Abstract
Recent research suggests that some people with aphasia preserve some ability to learn novel words and to retain them in the long-term. However, this novel word learning ability has been studied only in the context of single word-picture pairings. We examined the ability of people with chronic aphasia to learn novel words using a paradigm that presents new word forms together with a limited set of different possible visual referents and requires the identification of the correct word-object associations on the basis of online feedback. We also studied the relationship between word learning ability and aphasia severity, word processing abilities, and verbal short-term memory (STM). We further examined the influence of gross lesion location on new word learning. The word learning task was first validated with a group of forty-five young adults. Fourteen participants with chronic aphasia were administered the task and underwent tests of immediate and long-term recognition memory at 1 week. Their performance was compared to that of a group of fourteen matched controls using growth curve analysis. The learning curve and recognition performance of the aphasia group was significantly below the matched control group, although above-chance recognition performance and case-by-case analyses indicated that some participants with aphasia had learned the correct word-referent mappings. Verbal STM but not word processing abilities predicted word learning ability after controlling for aphasia severity. Importantly, participants with lesions in the left frontal cortex performed significantly worse than participants with lesions that spared the left frontal region both during word learning and on the recognition tests. Our findings indicate that some people with aphasia can preserve the ability to learn a small novel lexicon in an ambiguous word-referent context. This learning and recognition memory ability was associated with verbal STM capacity, aphasia severity and the integrity of the left inferior frontal region.
Keywords: New word learning, Aphasia, Short-term memory, Inferior frontal regions
1. Introduction
Humans have a remarkable word learning capacity. While most of their vocabulary is acquired effortlessly during childhood (Lindfors, 1991) adults can continue to integrate novel technological terms and new words of a second language into their already well-shaped mental lexicons (Davis & Gaskell, 2009; Grosjean & Li, 2013; Nation, 2001). Yet, access to this previously acquired vocabulary can be limited or severely impaired after brain insult (Goodglass & Wingfield, 1997) leading to the difficulties in retrieving and producing words commonly observed in aphasia (Nickels, 2002). The investigation of the residual ability for novel word learning may be crucial to further understand the mechanisms underlying language recovery in aphasia. Importantly, recent research has shown a relationship between new word learning ability and response to anomia therapy in chronic aphasic individuals (Dignam et al., 2016). Thus, methods that facilitate this new learning potential could be useful in the context of therapy (Kelly & Armstrong, 2009), as associative learning procedures could be suited for intensive training (Breitenstein et al., 2007) even when brain regions essential for language processing have been extensively damaged (Tuomiranta et al., 2014).
The existing literature on novel word learning in aphasia suggests that: (i) people with aphasia can preserve some ability to learn new word-referent associations despite residual language impairment (McGrane, 2006); (ii) this learning potential can be observed using different facilitation procedures (Marshall, Freed, & Karow, 2001) (iii) novel words and some pre-existing but inaccessible vocabulary can be trained and retained in the long-term (Tuomiranta, Rautakoski, Rinne, Martin, & Laine, 2012; Tuomiranta et al., 2014); and (iv) people with aphasia show large inter-individual variability in their capacity to learn and store novel word forms and to re-learn their affected vocabulary (Kelly & Armstrong, 2009). These previous studies have examined novel word learning in aphasia using single word-picture pairings. However, the relationship between a given referent and a lexical item is not unequivocal in natural language learning (Smith & Yu, 2008), nor in the case of some language therapy practice (Kelly & Armstrong, 2009). Natural language learning contexts provide a rich and complex input in terms of words and multiple possible referent candidates (Romberg & Saffran, 2010). Yet, both children and adult learners can successfully infer the correspondence between words and referents from highly ambiguous contexts (Markman, 1994; Yu, Smith, Klein, & Shiffrin, 2007). Learners can benefit from different cues and representational, social and attentional constraints (Markman, 1990; Smith, 2000; Tomasello, 2000) to establish word-to-object mappings in a single learning situation. However, this word-referent indeterminacy can also be resolved by infants and adults across multiple encounters and learning instances (Smith & Yu, 2008; Yu & Smith, 2007). Recent research has proposed cross-situational learning as a learning mechanism that allows computing the co-occurrences between words and referents while keeping track of multiple possible word-referent pairings simultaneously to finally converge on single mappings based on the accumulated statistical evidence (Smith & Yu, 2008; Yu & Smith, 2007). Another account for this type of learning is the propose-but-verify learning strategy (Trueswell, Medina, Hafri, & Gleitman, 2013) which suggests that learners formulate a single hypothesized meaning for a given word and maintain it to evaluate its consistency across the next learning instances to either confirm and strengthen it in memory or abandon it and postulate a new one for subsequent confirmation or rejection. Although the debate regarding the mechanisms that support word learning under referential ambiguity continues, there is clear evidence that adults can learn multiple word-referent mappings rapidly and effectively (Yu & Smith, 2007; Smith, Smith & Blythe, 2011) and retain this knowledge in the long term (Vlach & Sandhofer, 2014).
Word learning paradigms that simulate this word-referent ambiguity may thus provide an appropriate and ecologically valid method to study this ability in aphasia. To our knowledge, only one previous study (Breitenstein, Kamping, Jansen, Schomacher, & Knetcht, 2004) evaluated word learning in aphasia using a task with a higher statistical co-occurrence of the correct label-object pairings as compared to incorrect pairings, thus, simulating this word-referent ambiguity. The two aphasic participants described in this report could learn most of the correct word-picture associations. However, the sample was rather small and the novel words were paired with known visual referents. In the present multicenter study, we aimed to examine the ability of individuals with chronic aphasia to learn novel word-referent mappings using a more complex natural language learning approach. We employed a word learning paradigm (Magnuson, Tanenhaus, Aslin, & Dahan, 2003; Mirman, Magnuson, Graf Estes, & Dixon, 2008) that presents novel words combined with a limited set of different possible novel referents, calling for the evaluation of these word-object relationships on the basis of online visual feedback. Our first goal was to examine the learning performance of a group of aphasic participants as compared to that of a group of matched controls and a group of young adults with whom the word learning task was first validated. We also examined the ability of the participants with aphasia to remember the trained word-object associations in the short-term and one week after training without feedback. We further explored the influence of aphasia severity in the encoding of novel word-object associations during training and the later recognition of these associations.
Importantly, because the acquisition of novel words encompasses various cognitive abilities (Carroll, 1993; Gupta & Tisdale, 2009), the individual variability in word learning performance in aphasia may be associated with the cognitive and language processes available to encode and strengthen novel word memory traces after brain damage. Verbal short-term memory (STM) has been related to word learning ability during early language acquisition (Gathercole & Baddeley, 1989) and across the life-span (Gathercole, 2006; Gupta, 2003). It has been suggested that the acquisition of new words is constrained by the capacity to hold phonological codes in a temporary phonological memory (Gathercole, Service, Hitch, Adams, & Martin, 1999) that makes them available for long-term learning (Baddeley, Gathercole, & Papagno, 1998). Phonological STM as measured by nonword repetition and STM span tasks (Gathercole et al., 1999) has been associated with vocabulary knowledge in the native language (Gathercole & Addams, 1994; Michas & Henry, 1994) and the acquisition of foreign vocabulary (Papagno & Vallar, 1995; Service, 1992). However, semantic representations also contribute to verbal STM (Martin & Saffran, 1997, 1999; Martin, Wu, Freedman, Jackson, & Lesch, 2003), as the maintenance of verbal information in STM is affected by its lexical-semantic features such as lexical frequency (Roodenrys, Hulme, Alban, Ellis, & Brown, 1994), semantic category (Poirier & Saint-Aubin, 1995; Tse, 2009), and imageability (Bourassa & Besner, 1994). Moreover, a distinction between phonological STM and semantic STM has been proposed (Martin, Lesch, & Bartha, 1999; Shivde & Anderson, 2011) based on neuropsychological case studies suggesting that these are dissociable capacities (Freedman & Martin, 2001; Majerus, van der Linden, Poncelet, & Metz-Lutz, 2004; Martin, Shelton, & Yaffee, 1994). Therefore, it is possible that semantic STM and phonological STM make differential contributions to new word learning.
Phonological and lexical-semantic processing alone may also influence word learning ability. For instance, phonological knowledge of word forms may facilitate the acquisition of new words (Gupta & Tisdale, 2009). Likewise, semantic factors such as the number of semantic neighbors can influence the recall of recently learned word-object associations (Storkel & Adlof, 2009). In aphasia, previous research has demonstrated that phonological ability is related to familiar word learning when words in a list are low in frequency and imageability, whereas semantic ability is associated with learning performance when words present the opposite pattern (Martin & Saffran, 1999). Similarly, a relationship between word processing and novel word learning has been reported in aphasic individuals, with phonological abilities predicting phonological learning and lexical-semantic abilities predicting receptive recognition learning (Gupta, Martin, Abbs, Schwartz, and Lipinski, 2006). However, the evidence of the influence of phonological and semantic STM on novel word learning in aphasia is still limited. The present study also examined the involvement of phonological discrimination ability, lexical-semantic skills, and verbal STM capacity for phonological and lexical-semantic representations in the immediate and delayed recognition of newly acquired word-referent associations in aphasia. Finally, we also examined if recognition ability in aphasia was associated with gross lesion location.
2. Materials and methods
2.1. Participants
Participants were seventy-three individuals (45 female) recruited in three laboratories: Barcelona (Spain) (n = 57), Philadelphia (USA) (n = 8), and Turku (Finland) (n = 8). The total sample included three groups. The first group involved 45 undergraduate psychology students at the University of Barcelona (hereafter young adults). The young adults (39 female) had a mean age of 22.4 years (SD = 6) and their mean number of educational years was 14.02 (SD = 1.5). They were predominantly early bilinguals for Catalan and Spanish. This group was recruited to validate the experimental task and to ensure that the standard level of word learning across blocks was similar to previous research using this paradigm (Mirman et al., 2008). The second and third groups included 14 persons with stroke-induced chronic aphasia and 14 healthy controls (hereafter, “matched controls”), respectively.1 The participants in these two groups were matched by gender, age and years of education. There were 3 female participants in each group. The mean age was 65.36 years (SD = 8.28) for the aphasia group and 66.57 years (SD = 6.42) for the matched control group. The mean number of educational years was 12.71 (SD = 5.1) for the aphasic participants and 15.36 (SD = 4.4) for the matched controls. Each one of the aphasia and the matched control groups included 6 Spanish speakers, 4 English speakers, 2 Swedish speakers, and 2 Finnish speakers.
Table 1 presents the demographic and clinical information of the participants with aphasia. Participants in the aphasia group fulfilled the following inclusion criteria: i) age between 25 and 77 years, ii) first and single stroke confirmed by CT or MRI scan, iii) persistent stroke-induced aphasia as determined by formal speech and language assessment at 1 year or more from stroke onset, iv) preserved ability to understand and follow instructions to complete the experimental task. The aphasic participants had an average time of enrollment in the study of 53.8 months (SD = 48.3) after stroke onset. They were recruited as follows: the Spanish speakers were recruited from a database of patients who had been admitted to the stroke unit of the Hospital Universitari de Bellvitge in Barcelona, the English speakers were recruited from the subject pool of the Aphasia Rehabilitation Research Laboratory at Temple University in Philadelphia, the Finnish speakers were contacted through an aphasia association and the Swedish speakers through the Abo Akademi university speech therapy clinic in Turku.
Table 1.
Demographic and clinical background of participants with chronic aphasia.
| Case | Gender | Age (years) |
Education (years) |
Native language |
Time from stroke (months) |
Etiology of stroke |
Lesion location | Aphasia type/ severity (chronic stage) |
|---|---|---|---|---|---|---|---|---|
| AE | M | 66 | 11 | Spanish | 24 | I | Insular and opercular frontal regions, caudate and lenticular nucleus, and postcentral parietal regionsb |
Anomic/mild |
| JH | M | 54 | 16 | Catalan/Spanisha | 19 | I/H | Extensive MCA stroke/intracerebral hemorrhage (frontal regions, caudate nucleus)b |
Broca/moderate |
| AF | M | 69 | 8 | Catalan/Spanisha | 24 | I | Left MCA stroke (parietal perisylvian regions)c | Fluent aphasia/mild |
| AM | M | 72 | 10 | Spanish | 17 | I | Left MCA stroke (frontal regions, insula)b | Broca/severe |
| RS | M | 57 | 8 | Catalan/Spanisha | 15 | I | Left MCA stroke (caudate nucleus, putamen and internal capsule)c | Anomic/mild |
| AL | F | 75 | Reading/ writing |
Spanish | 19 | I/H | Left MCA stroke/intracerebral hemorrhage (insula, opercular frontal and temporal regions, BG)b |
Wernicke/moderate |
| CM | M | 50 | 12 | English | 53 | I | Left MCA stroke (parietal, bilateral subcortical lesions on the cerebrum brainstem, cerebellum and putamen as well as white matter lesions)c |
, Anomic/mild-moderate |
| FS | F | 59 | 12 | English | 82 | H | Left intracerebral hemorrhage within the temporal lobec | Conduction/moderate |
| QH | M | 61 | 18 | English | 55 | I/H | Left posterior temporal intracranial hemorrhage/left transverse sigmoid junction sinus thrombosis with venous infarct and hemorrhagic conversion (left hemicraniectomy)c |
Anomic/mild |
| KM | M | 67 | 16 | English | 192 | I | Left MCA-ACA stroke involving the left frontal, temporal and parietal lobes and left BGb |
Transcortical motor/ mild-moderate |
| BB | M | 73 | 18 | Swedish | 96 | I | Right MCA stroke | Anomic/moderate |
| BL | F | 63 | 16 | Swedish/Finnisha | 85 | I/H | Aneurysm rupture in the PCA leading to subarachnoid hemorrhage. The hemorrhage lead to a vasospasm resulting in infarction in the left temporal and parietal lobesc |
Fluent aphasia/mild |
| JS | M | 77 | 15 | Finnish | 36 | I | Left MCA stroke (temporal regions)c | Mixed/mild |
| EP | M | 72 | 18 | Finnish | 36 | H | Extensive left temporal intracranial hemorrhagec | Anomic/moderate |
The data of the participants with aphasia presented in this table have been previously reported elsewhere (Peñaloza et al., 2015).
Ed = education; M = male; F = female; I = ischemia; H = hemorrhage; MCA = middle cerebral artery; ACA = anterior cerebral artery; PCA = posterior cerebral artery; BG = basal ganglia.
Early bilingual.
Damaged frontal region.
Spared frontal region.
All participants in the young adult group were right-handed. Participants with aphasia and their matched controls were right-handed except for participant BB who was left-handed and had suffered a right hemisphere stroke. The young adults had normal vision and hearing, and no visual and auditory deficits were detected upon screening in the aphasia group and the matched control group. None of the participants had a history of neurological disorders (other than stroke for the aphasia group), mental illnesses, or learning impairments. All participants gave their written informed consent and all procedures were approved by the ethical committees of each participating institution.
2.2. Language processing and STM assessment
The diagnosis of aphasia and aphasia severity was determined using versions of the Boston Diagnostic Aphasia Examination (BDAE) for the Spanish (Goodglass, Kaplan, & Barresi, 2005), Swedish (Laine, Niemi, Koivuselkä-Sallinen, & Koivusalo, 1986) and Finnish aphasic speakers (Laine, Niemi, Koivuselkä-Sallinen, & Tuomainen, 1997). The language background testing of these participants included the following assessments. Spontaneous speech was assessed with the conversational and expository speech subtests of the BDAE. Verbal comprehension was evaluated with the Word comprehension, Commands, and Complex ideational material subtests of the BDAE, as well as with the Token Test (De Renzi & Faglioni, 1978). Naming ability was assessed with the Responsive naming and Animal naming subtests of the BDAE, and versions of the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 2005; Laine, Koivuselkä-Sallinen, Hänninen, & Niemi, 1997; Tallberg, 2005). Repetition ability was assessed using the Word repetition and the Sentence repetition subtests of the BDAE.
Because the Western Aphasia Battery-Revised (WAB-R; Kertesz, 2006) is traditionally used in North America for clinical practice, the aphasia profile and aphasia severity of the English-speaking participants were determined by this battery. The WAB-R subtests tapping on spontaneous speech, comprehension, naming and word finding, and repetition were used as part of their language background assessment. For each individual with aphasia, a general quotient was calculated for each of these language domains. The BNT was used to assess visual confrontation naming. In addition, the 5-point severity rating scale of the BDAE (Goodglass, Kaplan, & Barresi, 2001) was also applied with the participants in order to have comparable aphasia severity scores for the entire aphasia group to examine the relationship between word learning ability and aphasia severity. The speech and language profiles of the participants with aphasia are presented in Tables 2 and 3.
Table 2.
Speech and language profile of the Spanish, Finnish and Swedish speaking participants with chronic aphasia.
| Language measure | Participants with aphasia |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AE | JH | AF | AM | RS | AL | BB | BL | JS | EP | |
| BDAE | ||||||||||
| Severity rating | 5 | 3 | 5 | 1 | 5 | 3 | 3 | 2 | 4 | 3 |
| Spontaneous speech | ||||||||||
| Melodic line | 7/7 | 4/7 | 7/7 | 3/7 | 7/7 | 7/7 | 6/7 | 7/7 | 4/7 | 7/7 |
| Phrase length | 7/7 | 5/7 | 7/7 | 4/7 | 7/7 | 7/7 | 5/7 | 7/7 | 5/7 | 7/7 |
| Articulation rating | 6/7 | 3/7 | 6/7 | 3/7 | 6/7 | 6/7 | 6/7 | 6/7 | 3/7 | 7/7 |
| Grammar | 6/7 | 4/7 | 7/7 | 2/7 | 7/7 | 6/7 | 6/7 | 7/7 | 5/7 | 7/7 |
| Paraphasia in fluent speech | 6/7 | 5/7 | 6/7 | 5/7 | 6/7 | 5/7 | 6/7 | 7/7 | 7/7 | 6/7 |
| Auditory comprehension | ||||||||||
| Word comprehension | 36/37 | 35/37 | 37/37 | 24.5/37 | 35/37 | 33/37 | 66.5/72 | 72/72 | 68/72 | 56.5/72 |
| Commands | 14/15 | 14/15 | 15/15 | 12/15 | 15/15 | 12/15 | 8/15 | 15/15 | 15/15 | 12/15 |
| C. ideational material | 11/12 | 6/12 | 10/12 | 10/12 | 10/12 | 7/12 | 6/12 | 10/12 | 11/12 | 10/12 |
| Naming | ||||||||||
| Responsive naming | NA | 18/20 | 20/20 | 8/20 | 19/20 | 15/20 | 20/30 | 30/30 | 25/30 | 23/30 |
| Animal naming | 14 | 14 | 22 | 3 | 7 | 7 | 9 | 14 | 4 | 4 |
| BNT | 47/60 | 39/60 | 47/60 | 11/60 | 51/60 | 28/60 | 17/60 | 48/60 | 41/60 | 8/60 |
| Repetition | ||||||||||
| Word repetition | 10/10 | 8/10 | 10/10 | 6/10 | 9/10 | 6/10 | 8/10 | 9/10 | 9/10 | 10/10 |
| Sentence repetition | 8/10 | 2/10 | 9/10 | 0/10 | 9/10 | 2/10 | 2/8a 0/8b |
7/8a 5/8b |
7/8a 5/8b |
7/8a 6/8b |
| Other tests | ||||||||||
| Token test | 25/36 | 14/36 | 28/36 | 12/36 | 31/36 | 20/36 | 15/36 | 25/36 | 19.5/36 | 18.5/36 |
Scores below the 50th percentile are marked in bold (for participants BB, BL, JS and EP scores are marked in bold only when normative data were available for the reported measures).
C. ideational material = complex ideational material; NA = not administered.
Repetition of high-probability sentences.
Repetition of low-probability sentences.
Table 3.
Speech and language profile of the English speaking participants with chronic aphasia.
| Language measure | Participants with aphasia |
|||
|---|---|---|---|---|
| CM | FS | QH | KM | |
| WAB-R | ||||
| Spontaneous speech quotient | 18 | 18 | 17 | 13 |
| Auditory comprehension quotient | 9.5 | 9.2 | 9.9 | 8.4 |
| Repetition quotient | 7.7 | 6.7 | 8.2 | 8.6 |
| Naming and word finding quotient | 9.5 | 8.9 | 7.4 | 8 |
| WAB-R aphasia quotient | 89.3 | 85.5 | 84.9 | 76 |
| Other tests | ||||
| BNT | 42 | 32 | NA | NA |
| BDAE severity rating | 4 | 4 | 5 | 3 |
A selection of subtests of the Temple Assessment of Language and Short-term memory in Aphasia (TALSA; Martin, Kohen, & Kalinyak-Fliszar, 2010) available in English, Spanish and Finnish (Tuomiranta, Laine, & Martin, 2009) was administered to 11 participants with aphasia (see Table 4 for direct and composite scores). One Spanish speaker with aphasia was unavailable for testing and the Swedish speakers with aphasia were not evaluated with the TALSA battery, as it is currently unavailable in this language. The selection of tests included word processing tasks with STM manipulations (Phoneme discrimination, Rhyming judgements, Lexical comprehension, and Category judgements) and STM measures with lexical-semantic manipulations (Word pointing span, Digit pointing span, Word repetition span, and Digit repetition span) and without lexical-semantic support (Nonword repetition).2 A brief description of the individual measures and the composite scores derived from them is provided below.
Table 4.
Proportion of correct responses and composite scores of the participants with aphasia in the TALSA subtests.
| TALSA measure | AE | JH | AF | AM | RS | AL | CM | FS | QH | KM | JS | EP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phonological discriminationa | ||||||||||||
| Phoneme discrimination (1 secU) | NA | 1 | .95 | .45 | 1 | 1 | 1 | .90 | 1 | 1 | 1 | 1 |
| Phoneme discrimination (5 secU) | NA | 1 | 1 | .80 | .95 | .95 | 1 | .85 | 1 | .85 | .75 | 1 |
| Rhyming judgments (1 secU) | NA | .95 | 1 | .45 | 1 | .90 | .80 | .85 | .95 | .95 | .75 | .80 |
| Rhyming judgments (5 secU) | NA | .80 | 1 | .70 | 1 | .85 | .80 | .80 | 1 | 1 | 1 | .95 |
| Composite phonological score | NA | .94 | .99 | .60 | .99 | .93 | .90 | .85 | .99 | .95 | .91 | .94 |
| Lexical-semantic processesa | ||||||||||||
| Lexical comprehension (1 secU) | NA | 1 | 1 | .43 | 1 | .87 | 1 | .94 | 1 | 1 | .94 | .81 |
| Lexical comprehension (5 secU) | NA | 1 | .93 | .43 | 1 | .87 | 1 | 1 | 1 | 1 | .94 | .90 |
| Category judgments-words (1 secU) | NA | 1 | 1 | .80 | .95 | .90 | .95 | 1 | 1 | .95 | 1 | 1 |
| Category judgment-words (5 secU) | NA | 1 | 1 | .50 | 1 | .95 | .95 | .95 | 1 | .85 | .95 | .90 |
| Category judgments-pictures (1 secU) | NA | 1 | .95 | .65 | .95 | .85 | .95 | .90 | .95 | 1 | .90 | .95 |
| Category judgments-pictures (5 secU) | NA | 1 | .95 | .75 | .85 | .95 | .85 | .90 | .95 | .90 | .95 | .80 |
| Composite lexical-semantic score | NA | 1 | .97 | .59 | .96 | .90 | .95 | .95 | .98 | .95 | .95 | .89 |
| Verbal STM-nonword repetitiona | ||||||||||||
| Nonword repetition (1 secU) | NA | .07 | .20 | 0 | .73 | .26 | .40 | .13 | .93 | .80 | .60 | .80 |
| Nonword repetition (5 secU) | NA | .07 | .26 | 0 | .60 | .13 | .20 | .70 | .87 | .80 | .40 | .80 |
| Composite nonword repetition score | NA | .07 | .23 | 0 | .67 | .20 | .30 | .10 | .90 | .80 | .50 | .80 |
| Verbal STM-spansb | ||||||||||||
| Word repetition span | NA | 3.0 | 4.2 | 1.4 | 3.8 | 2.8 | 2.2 | 2.2 | 4.2 | 3.4 | 3.2 | 5.1 |
| Digit repetition span | NA | 3.2 | 5.2 | 2.0 | 4.8 | 2.6 | 3.2 | 3.2 | 5.6 | 3.8 | 4.0 | 6.1 |
| Composite repetition span score | NA | 3.1 | 4.7 | 1.7 | 4.3 | 2.7 | 2.7 | 2.7 | 4.9 | 3.6 | 3.6 | 5.6 |
| Word pointing span | NA | 2.2 | 4.0 | 1.8 | 4.2 | 2.2 | 3.0 | 3.0 | 4.4 | 2.4 | 3.1 | 3.2 |
| Digit pointing span | NA | 3.4 | 5.6 | 1.8 | 4.8 | 2.8 | 4.0 | 4.0 | 5.0 | 3.2 | 3.1 | 5.1 |
| Composite pointing span score | NA | 2.8 | 4.8 | 1.8 | 4.5 | 2.5 | 3.5 | 3.5 | 4.7 | 2.8 | 3.1 | 4.15 |
NA = Not administered; 1 secU = 1-sec unfilled condition; 5 secU = 5-sec unfilled condition; STM = short-term memory.
Proportion of correct responses is provided.
Span is provided (the maximum span for all subtests is 7, the number of string length conditions).
2.2.1. Phonological processing measures
The Phoneme discrimination subtest requires participants to hear two items (words or nonwords) and to judge whether they are the same or not. This probe includes 20 word and 20 nonword pairs. Words are concrete tokens of 1 or 2 syllables. Nonwords are generated by modifiying 1 or 2 phonemes of the words included. The Rhyming judgment subtest demands deciding whether a given pair of words or nonwords rhyme. The test includes 20 pairs of words and 20 pairs of nonwords (10 rhyming and 10 non-rhyming test pairs in each set).
2.2.2. Lexical-semantic processing measures
The Lexical comprehension subtest requires matching a spoken word to one of four pictures of the same semantic category. The Category judgments subtest involves the presentation of two items (words or pictures) and requires participants to determine whether the two items belong to the same semantic category. In the Word condition, words are presented auditorily and visually on the screen, whereas in the Picture condition, the images are presented on the screen but their labels are not provided.
2.2.3. Verbal STM measures
The Nonword repetition subtest requires participants to repeat 15 nonwords. The task includes 1, 2 and 3-syllable items, matched for length and CV structure. In addition, two types of STM span probes were administered. The Word repetition span and the Digit repetition span require the immediate repetition of 10 strings of either words or digits in each of 7 string length conditions (1 item, 2 items, etc.). The Word pointing span and the Digit pointing span demand the participant to listen to a sequence of words or digits and to point at the sequence on a visual array of 9 possible items (item position is randomized for each trial). Words and digit names are matched in syllable length, and sequences are generated from a finite set of 9 items. Sequences must be recalled in serial order, and a span size is calculated for each subtest using Shelton, Martin, & Yaffee (1992) formula: string length at which at least 50% of the strings are recalled + (.50 × proportion of strings recalled in the next string length).
2.2.4. Composite scores
In order to assess the relationship between word learning performance and the language and STM measures, we collapsed the scores of the participants with aphasia into five composite measures. Composite phonological processing summed up performance on the Phonological discrimination and the Rhyming judgments subtests, whereas composite lexical-semantic processing involved the Lexical comprehension and Category judgments subtests. These two composite scores reflected the status of the word processing abilities of the aphasic participants without speech output requirements. The STM composite scores were determined according to two factors: (i) whether measures involved phonological or lexical-semantic support, (ii) whether or not measures involved speech production demands. Accordingly, composite nonword repetition included nonword repetition subtests in both interval conditions measuring phonological STM with speech output, composite repetition span included the word and digit repetition span tests tapping lexical-semantic STM with speech output, and composite pointing span included the word and digit pointing span tests measuring lexical-semantic STM without speech output. This last composite does not require speech production which can be disrupted in aphasia, and therefore it can be considered as a more pure measure of lexical-semantic STM. The raw scores on each of the TALSA subtests were converted into percentages of correct responses and composite scores were obtained by calculating the mean percentage of correct performance of all the measures involved, except for composites pointing and repetition span which represent the average span of the STM subtests involved in each composite.
2.3. Object-word learning task
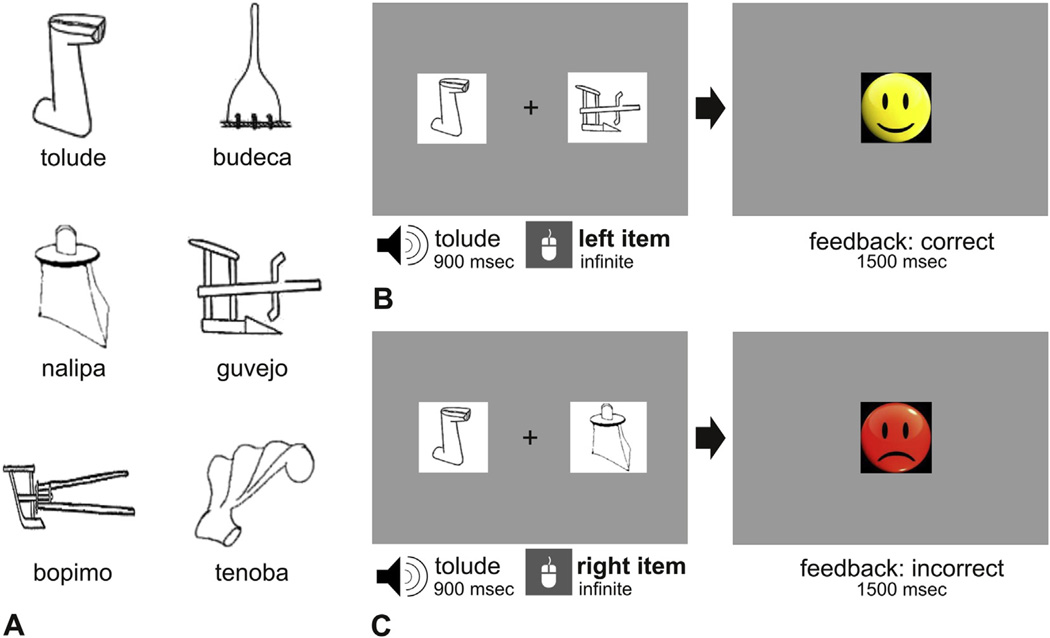
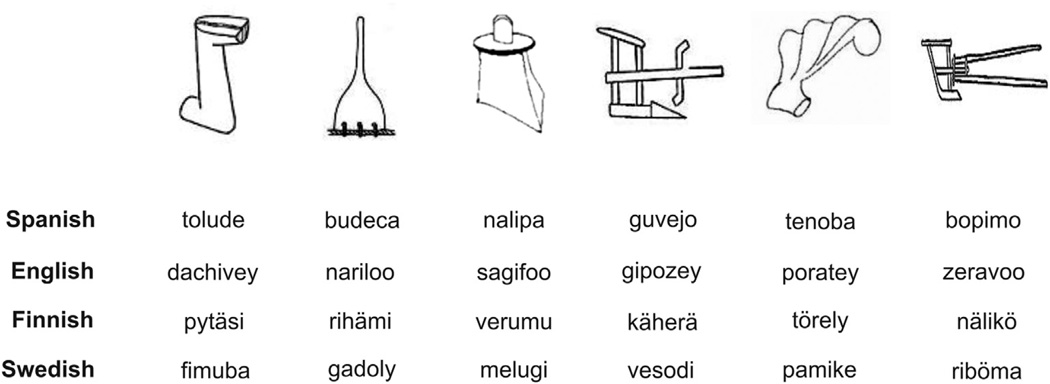
The experimental word learning task reported in this study was similar in structure to that reported in Magnuson et al. (2003) and Mirman et al. (2008). Fig. 1 depicts the experimental design of the task. The auditory stimuli consisted of six trisyllabic pseudo-words (hereafter words). A set of six words was developed according to the phonotactic rules of each of the native languages of the participants, ensuring that the syllables used to create the words were frequent at the first, second and third position of real words of each language. The words were matched in word length and number of syllables across languages. The audio files of these words were generated with MBROLA (Dutoit, Pagel, Pierret, Bataille, & van der Vreken, 1996) using a monotone male voice in all cases. All phonemes had the same duration (150 msec) and pitch (200 Hz; equal pitch rise and fall, with maximum pitch at 50% of the phoneme). The visual referents included six black and white outline drawings of objects from the Ancient Farming Equipment Paradigm (Laine & Salmelin, 2010). The same set of images was used across languages (see Fig. 1 for the set of images and the Appendix A for the words used in each language).
Fig. 1.
Word learning task. Panel A shows the stimuli used with the Spanish speaking participants. Panels B and C depict a sample of two learning trials with a correct and an incorrect response respectively. In each trial, two novel objects, a target and a foil were presented together with a spoken label and participants needed to decide whether the spoken word was the label for the picture on the left or the right side of the screen by pressing the corresponding mouse button (bold text). Visual feedback was provided following the correct (B) or incorrect (C) association between novel words and visual referents in each trial.
The word learning task consisted of 210 trials distributed across 7 learning blocks (30 trials per block). In each trial two novel objects (target and foil) appeared on the screen, one to the left and one to the right of a fixation cross. At the same time, the participant could hear the label (word) that corresponded to one of the two objects. The participant was required to decide whether the spoken word was the label for the object appearing on the left or on the right side of the screen by pressing the corresponding mouse button. Visual feedback was provided following the participant’s response in each trial: a yellow happy face appeared on the screen when the word-object association was correct, and a red sad face followed an incorrect association. The two visual referents remained on the screen until a response was given, and the visual feedback was presented for 1500 msec. After feedback offset, a fixation cross appeared at the center of the screen for 1000 msec signaling the beginning of the next trial. The 30 learning trials within each block resulted from the exhaustive combination of each object with the remaining 5 objects yielding 5 object pairs. These object pairs were exhaustively associated with the 6 to-be-learned words. Thus, during the 7 learning blocks, each object was presented 35 times as the correct visual referent for each word, and 35 times as the foil. The position of the target object on the screen was counterbalanced across trials. The order of trials was randomized separately for each participant. The recognition of the word-referent associations was evaluated immediately after training and at 1 week. Each of these assessments consisted of an additional block of 30 randomized trials. The trials in these testing blocks were similar to those of the learning phase but no feedback was provided for response accuracy.
The task was presented on E-prime 2.0 (Psychology Software Tools. Inc., PA, USA). At the beginning of the word learning task, participants were instructed to pay attention to the novel word they would hear and to carefully look at the pair of objects appearing simultaneously on the screen, as they were to decide which one of the two objects corresponded to the label provided. They were also told that a happy face would appear if their response was correct, and a sad face would indicate an incorrect label-object association. All participants were encouraged to respond as quickly and accurately as possible. In order to reduce fatigue effects due to the task length, short pauses (ca. 1–2 min) were held every two learning blocks (60 trials). Once the 7 learning blocks were completed and a short pause was held, participants underwent the first test block assessing the immediate recognition of the word-referent associations after learning. They were explained that this time they would not receive feedback after response; therefore they needed to be careful with their responses. All participants were available for re-testing at 1 week. At this time, they were reminded of the same instructions, and were administered the second test block of 30 trials without feedback.
3. Results
3.1. Learning curves
The learning curves were analyzed using multilevel regression (growth curve analysis, GCA: Mirman, 2014) with a second-order orthogonal polynomial model of change over time, fixed effects of time (block) and group, and random effects of participants for each of the time terms. Because the outcome variable is dichotomous (correct or incorrect response), the data were analyzed using logistic GCA. Model comparisons were used to evaluate overall group differences with respect to particular time terms. In orthogonal polynomial models, the intercept corresponds to overall average outcome, the linear term corresponds to the linear slope, and the quadratic term corresponds to the curvature.
3.1.1. Group differences
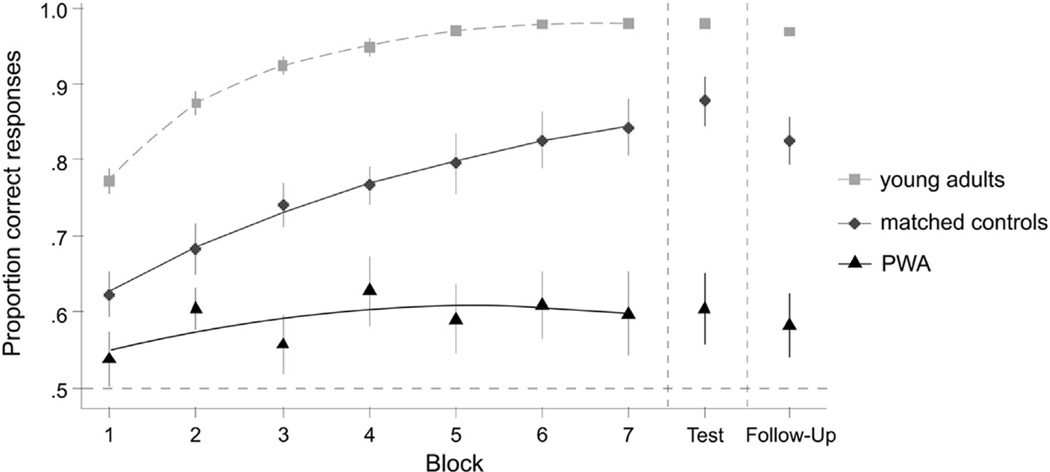
In order to study group differences, the matched control group was set as the reference level for the fixed effect of Group and parameters were estimated for the young adult and the patient group relative to the matched control group. The logistic GCA revealed statistically significant differences between groups in terms of overall accuracy during learning [Intercept term: χ2 (2) = 33, p < .001] and in terms of linear increase in accuracy [Linear term: χ2 (2) = 48, p < .001]. There was no overall group difference in curvature of the learning curves [Quadratic term: χ2 (2) = 4.13, p = .13]. The group learning curves with logistic GCA model fits are shown in Fig. 2.
Fig. 2.
Comparison of learning curves for the young adults, matched controls and participants with aphasia (PWA). The observed (symbols) and logistic GCA model fit (lines) learning curves are depicted for the three groups.
The parameter estimates capture pairwise group comparisons. These revealed that compared to the matched control group, the mean proportion correct responses during learning was significantly lower for the participants with aphasia (Estimate = −.863, SE = .296, p = .003) and was higher for the young adult group (Estimate = 1.95, SE = .25, p < .001). The linear slope of the learning curves was also shallower for the participants with aphasia compared with the matched controls (Estimate = −1, SE = .413, p = .015) and steeper for the young adults compared with the matched controls (Estimate = 1.77, SE = .385, p < .001). We found no significant differences in the curvature of accuracy increase for the participants with aphasia (Estimate = −.055, SE = .211, p > .05) or the young adults (Estimate = −.396, SE = .218, p > .05) compared with the matched controls.
3.1.2. Learning and aphasia severity
To examine the effect of aphasia severity on word learning, the analyses were restricted to just the participants with aphasia and the individual scores on the BDAE severity rating scale were added as a continuous fixed effect on each time term. As in the group comparisons above, the base model contained the fixed effects of each time term (intercept, linear, and quadratic) and random effects of participants for each of the time terms. The continuous fixed effect of severity on each time term was added to successively evaluate its continuation to model fit. Model comparisons revealed severity-related differences on the intercept term [overall percent correct: χ2 (1) = 8.33, p = .004] and the quadratic term [curvature: χ2 (1) = 5.54, p = .019]. These differences seemingly reflect differences in the learning performance of the participants with aphasia in the early part of the learning curves rather than the outcome of learning. In other words, while the less severely affected participants achieved fast learning that hit a plateau after 3–4 blocks, the more severely affected participants showed slow learning that reached the same level at the end of the 7 learning blocks.
3.2. Recognition tests
3.2.1. Immediate recognition test
Each of the three groups performed significantly above chance on the immediate recognition test (Table 5). Logistic regressions indicated that the differences between groups were also highly reliable with the participants with aphasia performing substantially worse than their matched controls (Estimate = − 1.55, SE = .18, p < .001) and the young adults performing much better than the matched controls (Estimate = 1.99, SE = .251, p < .001).
Table 5.
Recognition tests: group mean performance (95% confidence interval in parentheses). All means are above chance performance (.5) according to exact binomial test (all p < .001).
| Recognition test | Matched controls | Participants with aphasia | Young adults |
|---|---|---|---|
| Immediate | .879 (.843–.908) | .605 (.556–.652) | .981 (.973–.988) |
| Follow-up | .826 (.786–.861) | .583 (.535–.631) | .970 (.960–.979) |
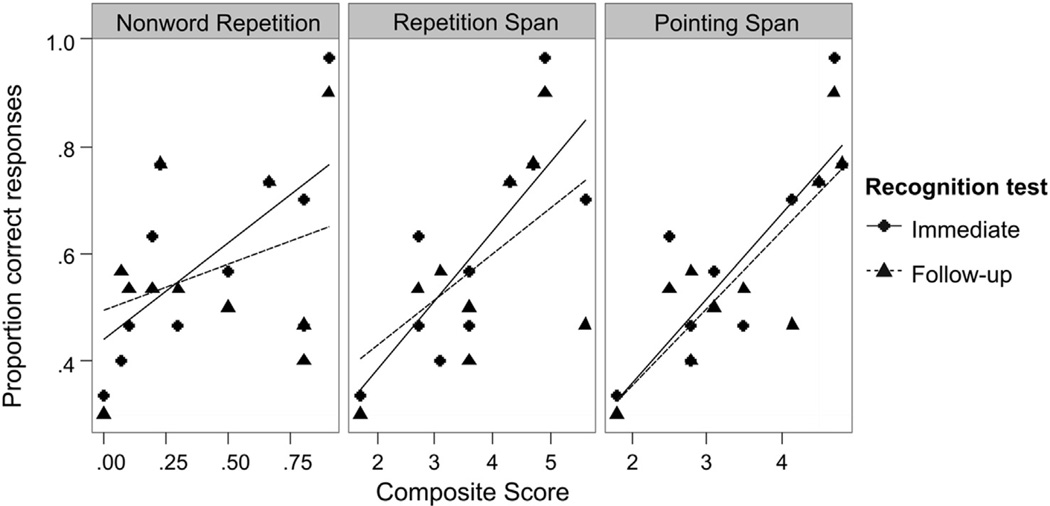
The correlations between word learning and the three STM composite scores are depicted in Fig. 3. Stepwise logistic regression was used to evaluate predictors of performance on the immediate recognition test for the participants with aphasia (n = 11) (Table 6). Individually, overall aphasia severity and each of the composite scores predicted word learning performance. However, after controlling for aphasia severity, only the verbal STM composite measures (nonword repetition, repetition span and pointing span) captured additional variance in performance. Note that composite nonword repetition and composite pointing span were marginally correlated (r = .53, p = .095), and composite repetition span was highly correlated with the composite nonword repetition (r = .76, p = .007) and with the composite pointing span (r = .83, p = .002). Therefore, the effects of STM measures on recognition performance should not be considered independent effects.
Fig. 3.
Correlations between recognition performance and composite scores of STM. Only phonological STM (nonword repetition) and lexical-semantic STM (repetition and pointing span) composite scores predicted the immediate and follow-up recognition of the newly acquired word-object associations beyond aphasia severity.
Table 6.
Predictors of recognition memory performance for participants with aphasia.
| Variable | Estimate | Std. error | z value | p-value | χ2(i) | p-value |
|---|---|---|---|---|---|---|
| Immediate recognition test | ||||||
| Aphasia severity | .251 | .086 | 2.926 | .003 | ||
| Composite phonological | 4.822 | 1.190 | 4.051 | .000 | 1.033 | .310 |
| Composite lexical-semantic | 3.001 | 1.076 | 2.790 | .005 | 2.208 | .137 |
| Composite nonword repetition | 1.563 | .377 | 4.145 | .000 | 6.826 | .009 |
| Composite pointing span | .698 | .132 | 5.288 | .000 | 7.783 | .005 |
| Composite repetition span | .579 | .112 | 5.150 | .000 | 10.538 | .001 |
| Follow-up recognition test | ||||||
| Aphasia severity | .308 | .086 | 3.564 | .000 | ||
| Composite phonological | 4.596 | 1.194 | 3.850 | .000 | .017 | .897 |
| Composite lexical-semantic | 4.060 | 1.156 | 3.511 | .000 | .343 | .558 |
| Composite nonword repetition | .720 | .358 | 2.011 | .044 | .000 | .984 |
| Composite pointing span | .622 | .128 | 4.843 | .000 | .929 | .335 |
| Composite repetition span | .359 | .104 | 3.439 | .001 | .411 | .521 |
Estimates correspond to tests of individual variables. The final two columns show the effect of composite scores after aphasia severity is already in the model.
3.2.2. Follow-up recognition test
There were only slight decreases in performance after one week (Table 5), with each group still showing above-chance performance and the group differences remaining statistically significant: the participants with aphasia still performed substantially worse than the matched controls (Estimate = −1.22, SE = .162, p < .001) and the young adults still performed much better than the matched controls (Estimate = 1.93, SE = .206, p < .001).
Table 6 shows tests of predictors of performance on the follow-up recognition test for the participants with aphasia. Individually, overall aphasia severity and each of the composite scores predicted performance, but none of the composite scores were significant after controlling for aphasia severity. However, the relationship between recognition test performance and composite verbal STM measures was quite consistent across the immediate and follow-up recognition tests. In order to directly test whether the relationship between verbal STM composite measures and recognition test performance changes from immediate to follow-up testing, we combined the data from both recognition tests and analyzed them using multilevel logistic regression. After effects of aphasia severity and test time (immediate us follow-up) were accounted for, there was an additional highly reliable main effect for each of the composite verbal STM composite measures [nonword repetition: χ2 (1) = 38.7, p < .0001; repetition span: χ2 (1) = 40.1, p < .0001; pointing span: χ2 (1) = 40.3, p < .0001] and a marginal STM by test time interaction [nonword repetition: χ2 (1) = 3.8, p = .05; repetition span: χ2 (1) = 3.5, p < .06; pointing span: χ2 (1) = 1.8, p > .18]. In other words, these verbal STM composite measures accounted for substantial variance in word learning beyond aphasia severity, although this effect weakened slightly between the immediate and follow-up recognition tests (except for pointing span which does not appear to weaken) as observed in Fig. 3.
3.3. Effects of lesion location
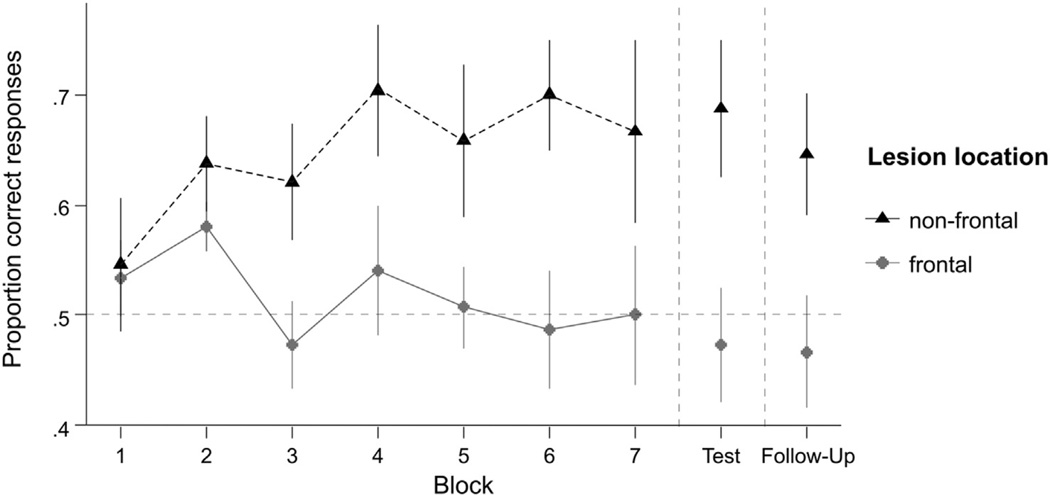
The learning and recognition performance of the participants with aphasia were compared according to gross lesion location (frontal lesion n = 5 vs non-frontal lesion n = 8). Participant BB was excluded from these analyses as he had a right hemisphere stroke. Fig. 4 shows that the frontal lesion group performed worse during both word learning and on recognition tests. The recognition test performance by lesion location is presented in Table 7, along with binomial tests showing that the frontal lesion group was not different from chance but the non-frontal lesion group was well above chance. During word learning, there was a significant effect of lesion location on overall accuracy [intercept term: χ2 (1) = 33.6, p < .001] and a marginal effect on the slope of the learning curve [linear term: χ2 (1) = 3.79, p = .052)]. During the recognition tests, after controlling for differences due to aphasia severity and test time, there was a significant effect of lesion location [χ2 (1) = 13, p = .0003], that was approximately constant across the immediate and follow-up recognition tests [no lesion location by test time interaction: χ2 (1) = .829, p = .36]. However, the lesion location effects were reduced when additionally controlling for verbal STM effects: they became non-significant after composite nonword repetition or composite repetition span was included in the model [both χ2 (1) < 1.0, p > .3] and only marginally significant after composite pointing span was in the model [χ2 (1) = 2.93, p = .087].
Fig. 4.
Effect of lesion location on learning curves and recall tests. The learning performance of the aphasic participants with frontal lesions and non-frontal lesions is shown for comparison. The mean (symbols) and SEM of each group is depicted at each time point over seven learning blocks and two test blocks. Note that the learning and recognition performance of the participants with aphasia with non-frontal lesions was clearly superior to the performance of the aphasic participants with frontal lesions.
Table 7.
Recognition tests: mean performance (with 95% confidence interval and p-value for difference from chance based on exact binomial test) by test and gross lesion location.
| Recognition Test |
Damaged frontal region |
Spared frontal region |
|---|---|---|
| Immediate | .473 (.391–.556) | .688 (.625–.746)*** |
| Follow-up | .467 (.385–.550) | .646 (.582–.706)*** |
Significant at the .001 probability level.
3.4. Lesion location and verbal STM
We used a Mann–Whitney U test to examine group differences in verbal STM according to lesion location. The verbal STM scores of participant AE with a frontal lesion and participant BL with a lesion that spared the frontal region were unavailable. This test showed that the non-frontal lesion group (Md = 4.15, n = 7) had significantly better verbal STM capacity than the frontal lesion group (Md = 2.65, n = 4) as measured by their composite pointing span scores (U = .00, z = −2.66, p = .008). The differences between these two groups in their composite repetition span and composite nonword repetition scores were non-significant (p > .010 in both cases).
3.5. Individual differences in learning performance in aphasia
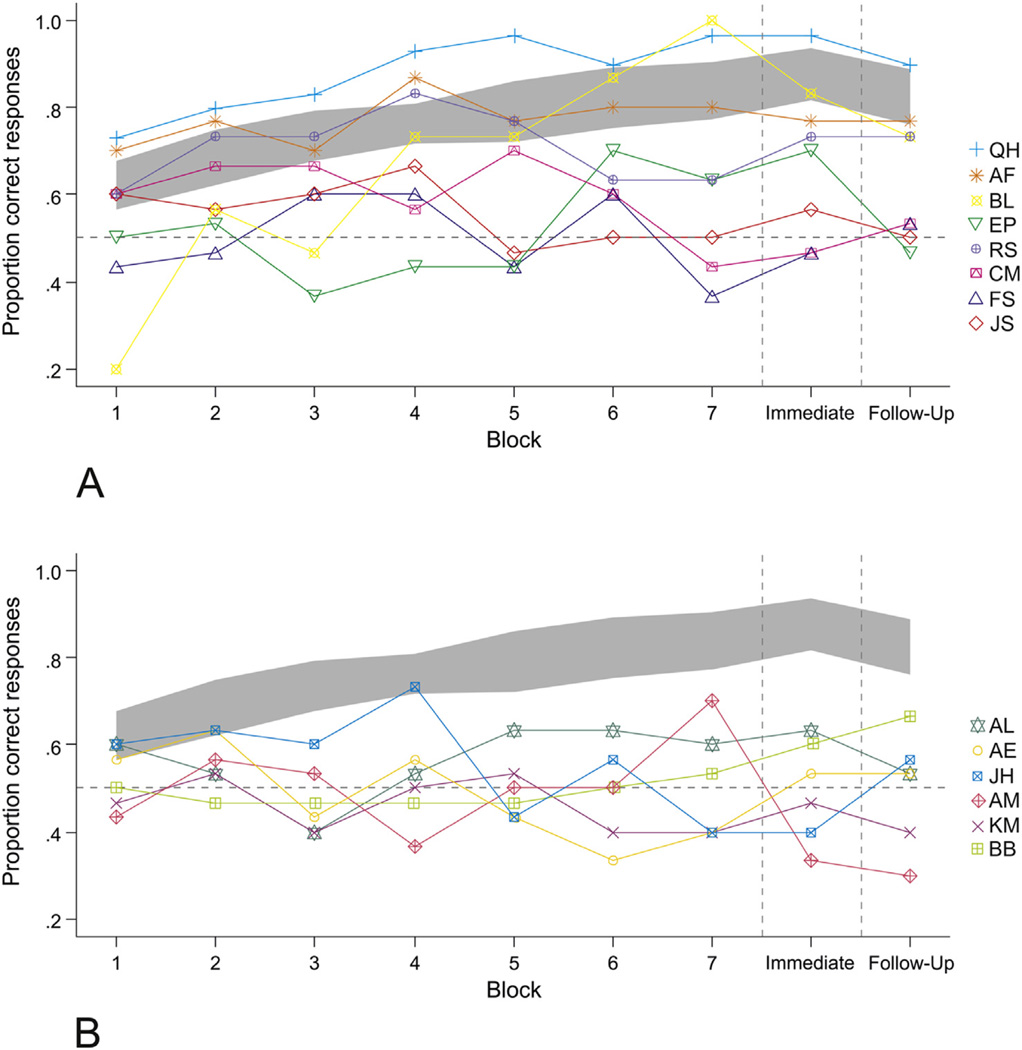
The individual learning curves of the participants with aphasia are depicted in Fig. 5. Individual differences in the outcome of learning performance were examined in the aphasia group using the exact binomial test to contrast response accuracy in the immediate and follow-up recognition tests against chance level. Participants AF, RS, QH, BL, and EP performed significantly above chance in the immediate recognition test (binomial test, p ≤ .042 in all cases) and all of these participants except EP performed significantly above chance in the follow-up test (binomial test, p ≤ .016 in all cases). All the remaining participants were not reliably different from chance level in both recognition tests (binomial test, p > .05 in all cases).
Fig. 5.
Individual variability in performance across learning and recognition testing blocks. Panel A and B depict the individual performances of participants with spared frontal region and participants with damaged frontal region respectively. Panel B also includes the learning performance of participant BB with a right hemisphere stroke. The individual learning curves of the participants with aphasia are displayed over the seven learning blocks with online feedback, and the immediate and follow-up recognition test blocks without feedback. The gray ribbon shows 95% confidence interval for the matched control participants (computed by nonparametric bootstrap) for comparison.
4. Discussion
The present study aimed to examine novel word learning ability in aphasia using a paradigm that presents novel words together with a limited set of novel visual referents, thus simulating the word-to-referent mapping ambiguity of natural language learning contexts. The logistic GCA revealed that the aphasia group showed a lower overall accuracy and slower learning performance than the matched control group. However, the immediate and follow-up recognition performances of the aphasia group were significantly above chance, indicating that at least some aphasic participants could successfully acquire the novel word-referent associations. Importantly, the subsequent individual analyses revealed that some people with chronic aphasia preserve the ability to learn novel word-object associations and maintain this newly acquired small lexicon 1 week after training, supporting previous reports of spared word learning ability in aphasia in single word-picture pairing tasks (McGrane, 2006; Tuomiranta et al., 2012, 2014) and in more referentially ambiguous learning conditions (Breitenstein et al., 2004).
A large range of new word learning performance is often observed in aphasia (Kelly & Armstrong, 2009). In our cohort, individual variability in new word learning was modulated by aphasia severity, phonological and lexical-semantic processing, and verbal STM composite measures. However, only verbal STM measures (not phonological or lexical-semantic processing measures) captured unique variance beyond aphasia severity, with phonological STM (i.e., composite nonword repetition) and lexical-semantic STM (i.e., composites repetition and pointing span) all predicting word learning. This is consistent with language-based models of verbal STM, which postulate that STM supports the maintenance of both phonological and lexical-semantic representations of verbal information (Martin & Saffran, 1997; Martin et al., 1999), and suggest that phonological and semantic components of STM are essential for the long-term learning of such representations (Freedman & Martin, 2001).
In our paradigm, learners necessarily depart from random correspondences between unrelated words and pictures which are nevertheless informative for subsequent attempts. Learning the correct word-referent associations may require the temporary retention of the phonological representations of the novel words while the learner evaluates the referent candidates presented in each trial and selects one on the basis of their perceptual features and the visual feedback of previous correct and incorrect associations. However, the retention of the novel semantic information (i.e.: visual referents) is important because even when one referent has been chosen, the two alternative referents provided in a given trial need to be held in the short-term until visual feedback is presented. Insofar as the novel referents constitute basic visual-semantic representations, these must be maintained in semantic STM in order for feedback to be used for learning (i.e., positive feedback strengthens selected referent, negative feedback strengthens alternative referent). In this way, verbal STM plays a crucial role in new word learning by holding the new phonological and semantic representations long enough to allow the formation of long-term associations (Freedman & Martin, 2001). With more trials, both phonological and semantic STM ensure that the correct mappings stay active and that their memory traces are strengthened over time, thus reducing the likelihood of mapping novel words onto wrong referents in subsequent trials. This is coherent with the mutual exclusivity constraint on word learning (Markman, 1990) which suggests that learning can be more efficient when one word can only be associated with one picture. In this way, learners can narrow down the possible candidates for unmapped words as otherwise they would persist in making randomly conflicting word-picture associations (Yu et al., 2007).
Importantly, because of this close relationship between verbal STM and word learning, factors that impact verbal STM capacity may also impair word learning ability. Reduced STM capacity for phonological or semantic codes involves their overly rapid decay from verbal STM (Freedman & Martin, 2001). Verbal STM is often affected in aphasia (Martin & Ayala, 2004; Martin & Saffran, 1999) and reduced capacity to retain phonological or semantic codes in the short-term can account for impaired ability to learn the corresponding novel phonological or semantic information in aphasic individuals (Freedman & Martin, 2001). Performance in the present word learning task should reflect the individual ability to map the novel word forms onto the right lexical-semantic representations and to recognize such representations when cued by the novel phonology at testing, thus drawing on both the lexical-semantic components of the associations and the active recall of the novel word phonology. We found that the participants with aphasia with more spared verbal STM also showed better word learning performance. Participants AF, QH, EP, RS and BL showed individual learning curves that resembled the matched controls’ average and achieved the highest scores on the recognition tests, and four of these participants also had the highest lexical-semantic STM spans (STM spans for BL were unavailable). Participants QH, EP and RS also had the highest phonological STM spans. In contrast, the remaining aphasic participants had lower scores in the recognition tests and were more impaired in their lexical-semantic and phonological STM.
Interestingly, while participant AF’s lexical-semantic STM and word learning performance were high in spite of his impaired phonological STM, participant KM whose phonological STM was amongst the highest in the aphasia group, had impaired lexical-semantic STM and word learning ability.3 This suggests that in aphasia, the retention of the novel lexical-semantic representations might be more crucial than the retention of the novel phonology for effective word learning in a highly ambiguous word-referent context. According to language-based models of STM, there are multiple levels of lexical representation that contribute to STM (Martin & Saffran, 1997; Martin et al., 1999) and previous studies have evidenced the dissociation of such components (Freedman & Martin, 2001; Majerus et al., 2004; Shivde & Thompson-Schill, 2004). The differences in word learning and verbal STM between participants AF and KM point in this direction. However, although our relatively pure measures of phonological (composite nonword repetition) and lexical-semantic STM (composite pointing span) were not significantly correlated, we had limited power to detect independent effects of these word learning predictors. Future research with a larger sample of aphasic individuals is needed to determine whether or not phonological and lexical-semantic STM capacity make independent contributions to novel word learning in aphasia in high word-referent ambiguity contexts.
We also found a significant effect of lesion location on word learning in aphasia, although this effect was reduced after controlling for verbal STM probably due to STM deficits resulting from anterior brain damage. The dorsal and ventral processing speech pathways have been related to the temporary maintenance of verbal information (Majerus, 2013). The dorsal speech pathway (Hickok & Poeppel, 2004, 2007), a left lateralized network for auditory-motor integration, is implicated in novel phonology processing (Kümmerer et al., 2013) and language acquisition (Scott & Wise, 2004; Rodríguez-Fomells, Cunillera, Mestres-Missé, & de Diego-Balaguer, 2009). This neural circuit also supports phonological verbal STM (Hickok & Poeppel, 2000) with the posterior superior temporal gyrus (pSTG) as the neural basis for phonological storage and the posterior inferior frontal regions (including Broca’s area, insula, and dorsal premotor regions) supporting the rehearsal of phonological codes (Hickok & Poeppel, 2004). The ventral speech pathway (Hickok & Poeppel, 2004, 2007) is a fronto-temporal network supporting the mapping of sounds to meanings (Saur et al., 2008). Within this language stream, the middle temporal cortex and a more anterior part of the left inferior frontal cortex are involved in the retention of semantic information (Martin et al., 2003; Shivde & Thompson-Schill, 2004). It has been suggested that while the storage of phonological information is commonly localized in posterior regions of the left cortex, the inferior frontal regions could support the retention and rehearsal of verbal information, be it semantic or phonological in nature (Martin et al., 2003). In our study, the aphasia group with lesions that spared the left frontal cortex had a significantly superior overall learning and recognition performance than the group with left frontal lesions who were clearly impaired in this ability. It has been proposed that people with lesions involving inferior frontal regions experience difficulty with tasks that draw on semantic and phonological STM, whereas individuals with posterior lesions mainly show deficits that selectively impair phonological STM (Martin et al., 2003). We found that lexical-semantic STM as measured by the composite pointing span was significantly more preserved in participants with non-frontal lesions which predominantly involved the left temporal and/or parietal cortex, than in participants with frontal lesions. It is likely that inferior frontal lesions lead to a rapid decay of the semantic representations of novel words, impairing the encoding and recognition of the correct novel word-referent associations. Conversely, aphasic individuals with spared frontal regions may preserve the ability to retain the correct semantic representations for encoding and recognition when cued by novel phonological input, as reflected by their superior word learning ability. The frontal and non-frontal lesion groups did not significantly differ in their composite repetition span scores, yet the composite pointing span provides a relatively more pure measure of lexical-semantic STM capacity because it is not influenced by verbal output capacity. We found no significant differences in phonological STM capacity between the frontal and the non-frontal lesion groups. Although the participants with non-frontal lesions showed higher performance than those with frontal lesions, their composite nonword repetition scores represented equal or below 50% correct performance (except for QH and EP who were largely spared in their phonological STM), probably due to damage to regions that support the phonological storage component of phonological STM. Nevertheless, substantial learning in the non-frontal lesion group may be due to more preserved lexical-semantic STM and only enough ability to retain the novel phonology, whereas word learning deficits in the frontal lesion group may be related to an impairment that affects both lexical-semantic and phonological STM. While it is likely that anterior frontal lesions impair novel word learning in aphasia by impairing verbal STM capacity as discussed here, our aphasic sample is not sufficiently large to tease apart contributions of lesion location and STM to word learning. The possibility that damage to anterior frontal regions impairs word learning per se, beyond its effect on verbal STM, should be further studied.
Although not directly examined in the present study, it is likely that attentional control and executive processes involved in complex storage and processing tasks (Majerus, 2013) are also engaged in novel word learning under referential ambiguity. Learners should focus their attention on the relevant aspects of the learning context to correctly identify the proper meanings of new words (Rodríguez-Fornells et al., 2009), while the erroneous competing associations of alternative word-object couplings are inhibited and progressively suppressed during this lexical acquisition process (Yu & Smith, 2007). Frontal lesions can impair executive functioning affecting verbal memory performance in aphasia (Beeson, Bayles, Rubens, & Kaszniak, 1993). Moreover, impaired executive control of inhibition processes may lead to deficits in the retention of semantic representations in STM in aphasia due to increased interference from irrelevant information (Martin & Allen, 2008). Executive dysfunction may also impair learning in our participants with frontal lesions by compromising their ability to monitor response accuracy and feedback processing, two cognitive aspects that contribute to learning mechanisms (McCandliss, Fiez, Protopapas, Conway, & McClelland, 2002; McClelland, Thomas, McCandliss, & Fiez, 1999). External feedback may be effectively used to increase the production of target responses of aphasic individuals in word learning tasks (but see Breitenstein et al., 2004 for evidence of learning without feedback) and it can be helpful to control for the difficulty of learning in errorful situations (McCandliss et al., 2002). Yet, the production of errors may reinforce incorrect associations when feedback processing is compromised (Fillingham, Hodgson, Sage, & Lambon Ralph, 2003). In our study, performance feedback signaled response correctness to reduce the production of erroneous word-referent associations, as referential ambiguity could otherwise increase the possibility of forming incorrect associations. Nevertheless, the production of erroneous associations in the absence of optimal feedback modulation mechanisms may have hindered learning by increasing their likelihood of occurrence in subsequent learning trials due to their reactivation through familiarity-based implicit memory mechanisms (Anderson & Craik, 2006; Baddeley & Wilson, 1994). The fact that the early part of the learning curves was influenced by aphasia severity also suggests that the decreased availability of verbal STM and other cognitive resources impairs rapid word learning in aphasia.
Our study also contributes to the existing evidence of the overall decrement in learning ability associated with healthy aging. We found a clear learning advantage for young adults relative to their older counterparts who had a slower and less successful learning performance. Previous studies have shown that older adults perform worse than young adults when learning paired-associates (Service & Craik, 1993), single word-picture associations and more complex word-referent relationships in fast-mapping paradigms (Greve, Cooper, & Henson, 2014). These differences have been attributed to an age-related decline in episodic memory that affects the ability to create associations between individual items and contexts during encoding (Naveh-Benjamin, 2000; Naveh-Benjamin, Guez, Kilb, & Reedy, 2004; Overman & Becker, 2009). Moreover, such decreased learning performance in older adults has been related to a reduction of hippocampal grey matter volume (Greve et al., 2014). It has been proposed that younger subjects rely on lexical-semantic learning more effectively than older adults, using their existing lexicon to rapidly establish semantic associations between novel items and their familiar vocabulary to mediate new word learning, which in turn may reduce the need of maintaining previously unknown words in phonological STM (Service & Craik, 1993). This interpretation seems to be supported by the fact that the associative deficit observed in older adults can be reduced when the information to be held in memory can be supported by existing semantic connections (Naveh-Benjamin, Hussain, Guez, & Bar-On, 2003). Finally, our findings are in line with previous studies demonstrating age-related differences in adult learners in errorful or feedback-based learning situations (Anderson & Craik, 2006; Ferdinand & Kray, 2013). It has been shown that this type of learning promotes better recollection in young adults probably by engaging explicit memory mechanisms which allow for correct memory reinforcement and explicit error elimination. In contrast, older adults who depend more on automatic, implicit familiarity-based processes, may less effectively oppose familiarity-based errors due to a decline in explicit memory (Anderson & Craik, 2006). Moreover, older adults rely more on positive than negative feedback during learning as compared to young adults possibly due to their reduced working memory capacity (Ferdinand & Kray, 2013).
Regarding the existing proposals about the possible learning mechanisms underlying word learning under referential uncertainty (Trueswell et al., 2013; Yu et al. 2007; Vlach & Sandhofer, 2014), the present data cannot conclusively speak in favor of one versus the others. Our word learning paradigm based on Mirman et al. (2008) differed from those used in previous studies in several aspects: (i) the experimental manipulation of the number of learning conditions (i.e.: Vlach & Sandhofer, 2014), (ii) the inclusion of high and low informative learning instances (i.e.: Trueswell et al., 2013), (iii) the constraints placed on the order of presentation of each word learning instance (i.e.: Trueswell et al., 2013; Smith et al., 2011) and (iv) the exclusion of feedback (i.e.: Smith et al., 2011; Trueswell et al., 2013; Vlach & Sandhofer, 2014; Yu & Smith, 2007; Yu et al., 2007). Such methodological differences prevent these and our study from being directly comparable. Nevertheless, some considerations are worth taking into account. Both the cross-situational (Yu & Smith, 2007; Yu et al., 2007) and the propose-but-verify learning strategy (Trueswell et al., 2013) explicitly suggest that the hypothesized word-referent mappings need to be retained across learning instances. Regardless which account proves right, either tracking simultaneous co-occurrences for many words and referents (Yu & Smith, 2007; Yu et al., 2007) or testing single word-referent hypotheses (e.g.: because words appear interleaved across learning instances), learning in situations with referential ambiguity seems to rely also on STM capacity to some extent. It has been suggested that STM may modulate the ability to hold relevant information for successful word-object mapping under referential uncertainty, and the ability to retrieve such information during learning seems to lead to stronger retention of the acquired mappings in the long term (Vlach & Sandhofer, 2014).
There are of course some limitations in the present study. While examining the influence of verbal STM on new word learning in aphasia is important because STM deficits are common in this pathology, we did not address this relationship in the groups of healthy participants. Current research with healthy adults proposes that STM may modulate learning ability in high referential ambiguity conditions (Vlach & Sandhofer, 2014). Future studies are needed to determine whether and how verbal STM capacity in healthy individuals is related to the ability to learn new word-meaning mappings in such learning contexts. Furthermore, the influence of other cognitive processes not addressed in the present study (e.g.: executive functioning) on this type of word learning ability should also be examined in both aphasic and healthy individuals in the future. Finally, the influence of linguistic background and bilingualism in novel word learning in aphasia was beyond the scope of our study and the small size of the aphasia sample precluded any comparisons on word learning performance across the four languages involved in this study. It has been suggested that bilingualism may facilitate learning the phonology of novel words in healthy adult learners (Kaushanskaya & Marian, 2009), however, it remains unknown whether the same holds when learning ambiguous word-referent mappings and how this extends to people with aphasia. Further research may help revealing whether linguistic background in aphasic individuals also impacts their novel word learning possibilities.
5. Conclusions
The present study demonstrates that some people with aphasia can successfully acquire novel words even in word-referent ambiguity conditions, and maintain this lexical knowledge in the long-term in the absence of training and feedback. We suggest that verbal STM makes a unique contribution to new word learning in aphasia, with learning ability being critically influenced by the functionality of lexical-semantic and phonological STM mechanisms. Our findings further propose the integrity of the left inferior frontal region as the crucial component of the language-verbal STM network supporting the maintenance and the ultimate acquisition of novel linguistic material. Future studies should determine whether language rehabilitation could benefit from the use of more natural language learning approaches to potentiate language re-learning in aphasic individuals. Further contributions to this field should consider minimizing the cognitive demands (verbal STM, and possibly attentional and executive requirements) of learning tasks involving referential uncertainty. The use of errorless learning methods (Fillingham et al., 2003) and high frequency exposure to word-referent co-occurrence without feedback (Breitenstein et al., 2004) could be explored as potential ways to boost the participation of implicit memory mechanisms in the word-learning process, and decrease overall STM load and cognitive effort so that learning can be maximized in people with aphasia.
Acknowledgments
We thank all participants and especially the participants with aphasia and their relatives for their commitment to this study. The study conducted in Philadelphia was supported by the National Institutes of Health/National Institute on Deafness & Other Communication Disorders (NIHSS/NIDCD) under grant R01 DC01924-15 awarded to Nadine Martin. The study conducted in Turku was supported by a grant from the Academy of Finland (#135688) awarded to Matti Laine. In Spain, the study was supported by the Spanish Government under grant MICINN, PSI2011-29219 awarded to Antoni Rodríguez-Fornells. Claudia Peñaloza has been sponsored by an IDIBELL predoctoral fellowship.
Appendix A. Pictures and words used in the learning task according to the language group of the participants
Footnotes
The people with aphasia, their matched controls and most participants in the young adults group whose data is reported in the present work had been recruited for an earlier study on speech segmentation in aphasia (Peñaloza et al., 2015). Their demographic information as well as their background language testing provided in the earlier study is also reported here.
The subtests Phoneme discrimination, Rhyming judgements, Lexical comprehension, Category judgments, and Nonword repetition were administered under two conditions that varied memory load. In the 1-sec unfilled interval, the presentation of the stimuli of each pair is separated by 1-sec delay (Phoneme discrimination, Rhyming judgements, and Category judgments), or a response is required 1 sec after stimuli presentation (Lexical comprehension and Word-nonword repetition). In the 5-sec unfilled interval, a 5-sec gap is included between the presentation of each stimulus of a pair (Phoneme discrimination, Rhyming judgements, and Category judgments) or a response is required 5 sec after stimuli presentation (Lexical comprehension and Nonword repetition), thus allocating an additional load on the verbal short-term store.
Participant AF showed high word learning performance in spite of his impaired phonological STM capacity (composite nonword repetition expressed in proportion of correct responses = .23/1). His lexical-semantic STM capacity was well above 50% correct performance (composite STM pointing span = 4.8/7; composite STM repetition span = 4.7/7). Conversely, participant KM showed impaired word learning performance in spite of his largely spared phonological STM capacity (composite nonword repetition expressed in proportion of correct responses = .80/1). However, he also showed reduced lexical-semantic STM capacity (composite STM pointing span = 2.8/7; composite STM repetition span = 3.6/7).
Conflict of interest
The authors declare no competing financial interests.
REFERENCES
- Anderson N, Craik FIM. The mnemonic mechanisms of errorless learning. Neuropsychologia. 2006;44(14):2806–2813. doi: 10.1016/j.neuropsychologia.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Gathercole SE, Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105(1):158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Wilson BA. When implicit learning fails: amnesia and the problem of error elimination. Neuropsychologia. 1994;32(1):53–68. doi: 10.1016/0028-3932(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Beeson PM, Bayles KA, Rubens AB, Kaszniak AW. Memory impairments and executive control in individuals with stroke-induced aphasia. Brain and Language. 1993;45(2):253–275. doi: 10.1006/brln.1993.1045. [DOI] [PubMed] [Google Scholar]
- Bourassa D, Besner D. Beyond the articulatory loop: a semantic contribution to serial order recall of subspan lists. Psychonomic Bulletin and Review. 1994;1(1):122–125. doi: 10.3758/BF03200768. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Kamping S, Jansen A, Schomacher M, Knetcht S. Word learning can be achieved without feedback: implications for aphasia therapy. Restorative Neurology and Neuroscience. 2004;22(6):445–458. [PubMed] [Google Scholar]
- Breitenstein C, Zwitserlood P, de Vries MH, Feldhues C, Knecht S, Dobel C. Five days versus a lifetime: intense associative vocabulary training generates lexically integrated words. Restorative Neurology and Neuroscience. 2007;25(5–6):493–500. [PubMed] [Google Scholar]
- Carroll JB. Human cognitive abilities: a survey of factor-analytic studies. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Davis MH, Gaskell MG. A complementary systems account of word learning: neural and behavioural evidence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1536):3773–3800. doi: 10.1098/rstb.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzi E, Faglioni P. Normative data and screening power of a shortened version of the Token Test. Cortex. 1978;14(1):41–49. doi: 10.1016/s0010-9452(78)80006-9. [DOI] [PubMed] [Google Scholar]
- Dignam J, Copland D, Rawlings A, O’Brien K, Burfein P, Rodriguez AD. The relationship between novel word learning and anomia treatment success in adults with chronic aphasia. Neuropsychologia. 2016;81:186–197. doi: 10.1016/j.neuropsychologia.2015.12.026. [DOI] [PubMed] [Google Scholar]
- Dutoit T, Pagel N, Pierret F, Bataille O, van der Vreken O. The MBROLA project: towards a set of high-quality speech synthesizers free of use for non-commercial purposes. CSLP, Fourth International Conference on Spoken Language; Philadelphia. 1996. [Google Scholar]
- Ferdinand NJ, Kray J. Age-related changes in processing positive and negative feedback: is there a positivity effect for older adults. Biological Psychology. 2013;94(2):235–241. doi: 10.1016/j.biopsycho.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Fillingham JK, Hodgson C, Sage K, Lambon Ralph MA. The application of errorless learning to aphasic disorders: a review of theory and practice. Neuropsychological Rehabilitation. 2003;13(13):337–363. doi: 10.1080/09602010343000020. [DOI] [PubMed] [Google Scholar]
- Freedman ML, Martin RC. Dissociable components of short-term memory and their relation to long-term learning. Cognitive Neuropsychology. 2001;18(3):193–226. doi: 10.1080/02643290126002. [DOI] [PubMed] [Google Scholar]
- Gathercole SE. Nonword repetition and word learning the nature of the relationship. Applied Psycholinguistics. 2006;27(4):513–543. [Google Scholar]
- Gathercole SE, Addams A. Children’s phonological working memory: contributions of long-term knowledge and rehearsal. Journal of Memory and Language. 1994;33(5):672–688. [Google Scholar]
- Gathercole SE, Baddeley AD. Evaluation of the role of phonological STM in the development of vocabulary in children: a longitudinal study. Journal of Memory and Language. 1989;28(2):200–213. [Google Scholar]
- Gathercole SE, Service E, Hitch GJ, Adams AM, Martin AJ. Phonological short-term memory and vocabulary development: further evidence on the nature of the relationship. Applied Cognitive Psychology. 1999;13:65–77. [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. The assessment of aphasia and related disorders. 3rd. Baltimore: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. Evaluación de la afasia y los trastornos relacionados [The assessment of aphasia and related disorders, Spanish adaptation] 3rd. Madrid: Panamericana; 2005. [Google Scholar]
- Goodglass H, Wingfield A. Word-finding deficits in aphasia: brain-behavior relations and clinical symptomatology. In: Goodglass H, Wingfield A, editors. Anomia: Neuroanatomical and cognitive correlates. San Diego: Academic Press; 1997. pp. 3–30. [Google Scholar]
- Greve A, Cooper E, Henson RN. No evidence that fast-mapping benefits novel learning in healthy older adults. Neuropsychologia. 2014;60:52–59. doi: 10.1016/j.neuropsychologia.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean F, Li P. The psycholinguistics of bilingualism. New York: John Wiley & Sons, Inc; 2013. [Google Scholar]
- Gupta P. Examining the relationship between word learning, nonword repetition, and immediate serial recall in adults. The Quarterly Journal of Experimental Psychology. 2003;56(7):1213–1236. doi: 10.1080/02724980343000071. [DOI] [PubMed] [Google Scholar]
- Gupta P, Martin N, Abbs B, Schwartz M, Lipinski J. New word learning in aphasic patients: dissociating phonological and semantic components. Brain and Language. 2006;99:8–9. [Google Scholar]
- Gupta P, Tisdale J. Word learning, phonological short-term memory, phonotactic probability and long-term memory: towards an integrated framework. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1536):3755–3771. doi: 10.1098/rstb.2009.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitiue Science. 2000;4(4):131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reuietus Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. 3rd. Madrid: Panamericana; 2005. [Google Scholar]
- Kaushanskaya M, Marian V. The bilingual advantage in novel word learning. Psychonomic Bulletin and Review. 2009;16(4):705–710. doi: 10.3758/PBR.16.4.705. [DOI] [PubMed] [Google Scholar]
- Kelly H, Armstrong L. New word learning in people with aphasia. Aphasiology. 2009;23(12):1398–1417. [Google Scholar]
- Kertesz A. Western aphasia battery-revised. San Antonio: Pearson; 2006. [Google Scholar]
- Kümmerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Klöppel S, et al. Damage to ventral and dorsal language pathways in acute aphasia. Brain. 2013;136(2):619–629. doi: 10.1093/brain/aws354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine M, Koivuselkä-Sallinen P, Hänninen R, Niemi J. Bostonin nimentätesti [Finnish translation of the Boston Naming Test] Helsinki: Psykologien Kustannus Oy; 1997. [Google Scholar]
- Laine M, Niemi J, Koivuselkä-Sallinen P, Koivusalo A. The experimental Swedish version of the Boston Diagnostic Aphasia Examination. 2nd. 1986. Unpublished manuscript. [Google Scholar]
- Laine M, Niemi J, Koivuselkä-Sallinen P, Tuomainen J. Bostonin diagnostinen afasiatutkimus [The standardized Finnish version of the Boston Diagnostic Aphasia Examination] Helsinki: Psykologien Kustannus; 1997. [Google Scholar]
- Laine M, Salmelin R. Neurocognition of new word learning in the native tongue: lessons from the ancient farming equipment paradigm. Language Learning. 2010;60:25–44. [Google Scholar]
- Lindfors JW. Children’s language and learning. 2nd. Boston: Allyn and Bacon; 1991. [Google Scholar]
- Magnuson JS, Tanenhaus MK, Aslin RN, Dahan D. The microstructure of spoken word recognition: studies with artificial lexicons. Journal of Experimental Psychology: General. 2003;132(2):202–227. doi: 10.1037/0096-3445.132.2.202. [DOI] [PubMed] [Google Scholar]
- Majerus S. Language repetition and short-term memory: an integrative framework. Frontiers in Human Neuroscience. 2013;7:1–16. doi: 10.3389/fnhum.2013.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus S, van der Linden M, Poncelet M, Metz-Lutz MN. Can phonological and semantic short-term memory be dissociated? Further evidence from Landau-Kleffner Syndrome. Cognitive Neuropsychology. 2004;21(5):491–512. doi: 10.1080/02643290342000104. [DOI] [PubMed] [Google Scholar]
- Markman EM. Constraints children place on word meanings. Cognitive Science. 1990;14:57–77. [Google Scholar]
- Markman EM. Constraints on word learning in early language acquisition. Lingua. 1994;92:199–277. [Google Scholar]
- Marshall RC, Freed D, Karow CM. Learning of subordinate category names by aphasic subjects: a comparison of deep and surface-level training methods. Aphasiology. 2001;15(6):585–598. [Google Scholar]
- Martin R, Allen C. A disorder of executive function and its role in language processing. Seminars in Speech and Language. 2008;29(3):201–210. doi: 10.1055/s-0028-1082884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Ayala J. Measurements of auditory-verbal STM span in aphasia: effects of item, task, and lexical impairment. Brain and Language. 2004;89(3):464–483. doi: 10.1016/j.bandl.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Martin N, Kohen F, Kalinyak-Fliszar M. A processing approach to the assessment of language and verbal short-term memory abilities in aphasia. Clinical Aphasiology Conference; Charleston, SC. 2010. [Google Scholar]
- Martin N, Saffran EM. Language and auditory-verbal short-term memory impairments: evidence for common underlying processes. Cognitiue Neuropsychology. 1997;14(5):641–682. [Google Scholar]
- Martin N, Saffran EM. Effects of word processing and short-term memory deficits on verbal learning: evidence from aphasia. International Journal of Psychology. 1999;34(5–6):339–346. [Google Scholar]
- Martin RC, Lesch MF, Bartha MC. Independence of input and output phonology in word processing and short-term memory. Journal of Memory and Language. 1999;41:3–29. [Google Scholar]
- Martin RC, Shelton J, Yaffee LS. Language processing and working memory: neuropsychological evidence for separate phonological and semantic capacities. Journal of Memory and Language. 1994;33(1):83–111. [Google Scholar]
- Martin RC, Wu D, Freedman M, Jackson EF, Lesch M. An event-related fMRI investigation of phonological versus semantic short-term memory. Journal of Neurolinguistics. 2003;16(4–5):341–360. [Google Scholar]
- McCandliss B, Fiez J, Protopapas A, Conway M, McClelland J. Success and failure in teaching the/r/-/l/ contrast to Japanese adults: tests of a Hebbian model of plasticity and stabilization in spoken language perception. Cognitive, Affective and Behavioural Neuroscience. 2002;2(2):89–108. doi: 10.3758/cabn.2.2.89. [DOI] [PubMed] [Google Scholar]
- McClelland J, Thomas AG, McCandliss B, Fiez J. Understanding failures of learning: Hebbian learning, competition for representational space and some preliminary data. Progress in Brain Research. 1999;121:75–80. doi: 10.1016/s0079-6123(08)63068-x. [DOI] [PubMed] [Google Scholar]
- McGrane H. PhD thesis. 2006. An investigation into the ability of adults with post-stroke aphasia to learn new vocabulary. http://www.qmu.ac.uk/ssrc/pubs/McGrane2006PhD.pdf. [Google Scholar]
- Michas IC, Henry LA. The link between phonological memory and vocabulary acquisition. British Journal of Developmental Psychology. 1994;12(2):147–164. [Google Scholar]
- Mirman D. Growth curve analysis and visualization using R. Boca. Raton: Chapman & Hall/CRC Press; 2014. [Google Scholar]
- Mirman D, Magnuson JS, Estes KG, Dixon JA. The link between statistical segmentation and word learning in adults. Cognition. 2008;108(1):271–280. doi: 10.1016/j.cognition.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation ISP. Learning vocabulary in another language. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Naveh-Benjamin M. Adult age difference in memory performance: tests of an associative deficit hypothesis. Journal of Experimental Psychology, Learning, Memory and Cognition. 2000;26(5):1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative memory deficit in older adults: further support using face-name associations. Psychology and Aging. 2004;19(3):541–546. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Hussain Z, Guez J, Bar-On M. Adult age differences in episodic memory: further support for an associative-deficit hypothesis. Journal of Experimental Psychology, Learning, Memory and Cognition. 2003;29(5):826–837. doi: 10.1037/0278-7393.29.5.826. [DOI] [PubMed] [Google Scholar]
- Nickels L. Therapy for naming disorders: revisiting, revising, and reviewing. Aphasiology. 2002;16(10–11):936–979. [Google Scholar]
- Overman Becker. The associative deficit in older adults memory: recognition of pairs is not improved by repetition. Psychology and Aging. 2009;24(2):501–506. doi: 10.1037/a0015086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagno C, Vallar G. Verbal short-term memory and vocabulary learning in polyglots. Quarterly Journal of Experimental Psychology. 1995;48(1):98–107. doi: 10.1080/14640749508401378. [DOI] [PubMed] [Google Scholar]
- Peñaloza C, Benetello A, Tuomiranta L, Heikius IM, Järvinen S, Majos MC, et al. Speech segmentation in aphasia. Aphasiology. 2015;29(6):724–743. doi: 10.1080/02687038.2014.982500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier M, Saint-Aubin J. Memory for related and unrelated words: further evidence of the influence of semantic factors in immediate serial recall. Quarterly Journal of Experimental Psychology. 1995;48(2):384–404. doi: 10.1080/14640749508401396. [DOI] [PubMed] [Google Scholar]
- Rodíguez-Fornells A, Cunillera T, Mestres-Missé A, de Diego-Balaguer R. Neurophysiological mechanisms involved in language learning in adults. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1536):3711–3735. doi: 10.1098/rstb.2009.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg AR, Saffran JR. Statistical learning and language acquisition. Wiley Interdisciplinary Reviews: Cognitive Science. 2010;1(6):906–914. doi: 10.1002/wcs.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodenrys S, Hulme C, Alban J, Ellis A, Brown GD. Effects of word frequency and age of acquisition on short-term memory span. Memory and Cognition. 1994;22(6):695–701. doi: 10.3758/bf03209254. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, et al. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Wise RJS. The functional neuroanatomy of prelexical processing in speech perception. Cognition. 2004;92(1–2):13–45. doi: 10.1016/j.cognition.2002.12.002. [DOI] [PubMed] [Google Scholar]
- Service E. Phonology, working memory, and foreign-language learning. Quarterly Journal of Experimental Psychology. 1992;45(1):21–50. doi: 10.1080/14640749208401314. [DOI] [PubMed] [Google Scholar]
- Service E, Craik FIM. Differences between young and older adults in learning a foreign vocabulary. Journal of Memory and Language. 1993;32(5):608–623. [Google Scholar]
- Shelton J, Martin R, Yaffee L. Investigating a verbal short-term memory deficit and its consequences for language processing. In: Margolin D, editor. Cognitive neuropsychology in clinical practice. New York: Cambridge University Press; 1992. pp. 131–167. [Google Scholar]
- Shivde G, Anderson MC. On the existence of a working memory system for the active maintenance of semantics. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37(6):1342–1370. doi: 10.1037/a0024832. [DOI] [PubMed] [Google Scholar]
- Shivde G, Thompson-Schill SL. Dissociating semantic and phonological maintenance using fMRI. Cognitive, Affective & Behavioral Neurosicence. 2004;4(1):10–19. doi: 10.3758/cabn.4.1.10. [DOI] [PubMed] [Google Scholar]
- Smith LB. How to learn words: an associative crane. In: Golinkoff R, Hirsh-Pasek K, editors. Breaking the word learning barrier. Oxford: Oxford University Press; 2000. pp. 51–80. [Google Scholar]
- Smith K, Smith ADM, Blythe RA. Cross-situational learning: an experimental study of word-learning mechanisms. Cognitive Science. 2011;35(3):480–498. [Google Scholar]
- Smith L, Yu C. Infants rapidly learn word-referent mappings vial cross-situational statistics. Cognition. 2008;106(3):1558–1568. doi: 10.1016/j.cognition.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkel HL, Adlof SM. The effect of semantic size on word learning by preschool children. Journal of Speech, Language and Hearing Research. 2009;52(2):306–320. doi: 10.1044/1092-4388(2009/07-0175). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallberg IM. The Boston naming test in Swedish, normative data. Brain and Language. 2005;94:19–31. doi: 10.1016/j.bandl.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Tomasello M. Perceiving intentions and learning words in the second year of life. In: Bowerman M, Levinson S, editors. Language acquisition and conceptual development. Cambridge: Cambridge University; 2000. pp. 111–128. [Google Scholar]
- Trueswell JC, Medina TN, Hafri A, Gleitman LR. Propose but verify: fast mapping meets cross-situational word learning. Cognitive Psychology. 2013;66(1):126–156. doi: 10.1016/j.cogpsych.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse CS. The role of associative strength in the semantic relatedness effect on immediate serial recall. Memory. 2009;17(8):874–891. doi: 10.1080/09658210903376250. [DOI] [PubMed] [Google Scholar]
- Tuomiranta L, Càmara E, Froudist Walsh S, Ripollés P, Saunavaara J, Parkkola R, et al. Hidden word learning capacity through orthography in aphasia. Cortex. 2014;50:174–191. doi: 10.1016/j.cortex.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomiranta L, Laine M, Martin N. Adaptation of the Temple assessment of language and short-term memory in aphasia (TALSA) into the Finnish language. 2009 (Unpublished manuscript) [Google Scholar]
- Tuomiranta L, Rautakoski P, Rinne JO, Martin N, Laine M. Long-term maintenance of novel vocabulary in persons with chronic aphasia. Aphasiology. 2012;26(8):1053–1073. [Google Scholar]
- Vlach HA, Sandhofer CM. Retrieval dynamics and retention in cross-situational statistical word learning. Cognitive Science. 2014;38(4):757–774. doi: 10.1111/cogs.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Smith L. Rapid word learning under uncertainty via cross-situational statistics. Psychological Science. 2007;18(5):414–420. doi: 10.1111/j.1467-9280.2007.01915.x. [DOI] [PubMed] [Google Scholar]
- Yu C, Smith LB, Klein K, Shiffrin RM. Hypothesis testing and associative learning in cross-situational word learning: are they one and the same?. 29th cognitive science society conference; Nashville, TN. 2007. [Google Scholar]