Abstract
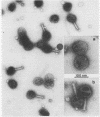
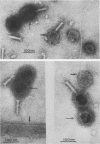
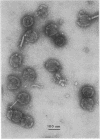
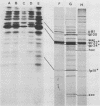
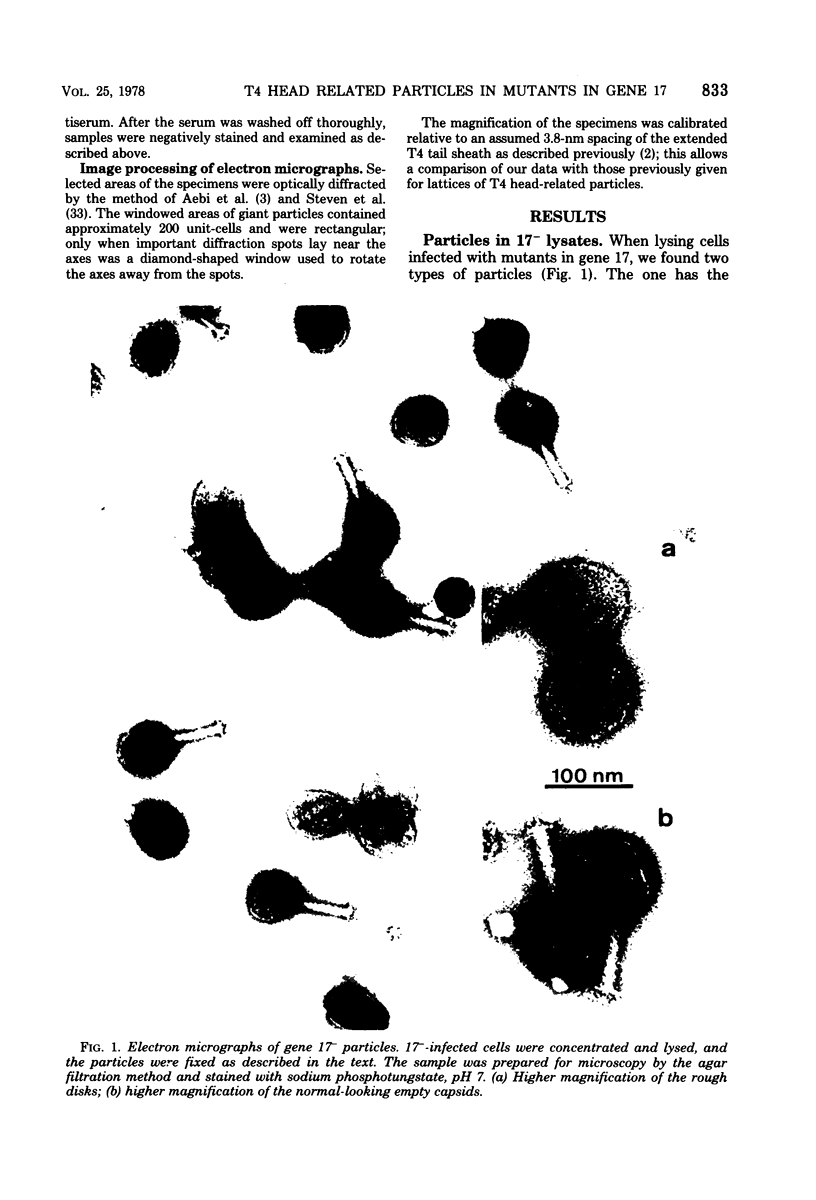
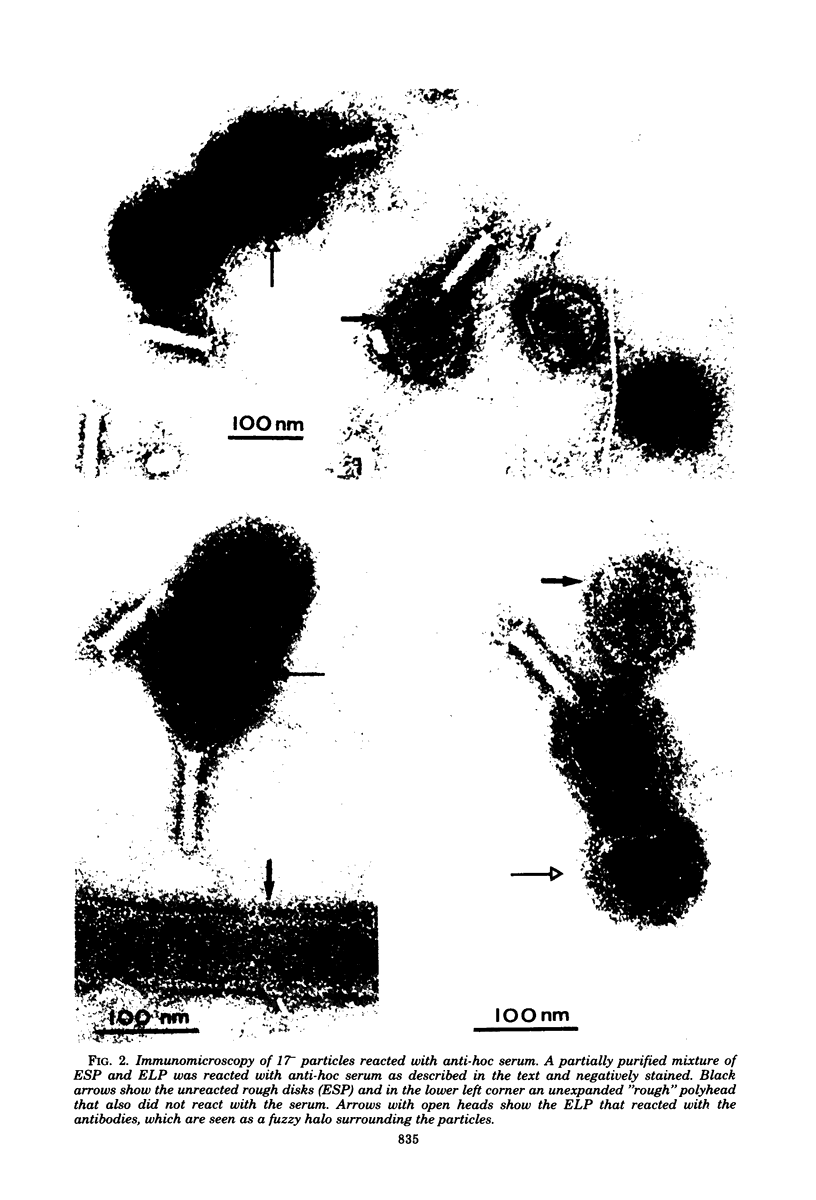
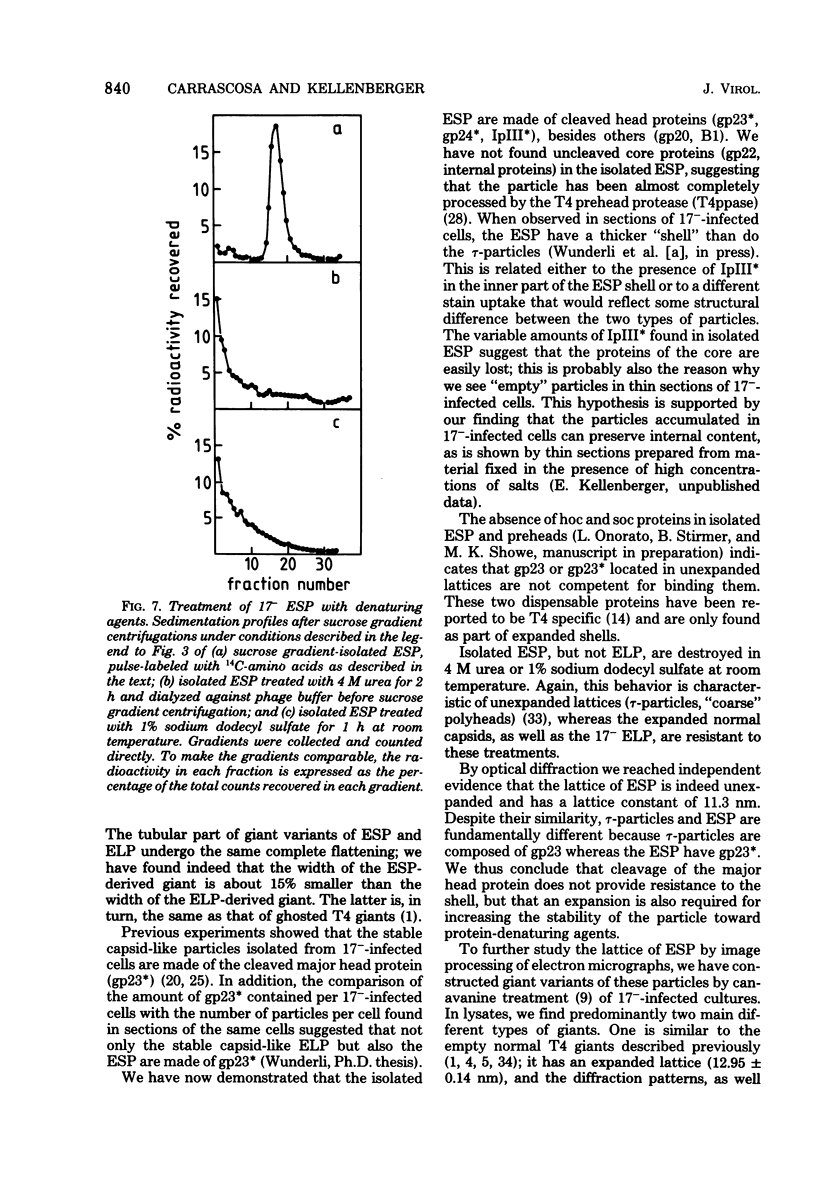
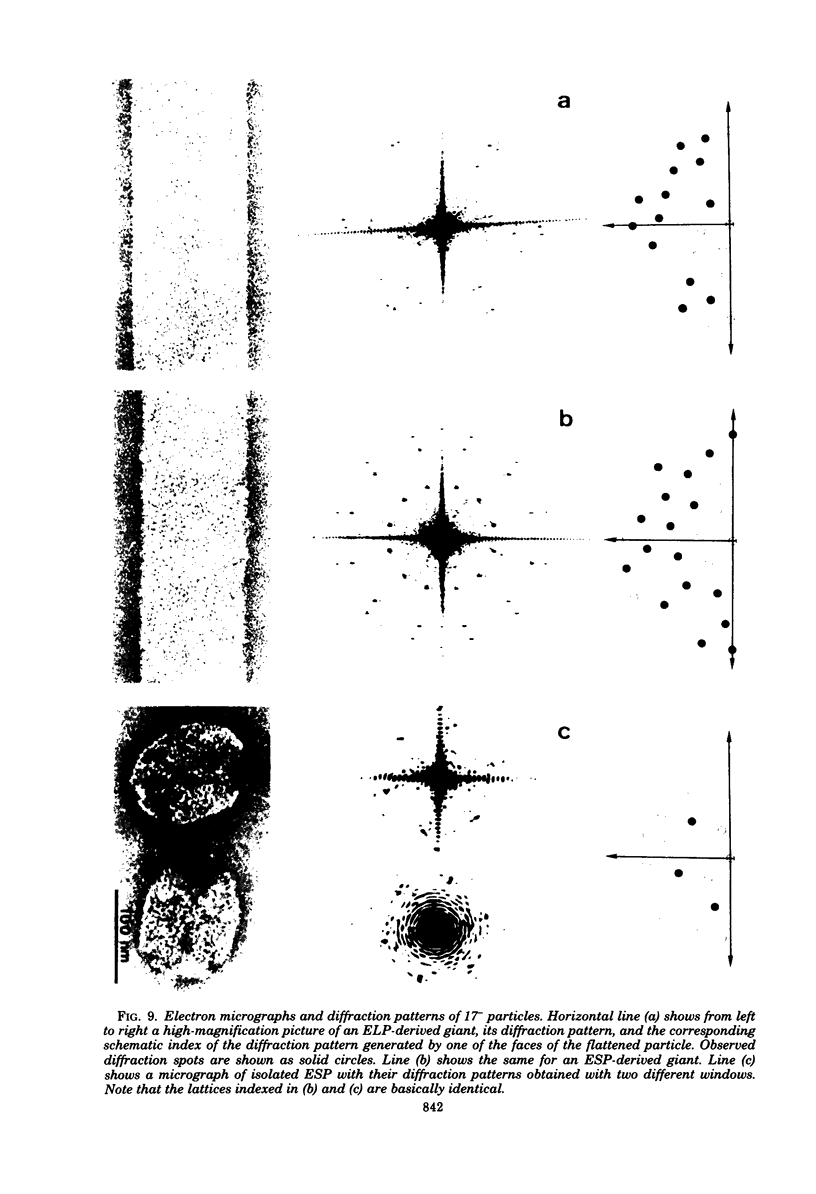
We have isolated and characterized two types of particles produced in comparable amounts by mutants in gene 17: the empty large particle and the empty small particle. Dimensions, morphology, stability, and protein composition of the empty large particle are very similar to those of the capsids or empty heads of mature phage. The other type of particle (empty small particle) is very similar in dimensions and stability to the prehead, but differs in that it is composed of processed proteins (gp23, gp24, IpIII). Structural analysis has shown that the protein subunits of the empty small particles are arranged in an unexpanded type of lattice (11.2 to 11.3 nm), whereas the empty large particles have an expanded lattice (13 nm). The characterization of the empty small particle as being composed of cleaved proteins, but still unexpanded, shows that the expansion of the T4 head shell is not necessarily linked to the cleavage of the structural proteins.
Full text
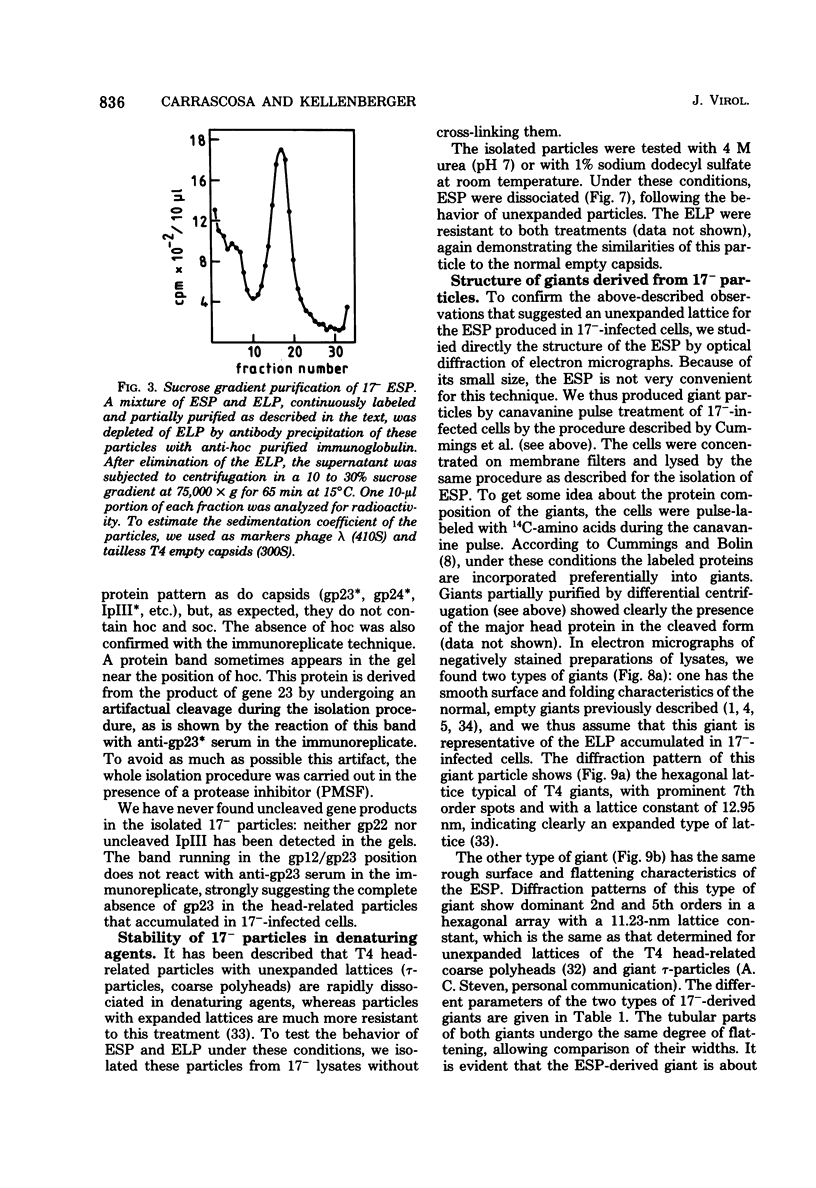
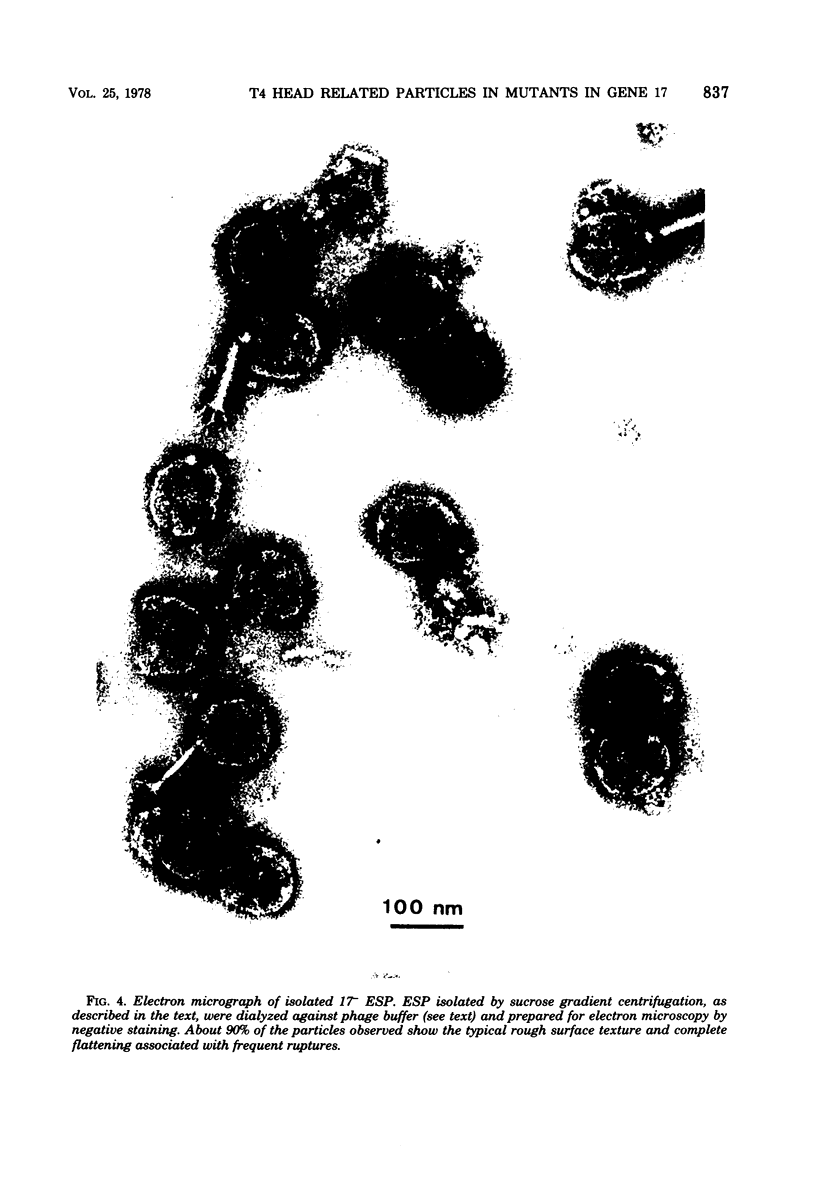
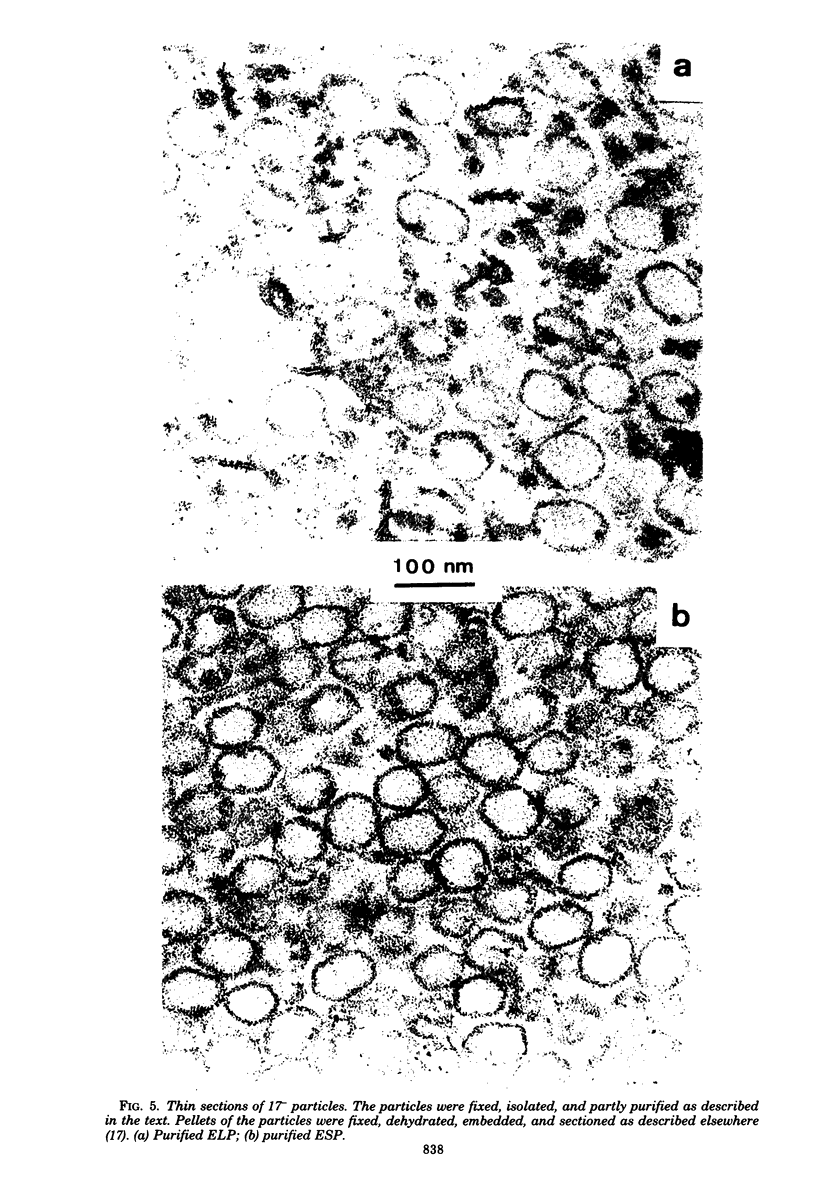
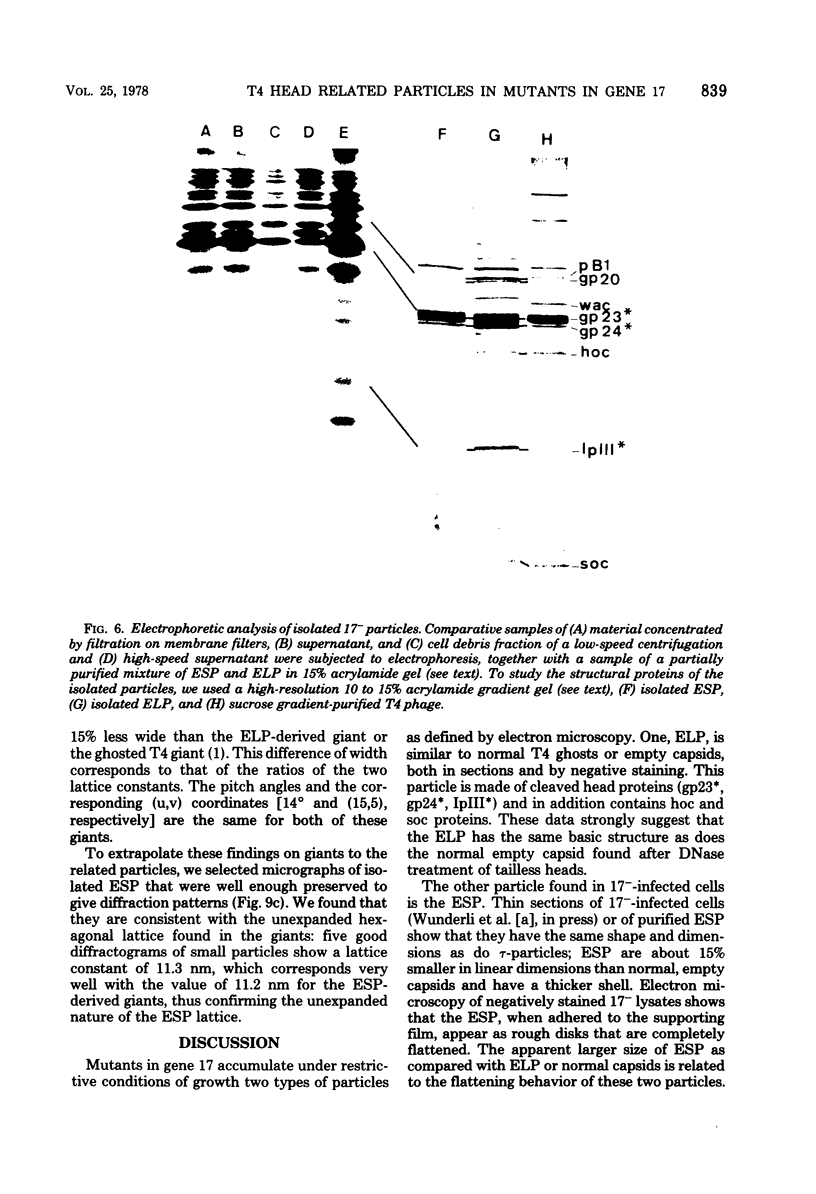
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Bijlenga R. K., ten Heggeler B., Kistler J., Steven A. C., Smith P. R. Comparison of the structural and chemical composition of giant T-even phage heads. J Supramol Struct. 1976;5(4):475–495. doi: 10.1002/jss.400050406. [DOI] [PubMed] [Google Scholar]
- Aebi U., Bijlenga R., v d Broek J., v d Broek H., Eiserling F., Kellenberger C., Kellenberger E., Mesyanzhinov V., Müller L., Showe M. The transformation of tau particles into T4 heads. II. Transformations of the surface lattice and related observations on form determination. J Supramol Struct. 1974;2(2-4):253–275. doi: 10.1002/jss.400020218. [DOI] [PubMed] [Google Scholar]
- Aebi U., Smith P. R., Dubochet J., Henry C., Kellenberger E. A study of the structure of the T-layer of Bacillus brevis. J Supramol Struct. 1973;1(6):498–522. doi: 10.1002/jss.400010606. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Cummings D. J. Structural aberrations in T-even bacteriophage. VIII. Surface morphology of T4 lollipops. Virology. 1977 Feb;76(2):767–780. doi: 10.1016/0042-6822(77)90257-4. [DOI] [PubMed] [Google Scholar]
- Bijlenga R. K., Aebi U., Kellenberger E. Properties and structure of a gene 24-controlled T4 giant phage. J Mol Biol. 1976 May 25;103(3):469–498. doi: 10.1016/0022-2836(76)90213-8. [DOI] [PubMed] [Google Scholar]
- Bijlenga R. K., Broek R vd, Kellenberger E. The transformation of rho-particles into T4 heads. I. Evidence for the conservative mode of this transformation. J Supramol Struct. 1974;2(1):45–59. doi: 10.1002/jss.400020106. [DOI] [PubMed] [Google Scholar]
- Bijlenga R. K., Scraba D., Kellenberger E. Studies on the morphopoiesis of the head of T-even phage. IX. Gamma-particles: their morphology, kinetics of appearance and possible precursor function. Virology. 1973 Nov;56(1):250–267. doi: 10.1016/0042-6822(73)90304-8. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Bolin R. W. Head length control in T4 bacteriophage morphogenesis: effect of canavanine on assembly. Bacteriol Rev. 1976 Jun;40(2):314–359. doi: 10.1128/br.40.2.314-359.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S., Couse N. L. Structural aberrations in T-even bacteriophage. 3. Induction of "lollipops" and their partial characterization. Virology. 1973 Jul;54(1):245–261. doi: 10.1016/0042-6822(73)90134-7. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Minagawa T. Genetic control of the DNA maturation in the process of phage morphogenesis. Virology. 1971 Jul;45(1):280–291. [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Yanagida M. The two dispensable structural proteins (soc and hoc) of the T4 phage capsid; their purification and properties, isolation and characterization of the defective mutants, and their binding with the defective heads in vitro. J Mol Biol. 1977 Feb 5;109(4):487–514. doi: 10.1016/s0022-2836(77)80088-0. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J., RYTER A. Electron microscopical studies of phage multiplication. IV. The establishment of the DNA pool of vegetative phage and the maturation of phage particles. Virology. 1959 Aug;8:478–498. doi: 10.1016/0042-6822(59)90050-9. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Bitterli D. Preparation and counts of particles in electron microscopy: application of negative stain in the agarfiltration method. Microsc Acta. 1976 May;78(2):131–148. [PubMed] [Google Scholar]
- Kellenberger E., Der Kamp C. K.-V. On a modification of the gene product P23 according to its use as subunit of either normal capsids of phage T4 or of polyheads. FEBS Lett. 1970 Jun 1;8(3):140–144. doi: 10.1016/0014-5793(70)80247-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Amos L. A., Klug A. Correlation between structural transformation and cleavage of the major head protein of T4 bacteriophage. Cell. 1976 Feb;7(2):191–203. doi: 10.1016/0092-8674(76)90018-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Paulson J. R., Hitchins V. Maturation of the head of bacteriophage T4. V. A possible DNA packaging mechanism: in vitro cleavage of the head proteins and the structure of the core of the polyhead. J Supramol Struct. 1974;2(2-4):276–301. doi: 10.1002/jss.400020219. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Teaff N., D'Ambrosia J. Maturation of the head of bacteriophage T4. III. DNA packaging into preformed heads. J Mol Biol. 1974 Oct 5;88(4):749–765. doi: 10.1016/0022-2836(74)90397-0. [DOI] [PubMed] [Google Scholar]
- Lickfeld K. G., Menge B., Wunderli H., van den Broek J., Kellenberger E. The interpretation and quantitation of sliced intracellular bacteriophages and phage-related particles. J Ultrastruct Res. 1977 Aug;60(2):148–168. doi: 10.1016/s0022-5320(77)80062-2. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. II. Studies on the maturation of gene 49-defective head intermediates. J Virol. 1972 Feb;9(2):377–389. doi: 10.1128/jvi.9.2.377-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. IV. Comparison of gene 16-, 17-, and 49-defective head structures. J Virol. 1972 Sep;10(3):545–554. doi: 10.1128/jvi.10.3.545-554.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa T. Endonuclease of T4 ghosts. Virology. 1977 Jan;76(1):234–245. doi: 10.1016/0042-6822(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Riva S., Cascino A., Geiduschek E. P. Uncoupling of late transcription from DNA replication in bacteriophage T4 development. J Mol Biol. 1970 Nov 28;54(1):103–119. doi: 10.1016/0022-2836(70)90448-1. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Isobe E., Onorato L. Bacteriophage T4 prehead proteinase. I. Purification and properties of a bacteriophage enzyme which cleaves the capsid precursor proteins. J Mol Biol. 1976 Oct 15;107(1):35–54. doi: 10.1016/s0022-2836(76)80016-2. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Isobe E., Onorato L. Bacteriophage T4 prehead proteinase. II. Its cleavage from the product of gene 21 and regulation in phage-infected cells. J Mol Biol. 1976 Oct 15;107(1):55–69. doi: 10.1016/s0022-2836(76)80017-4. [DOI] [PubMed] [Google Scholar]
- Simon L. D. Infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope: T4 head morphogenesis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):907–911. doi: 10.1073/pnas.69.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A., Traut R. R. Diagonal polyacrylamide-dodecyl sulfate gel electrophoresis for the identification of ribosomal proteins crosslinked with methyl-4-mercaptobutyrimidate. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3946–3950. doi: 10.1073/pnas.71.10.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Aebi U., Showe M. K. Folding and capsomere morphology of the P23 surface shell of bacteriophage T4 polyheads from mutants in five different head genes. J Mol Biol. 1976 Apr 15;102(3):373–400. [PubMed] [Google Scholar]
- Steven A. C., Couture E., Aebi U., Showe M. K. Structure of T4 polyheads. II. A pathway of polyhead transformation as a model for T4 capsid maturation. J Mol Biol. 1976 Sep 5;106(1):187–221. doi: 10.1016/0022-2836(76)90307-7. [DOI] [PubMed] [Google Scholar]
- Yanagida M., Ahmad-Zadeh C. Determination of gene product positions in bacteriophage T4 by specific antibody association. J Mol Biol. 1970 Jul 28;51(2):411–421. doi: 10.1016/0022-2836(70)90151-8. [DOI] [PubMed] [Google Scholar]
- Yanagida M. Molecular organization of the shell of T-even bacteriophage head. II. Arrangement of subunits in the head shell of giant phages. J Mol Biol. 1977 Feb 5;109(4):515–537. doi: 10.1016/s0022-2836(77)80089-2. [DOI] [PubMed] [Google Scholar]