Abstract
Despite advances in metabolite profiling, a full picture of the metabolic landscape of the cell has been limited by sub-cellular compartmentalization, which segregates distinct nutrient pools into membrane-bound organelles. Now, Chen et al. describe methods for overcoming this hurdle and provide a new quantitative picture of the mitochondrial metabolome.
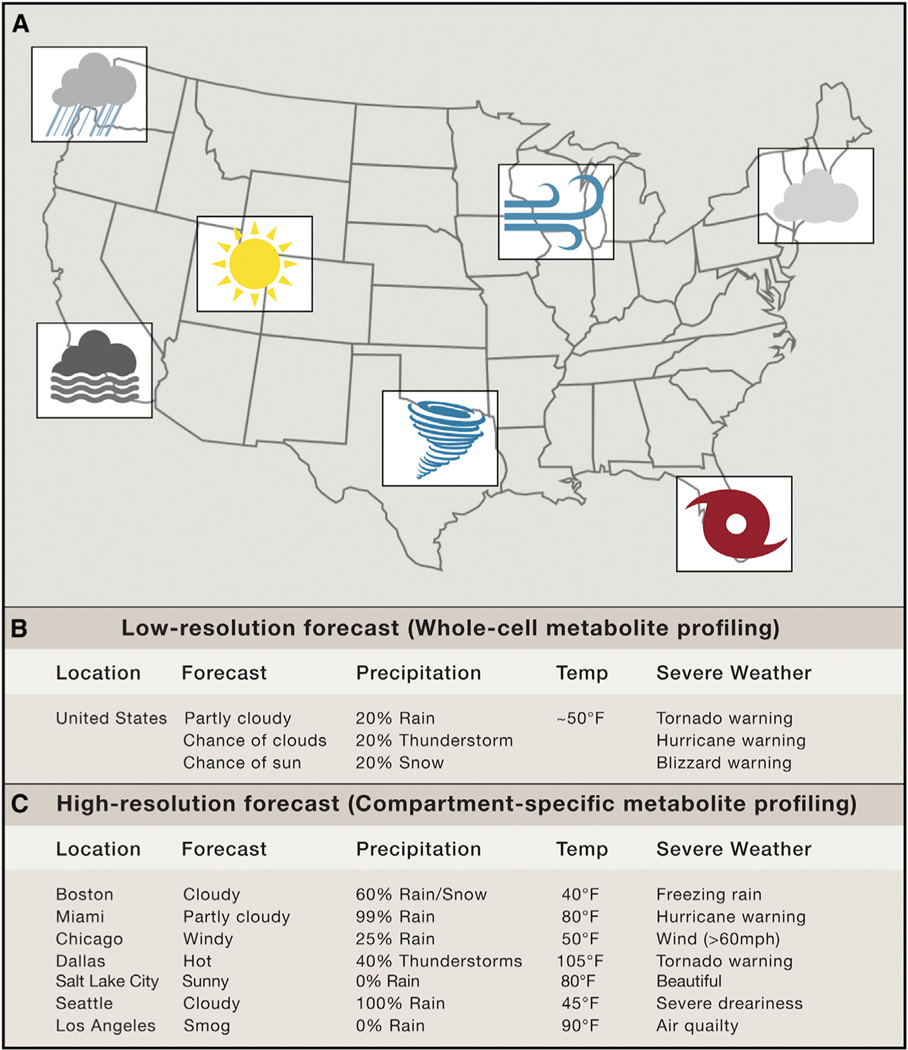
Wake up. 6 AM. You need to get to the lab. Eat breakfast. Brush your teeth. Check the weather. “Today in the USA, it will be an average temperature of 50° F. There is a 25% chance of sunshine, a 25% chance of rain, a 25% chance of thunderstorms, a 20% chance of snow, and a 5%chance of fog.” USA? What good does the average weather across the entire country do when you are trying to decide if you need to take your raincoat to work (Figure 1)? You need to know what the weather will be like in the city, or at least the region, in which you live. The same goes for cellular metabolite profiling, which allows for the relative quantitation of hundreds of metabolites using mass spectrometry. Shooting whole-cell extract through a mass spectrometer only tells you the average metabolite content across all of the diverse and highly specialized cellular compartments. Herein lies the problem. Fortunately, Chen et al. have a solution (Chen et al. 2016, this issue).
Figure 1. The Value of Spatial Resolution in Weather Forecasting.
(A) A weather map describing the forecast of a number of cities across North America.
(B) A low-resolution forecast of the United States of America (analogous to whole-cell metabolite profiling).
(C) A high-resolution forecast of the United States of America (analogous to compartment-specific metabolite profiling).
Metabolite profiling has made it possible to rapidly and simultaneously measure metabolite content in whole cells and tissues. In addition to defining the human metabolome, metabolite profiling has highlighted the metabolic perturbations and adaptations that occur during the course of normal physiology and in the context of disease states, thereby painting a more accurate picture of the highly dynamic metabolic network. Despite having a large impact on biological research, metabolite profiling has painted an incomplete picture of metabolism due to its inability to address the inherent problem of compartmentalization in eukaryotic cells. In addition to the cytosol, numerous membrane-bound organelles segregate independent pools of cellular metabolites and control the distribution of these metabolites using dozens of highly conserved metabolite transporters.
Mitochondria, which are enclosed by two membranes, are the primary sites for a number of vital metabolic processes, including fatty acid oxidation, amino acid biosynthesis, and respiratory chain (RC)-dependent ATP generation, as well as scores of others. As such, mitochondrial function is essential for maintaining normal cellular physiology, and defects result in a vast array of disease phenotypes (Pagliarini and Rutter, 2013). Despite the importance of this organelle, there is relatively little known about its metabolite content in the context of a living cell. This is because it is impossible, in a standard metabolite profiling experiment, to distinguish citrate (acetyl-coA, NADH, pyruvate, or any other metabolite) in the mitochondria from citrate in the cytosol or peroxisome, in spite of the fact that the biological impact of location is enormous (Jiang et al., 2016). Furthermore, the small fraction of the total cellular volume occupied by mitochondria makes it impossible to infer information about its metabolite content from data that includes the massive cytosolic metabolite pools.
In order to reliably characterize the mitochondrial metabolome, mitochondria must first be isolated from the cell. While this is trivial when interrogating mitochondrial proteins and other macromolecules, previous methods have not been amenable to metabolite profiling experiments due to technical challenges. Traditional mitochondrial isolation protocols either take too much time (resulting in loss of metabolites through export and chemical reactions) or lack specificity (resulting in contamination with metabolites from the cytosol and other organelles). In order to overcome this problem, Chen et al. (2016) developed a method, using an HA-tagged EGFP targeted to the mitochondrial outer membrane, allowing rapid mitochondrial immunocapture and organelle-specific metabolite profiling.
Initially, the authors applied this new technology to defining and quantifying the steady-state mitochondrial metabolome in normal growth conditions. Despite this important advance, the real utility of this method is the ability to overcome the problem of compartmentalization and specifically interrogate the dynamic responses of mitochondrial metabolism. The authors, using RC inhibition as a model, demonstrate that whole-cell metabolite profiling is, indeed, an inadequate readout of mitochondrial metabolism. Two important redox couples—NADH/NAD and GSH/GSSG—perhaps best exemplify this phenomenon. When interrogating whole cells, inhibition of the respiratory chain causes subtle, if any, perturbations in the ratio of NADH/NAD and GSH/GSSG. However, upon mitochondrial immunocapture and metabolite profiling, it becomes clear that these ratios markedly increase in the mitochondrial matrix.
It is important to note that compartmentalization is not always a problem. Take, for instance, SDH- and FH-deficient tumor cells, which massively accumulate succinate to a degree that it can be easily detected by whole-cell metabolite profiling, in spite of the fact that the succinate originates from the mitochondria (King et al., 2006). However, there are also instances like those highlighted above in which whole-cell measurements are not adequate. For years it was known that super-physiological levels of pyruvate could support proliferation in cells deficient in RC activity (King and Attardi, 1989). Two recent papers found that pyruvate serves as an electron acceptor to regenerate NAD+ pools in the cytosol, thereby supporting an alternate cytosolic biosynthetic pathway for aspartate, which is not dependent on RC function. At the time, the authors found that whole-cell NADH/NAD+ ratios were undisturbed but were unable to quantify those metabolites specifically in mitochondria. Now, it is clear that NAD+ was massively depleted in the mitochondria (which inhibited mitochondrial NAD+-dependent aspartate biosynthesis) but that the cytosolic NADH/NAD+ pool, which is much larger, masked this perturbation (Birsoy et al., 2015; Sullivan et al., 2015).
Overcoming the hurdle of compartmentalization has the potential to initiate a new era in the study of eukaryotic metabolism. For the first time, it is possible to reliably quantify the metabolite content in an isolated organelle. We need to move beyond the constraining requirement for expression of an organelle-localized fusion protein, thereby enabling organelle-specific metabolic profiling in any cell type from experimental models to human subjects. This would shine a new light on the degree to which metabolite compartmentalization dictates normal physiology and the metabolic basis for human disease.
REFERENCES
- Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. Cell. 2015;162:540–551. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WW, Freinkman E, Wang T, Birsoy K, Sabatini DM. Cell. 2016;166:1324–1337. doi: 10.1016/j.cell.2016.07.040. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Shestov AA, Swain P, Yang C, Parker SJ, Wang QA, Terada LS, Adams ND, McCabe MT, Pietrak B, et al. Nature. 2016;532:255–258. doi: 10.1038/nature17393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MP, Attardi G. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- King A, Selak MA, Gottlieb E. Oncogene. 2006;25:4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- Pagliarini DJ, Rutter J. Genes Dev. 2013;27:2615–2627. doi: 10.1101/gad.229724.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Cell. 2015;162:552–563. doi: 10.1016/j.cell.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]