole cell recording
All recordings were collected at 32°C (controlled by TC-344B, Warner Instrument Corporation, Hamden, Connecticut) in the dorsomedial NAcore. Inhibitory synaptic transmission was blocked with picrotoxin (50 µM). Multiclamp 700B (Axon Instruments, Union City, CA) was used to record excitatory postsynaptic currents (EPSCs) in whole cell patch-clamp configuration. Glass microelectrodes (1–2 MΩ) were filled with cesium-based internal solution (in mM: 124 cesium methanesulfonate, 10 HEPES potassium, 1 EGTA, 1 MgCl2, 10 NaCl, 2.0 MgATP, and 0.3 NaGTP, 1 QX-314, pH 7.2–7.3, 275 mOsm). Data were acquired at 10 kHz, and filtered at 2 kHz using AxoGraph X software (AxoGraph Scientific, Sydney, Australia). To evoke EPSCs a bipolar stimulating electrode (FHC, Bowdoin, Maine) was placed ~300 µm dorsomedial to the recorded cell to maximize chances of stimulating prelimbic afferents. The stimulation intensity was set to evoke an EPSC of 200–500 pA which was usually 30–70% of maximal EPSC. Recordings were collected every 20 sec. Series resistance (Rs) measured with a 2 mV hyperpolarizing step (10 ms) given with each stimulus and holding current were always monitored online. Recordings with unstable Rs, or when Rs exceeded 10 MΩ were aborted.
Measuring the AMPA/NMDA ratio
Recordings started no earlier than 10 min after the cell membrane was ruptured, to allow diffusion of the internal solution into the cell. AMPA currents were first measured at −80 mV to ensure stability of response. The membrane potential was then gradually increased to +40 mV. Recording of currents resumed 5 min after reaching +40 mV to allow stabilization of cell parameters. Currents composed of both AMPA and NMDA currents were then obtained. Then D-AP5 was bath-applied (50 µM) to block NMDA currents and recording of AMPA currents at +40 mV was started after 2 min. NMDA currents were obtained by subtracting the AMPA currents from the total current at +40 mV.
Data analysis
Statistics were performed using Prism (GraphPad Software). Reinstatement sessions were compared between extinction pressing, cue-induced reinstatement without laser, and cue-induced reinstatement with laser, using a two-tailed paired Student’s t-test or a one-way ANOVA with probability values adjusted according to the method of Bonferroni. All spine density and diameter data were first analyzed by averaging the values for all segments counted in all animals and again by obtaining the mean for all segments obtained each individual animal and treating each animal as an individual data point (Figure 2C and D, right and left panels). For electrophysiological measurements data were analyzed as individual cells from >4 animals per group. Significance was set at p ≤ 0.05 and data are presented as mean ± SEM.
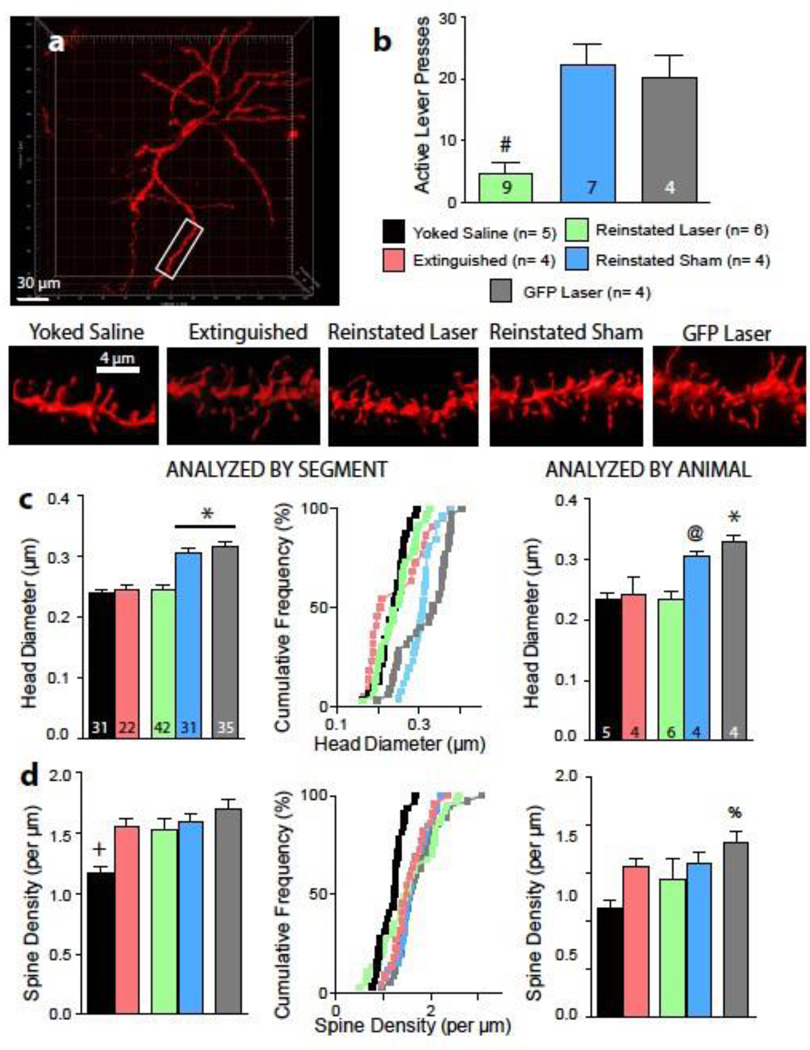
Figure 2. Optical inhibition of PL-to-NAcore projection reduces spine head diameter.
A) Representative neuron and dendrites from diolistically labeled NAcore neurons representative of each condition. B) Fifteen minutes of optical inhibition of the PL-to-NAcore projection reduced reinstated lever pressing. Numbers in bars represent number of animals in each condition, and include animals used for experiments in figures 2 and 3. C) Optical inhibition for 15 minutes significantly reduces spine head diameter in cocaine-treated animals. Numbers in left bar graph are representative of the number of neurons analyzed in each condition, numbers in right bar graph are of the same data, analyzed by each animal’s mean. Data are shown as mean ± SEM (left panel) or as cumulative frequency (center panel). D) Cocaine experience significantly increases spine density in the NAcore. Data are shown as mean ± SEM (left and right panels) or as cumulative frequency (center panels).
# p< 0.05, comparing reinstated laser to all other groups.
* p< 0.05, comparing reinstated sham or GFP laser to yoked saline, extinction, and reinstated laser groups.
+ p< 0.05, comparing yoked saline with all other treatment groups
@ p<0.05, comparing reinstated sham with yoked saline and reinstated laser groups
% p< 0.05 comparing GFP laser and yoked saline groups
RESULTS
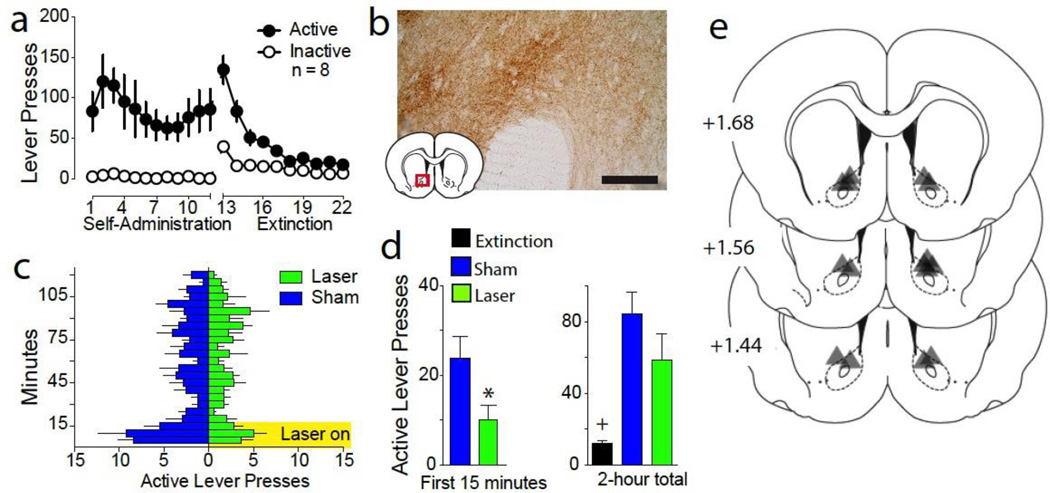
An AAV coding for the light-activated inhibitory proton pump ArchT (Chow et al. 2010) or a control virus expressing GFP only was microinjected into the PL and fiber optics were implanted above projection terminals in the NAcore. Following self-administration and extinction training (Figure 1A), animals underwent cue-induced reinstatement in the presence or absence of light delivered to the NAcore using a counterbalanced, within-subjects design. Figure 1B shows DAB staining for ArchT-GFP-expressing terminal fibers and boutons in the NAcore. Optical activation of ArchT reduced active lever pressing compared to sham animals (no laser delivery) (Figure 1C, 1D, Student’s t(7)= 2.27, p= 0.029). Over the 2-hour reinstatement session statistically equivalent reinstatement was observed in both sham and laser groups compared to extinguished lever pressing (Figure 1D, F(2,7)= 10.74, p= 0.007). We previously reported that optical inhibition of the NAcore does not alter locomotion and animals receiving a control GFP virus show no difference in lever pressing regardless of light delivery (Stefanik and Kalivas 2013; Stefanik et al. 2013a; Stefanik et al. 2013b). Figure 1E shows the location of optical fiber termination in the NAcore. The area of illumination was estimated from the tip of the histologically identified fiber tip to expand in a cone shape for ~0.5 mm from the most ventral penetration (Yizhar et al. 2011). Fiber implantations in the NAcore were localized primarily to the dorsal portion of the nucleus.
Figure 1. 15-minute inhibition of the PL-to-NAcore projection reduces reinstatement.
A) Lever pressing during self-administration and extinction corresponding to the data in figure 1 (n = 8). B) Immunoreactivity of ArchT expressing fibers in the NAc after virus injection in the PL. C) Reinstatement sessions for laser and sham were conducted using a randomized cross-over design, and active lever pressing broken down into 5-minute bins to reveal the inhibition of behavior while laser is on for 15 min. D) Optical inhibition of PL fibers in NAcore significantly reduced lever pressing during the first 15 minutes of the reinstatement session, which was not manifested over the entire 2-hour session. E) Location of bilateral fiber tips in the NAcore of animals examined in figure 1, according to the atlas of Paxinos (Paxinos and Watson 2007).
* p< 0.05, comparing reinstated laser and sham groups.
+ p< 0.05, comparing extinction with laser and sham
A separate group of animals was treated identically to rats described above but sacrificed after 15 minutes of cue-induced reinstatement in the presence or absence of optical inhibition. We quantified synaptic plasticity as changes in dendritic spine morphology and AMPA/NMDA at NAcore MSN synapses. Neurons were diolistically labeled with the lipophilic dye DiI, and dendritic spine density and spine head diameter were quantified from three-dimensional confocal images of dendrite segments of labeled neurons (Figure 2A). Cue-induced reinstatement was blocked in animals receiving optical inhibition (Figure 2B, F(2,17) = 14.39, p = 0.0002). Optical inhibition of the PL projection to the NAcore in the ArchT-expressing rats elicited a parallel inhibition of the increase in spine head diameter produced by cued reinstatement (Figure 2C, F(4,147) = 20.06, p < 0.0001). Thus, sham animals and GFP controls had larger mean head diameter than animals exposed to 15 min of cue reinstatement plus light delivery, yoked saline controls, or animals extinguished from cocaine self-administration. While cocaine exposed animals showed a significant increase in spine density relative to yoked-saline controls (Figure 2D, F(4,147) = 6.900, p < 0.0001), optical inhibition failed to alter spine density. Thus, the increase in spine density after extinction from cocaine self-administration was not altered by 15 min of cued reinstatement, regardless of whether the PL afferents to the NAcore optically inhibited or underwent sham inhibition. Dendritic morphology was also unaltered by light delivery in the GFP control animals. Additional analyses were conducted averaging all neurons for each animal (Figure 2C, 2D, right panels). These analyses confirmed the significant reduction in spine head diameter from optical inhibition (F (4,18) = 8.894, p = 0.0004) observed in the segment analysis and suggest a trend toward a cocaine exposure-induced increase in dendritic spine density (F (4,18) = 2.2528, p = 0.077).
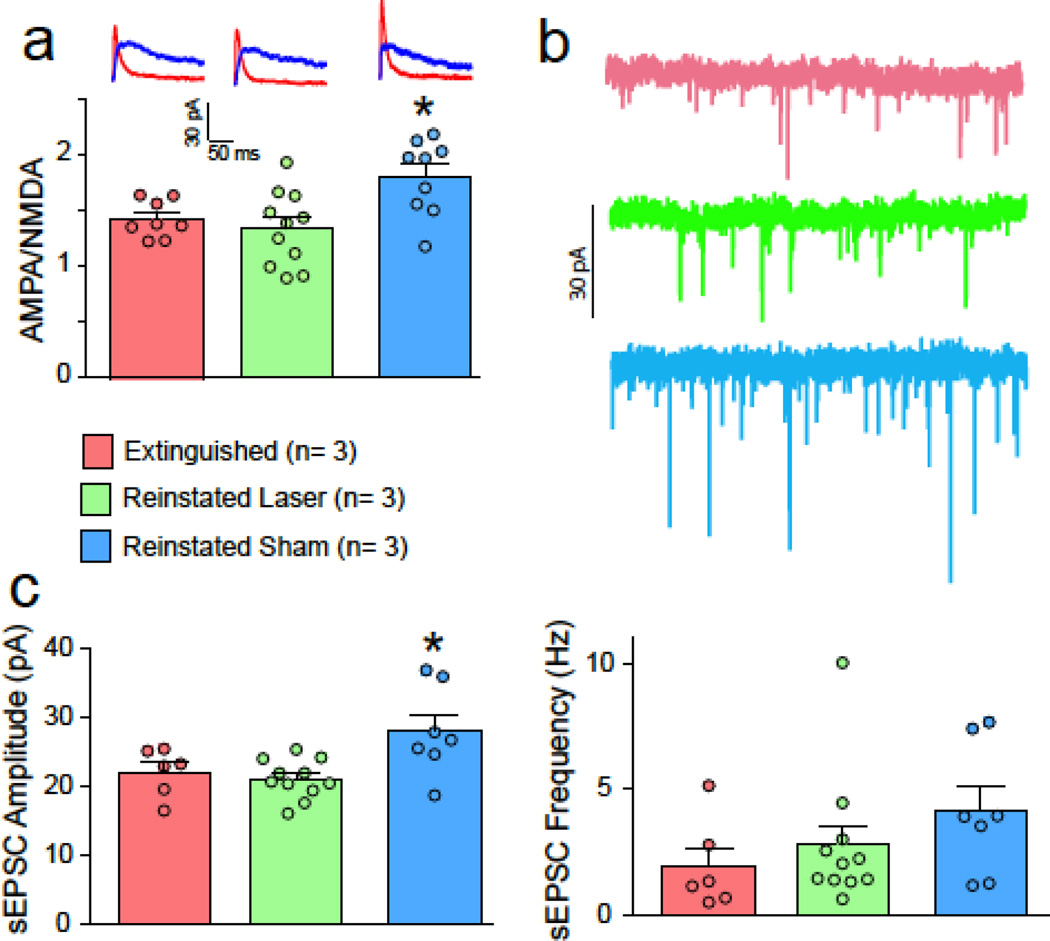
Paralleling spine head diameter, cue-induced reinstatement induced an increase in AMPA/NMDA in NAcore MSNs that was prevented by optically inhibiting PL afferents (Figure 3A, F(2,34) = 9.37, p < 0.001). Similarly, the sEPSC amplitude was elevated by cued reinstatement and reduced by optical inhibition (Figure 3B, F(2,21) = 5.95, p = 0.009). However, there was no difference in sEPSC frequency between treatment groups (Figure 3B, F(2,21) = 1.37, p = 0.276).
Figure 3. Optical inhibition of PL-to-NAcore projection prevents synaptic potentiation.
A) Increase in AMPA/NMDA after cue-induced reinstatement was blocked by 15 min optogenetic inhibition of PL terminals in the NAcore. Data shown as mean ± SEM ratio for each treatment group, with individual cells indicated in smaller circles. N refers to number of animals. Representative traces are shown for each treatment in the left panel. B) sEPSC amplitude was increased in the sham but not the laser group, supporting a postsynaptic potentiation mechanism. C) sEPSC frequency was not different between groups.
*p< 0.05, comparing reinstated sham to the other groups.
DISCUSSION
Cue-induced reinstatement of cocaine seeking is associated with t-SP in the NAcore during the first 15 min of reinstated behavior, and the intensity of cocaine seeking (active lever pressing) is correlated with the size of t-SP, as measured by spine head diameter and AMPA/NMDA (Gipson et al. 2013a). While pharmacological inactivation previously revealed that t-SP depends on neuronal activity in the PL (Gipson et al. 2013a), it is possible that other projections to the NAcore could have been responsible for observed results. The optogenetic strategy we employed allows for pathway-restricted and temporally precise manipulation of neurons within intact neural circuits (Adamantidis et al. 2011; Huff et al. 2013; Stuber et al. 2011; Tsai et al. 2009), and validated the necessity of PL projections to NAcore.
Since the peak behavioral response for cocaine seeking elicited by conditioned cues is in the first 15 min of the reinstatement session, we used 15 min of optical stimulation to selectively inhibit PL axons and terminals in the NAcore to show that these inputs were necessary for both reinstated behavior and t-SP. Thus, inhibiting PL terminals in the NAcore blocked cue-induced potentiation of spine head diameter and the AMPA/NMDA ratio in MSNs. Combined with the previously observed positive correlation between reinstated behavior and t-SP (Gipson et al. 2013a), these data further support the argument that PL afferents in NAcore are necessary for reinstated drug seeking and initiating t-SP. Moreover, t-SP occurs during reinstatement to different classes of drugs of abuse by different stimuli, including cue-induced nicotine seeking, heroin-induced heroin seeking, and cue-, context- and cocaine-induced cocaine seeking (Gipson et al. 2013b; Shen et al. 2011; Shen et al. 2014a; Stankeviciute et al. 2014). The fact that t-SP generalizes across different classes of addictive drug, and does not occur during reinstated sucrose seeking argues that t-SP is a form of neuroplasticity that is induced by addictive drugs, not natural rewards, and may be a reliable biological marker of reinstated or context-induced drug seeking.
While our data focus on t-SP induced by PL input to NAcore, it is important to note that multiple glutamatergic afferents provide excitatory drive to the NAcore that can facilitate reward seeking. Recent work using optogenetic stimulation of these pathways in the accumbens shell found that sufficient stimulation of different afferents was capable of eliciting cellular responses and reinforcing instrumental behavior (Britt et al. 2012). Moreover, optogenetic inhibition of glutamatergic amygdala inputs to the NAcore inhibits cue-induced reinstatement (Stefanik and Kalivas, 2013), akin to what was previously shown using a pharmacological inhibition strategy (Di Ciano and Everitt 2004). Based on this evidence, it is possible that the source of glutamate may be less important than the amount of released glutamate needed to reach a threshold to initiate behavior. One hypothesis is that for drug seeking, this threshold is reached when spillover of synaptically released glutamate excessively stimulates extrasynaptic receptors, such as the metabotropic glutamate 5 receptor (mGluR5) and NMDA receptors expressing the GluN2B subunit. Supporting this idea, self-administration of all drugs abuse examined to date produce a constitutive down-regulation of the glial glutamate transporter GLT-1 (Alhaddad et al. 2014; Gipson et al. 2013b; Reissner et al. 2014; Sari et al. 2011; Shen et al. 2014b), and a corresponding transient spillover of synaptic glutamate in NAcore during reinstatement, including alcohol, cocaine, methamphetamine, nicotine and heroin. Moreover, inhibiting either mGluR5 or GluN2B-containing NMDA receptors in the accumbens blocks reinstated cocaine or heroin seeking (Olive 2009; Shen et al. 2011; Shen et al. 2014b). Thus, initiating t-SP and reinstating behavior may not depend on the origin of glutamatergic afferents to the NAcore, and only require that the afferent release sufficient glutamate to overwhelm impaired glutamate transport, and thereby stimulate extrasynaptic mGluR5 and/or GluN2B receptors.
Consistent with previous work, we found that repeated cocaine administration elevated spine density of NAcore MSNs when data were analyzed by neuron number, and a strong trend towards an elevation when analyzed according to animal (Dietz et al. 2012; Dumitriu et al. 2012; Ferrario et al. 2005; Li et al. 2012; Robinson and Kolb 2004). However, cue-induced reinstatement did not alter the density of spines on MSN dendrites. Also, unlike the transient increase in spine head diameter and AMPA/NMDA induced by cocaine-conditioned cues, the 15- minute timeframe of optical inhibition was insufficient to alter the spine density. This profile of morphological and electrophysiological plasticity is consistent with the idea that changes in spine density tend to be more constitutive and indicative of enduring changes in synaptic strength, while changes in spine head diameter and AMPA receptor trafficking can occur rapidly and transiently (Bhatt et al. 2009; Golden and Russo 2012).
Our data definitively show that PL inputs to NAcore mediate cue-induced t-SP and the associated reinstatement of cocaine seeking. We also show that t-SP includes a PL-dependent increase in spine head diameter and AMPA/NMDA, but not spine density. In future studies it will be important to determine if the induction of t-SP is a property only of PL glutamatergic afferents, or if other afferents to the NAcore are also necessary for t-SP, as they are for cue-induced reinstatement of cocaine seeking.
Acknowledgments
This work was supported by National Institutes of Health T32 DA7288 and Grants DA015369, DA012513, and DA003906 (P.W.K.).
Footnotes
Author contributions: M.T.S. and P.W.K. designed research; M.T.S. and Y.M.K. performed research; M.T.S., Y.M.K., and P.W.K. analyzed data; M.T.S. and P.W.K. wrote the paper.
REFERENCES
- Adamantidis AR, et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nature Neuroscience. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, et al. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013a;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2013b;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Russo SJ. Mechanisms of psychostimulant-induced structural plasticity. Cold Spring Harbor perspectives in medicine. 2012;2 doi: 10.1101/cshperspect.a011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, Miller RL, Deisseroth K, Moorman DE, LaLumiere RT. Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3597–3602. doi: 10.1073/pnas.1219593110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. The European journal of neuroscience. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu N, Lu K, Zhang L, Gu J, Guo F, An S. Cocaine-induced dendritic remodeling occurs in both D1 and D2 dopamine receptor-expressing neurons in the nucleus accumbens. Neuroscience Letters. 2012;517:118–122. doi: 10.1016/j.neulet.2012.04.040. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th. Burlington: Elsevier Academic Press; 2007. [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addiction biology. 2014 doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;(47 Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends in neurosciences. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol and alcoholism. 2011;46:239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sesack SR, Toda S, Kalivas PW. Automated quantification of dendritic spine density and spine head diameter in medium spiny neurons of the nucleus accumbens. Brain structure & function. 2008;213:149–157. doi: 10.1007/s00429-008-0184-2. [DOI] [PubMed] [Google Scholar]
- Shen H-W, Gipson CD, Huits M, Kalivas PW. Prelimbic cortex and ventral tegmental area modulate synaptic plasticity in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology. 2014a doi: 10.1038/npp.2013.318. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Scofield MD, Boger H, Hensley M, Kalivas PW. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci. 2014b;34:5649–5657. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Current opinion in neurobiology. 2013;23:546–552. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Stamatakis AM, Phillips JL, Hovelso N, van Zessen R, Stuber GD. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nature protocols. 2012;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankeviciute NM, Scofield MD, Kalivas PW, Gipson CD. Rapid, transient potentiation of dendritic spines in context-induced relapse to cocaine seeking. Addiction biology. 2013 doi: 10.1111/adb.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankeviciute NM, Scofield MD, Kalivas PW, Gipson CD. Rapid, transient potentiation of dendritic spines in context-induced relapse to cocaine seeking. Addiction biology. 2014 doi: 10.1111/adb.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Frontiers in behavioral neuroscience. 2013;7:213. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Brown RM, Kalivas PW. Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013a;33:13654–13662. doi: 10.1523/JNEUROSCI.1570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, et al. Optogenetic inhibition of cocaine seeking in rats. Addiction biology. 2013b;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu T, Tabuchi S, Tanaka KF, Boyden ES, Tominaga M, Yamanaka A. Long-lasting silencing of orexin/hypocretin neurons using archaerhodopsin induces slow-wave sleep in mice. Behavioural brain research. 2013 doi: 10.1016/j.bbr.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends in neurosciences. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]