Abstract
OBJECTIVES
Since 2006, the British Columbia HIV/AIDS Drug Treatment Program (DTP) has expanded enrollment and dramatically increased its number of participants. We examined the effect this expansion has had on the underlying cause of death in HIV-infected individuals.
METHODS
We analyzed data from participants aged 18 years and older in the DTP to measure two-year mortality rates and causes of death from 2001–2012. We conducted tests of trend for all-cause and cause-specific mortality, and compared demographics and characteristics of individuals. Cox proportional hazard models were used to determine the risk of death.
RESULTS
8,185 participants received ART during the study period. Mortality declined from 3.88 per 100 person-years (PYRs) in 2001–2002 to 2.15 per 100 PYRs in 2011–2012 (p=0.019). We observed significant decreases in HIV-related deaths (2.34 to 0.56 per 100 PYRs; p=0.023) and deaths due to chronic liver disease (0.20 to 0.09 per 100 PYRs; p=0.013), cardiovascular disease (0.24 to 0.05 per 100 PYRs; p=0.026), and suicides (0.47 to 0 per 100 PYRs; p=0.003). Multivariate models, adjusted for age, gender, history of injection drug-use, AIDS diagnoses and baseline CD4 cell counts demonstrated that initiation of ART in all time periods after 2001–02 were independently associated with reduced mortality (p<0.001).
CONCLUSIONS
We observed declines in HIV-related mortality and certain non-HIV related causes of death among participants in the BC DTP over the study period. These findings suggest that there may be broader benefits to the increasingly liberal HIV treatment guidelines, including reductions in death due to cardiovascular disease and chronic liver disease.
INTRODUCTION
Since the introduction of highly active antiretroviral therapy (HAART) in 1996, there has been a significant reduction in HIV-related morbidity and mortality worldwide, in comparison to earlier time-periods.1 By 2006, the introduction of HAART was estimated to have saved at least 3 million life years in the United States, with recent studies suggesting that life expectancy of HIV-infected individuals now approaching that of the general population.2–4
During the early HAART implementation era, numerous studies in Europe and North America had demonstrated a significant reduction in mortality, AIDS, and AIDS-defining illnesses, along with large increases in life expectancy.5–7 However, early studies found HAART to reduce mortality primarily in patients with initial CD4 cell counts <200 cells/mm.3,8 Later studies have since demonstrated a mortality benefit for individuals initiating ART at higher CD4 cell counts, including >350 cells/mm,3,7,9,10 and even >500 cells/mm.3,11
These studies have led to serial revisions of clinical guidelines for HIV treatment, which have now recommended the initiation of HIV treatment earlier in the course of HIV disease.12–15 Recent studies suggest one-third of deaths in HIV-infected patients is AIDS-related.16 In British Columbia, revised treatment guidelines have dramatically increased the number of individuals receiving HAART since 2006. This expansion has also been associated with further decreases in new HIV diagnoses in the province, suggesting that reduced HIV transmission to be a secondary benefit of increased HAART coverage.17 However, the effects of this secondary HAART expansion on the clinical outcomes among HIV-infected individuals in BC has not been well studied. In this study, we undertook an analysis of all individuals who received HAART in BC over a 12-year period (ie. 2001–2012) in order to examine the impact of this secondary HAART expansion on all-cause mortality and cause-specific mortality.
METHODS
Since 1992, the BC HIV/AIDS Drug Treatment Program (DTP) has provided free antiretroviral medications to all medically eligible HIV-infected individuals residing in the province. Data for this study was drawn from the BC HIV/AIDS Drug Treatment Program database; the BC DTP database is a population-based cohort of antiretroviral-naïve HIV-infected adults aged 18 years and older enrolled in the DTP.18 The current dataset includes all individuals who initiated HAART between January 1, 2001 and December 31, 2012, with follow-up extending to the end of 2013. Ethical approval for the BC DTP database was provided by the University of British Columbia Research Ethics Board.
Details of the cohort have been described in more detail elsewhere.18 Briefly, physicians enrolling an HIV-infected individual in the DTP complete a drug request form, which included basic socio-demographic and clinical information. All viral load (VL) testing and most CD4 testing in British Columbia is conducted in laboratories at St. Paul’s Hospital and are uploaded regularly into the DTP database. Additional information including hepatitis C status, history of injection drug use (IDU) and CD4 cell counts for individuals who did not have their CD4 cell count testing performed at St. Paul’s Hospital are obtained from physician-reported values on the prescription refill forms. HAART therapy was defined as receiving at least three ARVs, with at least one nucleoside reverse transcriptase inhibitor (NRTI) and at least one ARV from another class (including non-nucleoside reverse transcriptase inhibitors or protease inhibitor). Medication adherence, expressed as a percentage, was estimated using pharmacy dispensing data by dividing the number of medication-days for which HAART was dispensed by the number of days of follow-up in the first year of treatment. Deaths were identified through record linkages with the BC Vital Statistics Agency and we used ICD-10 codes for the underlying cause of death (see Appendix 1).
We calculated all-cause and cause-specific mortality rates (per 100 person years) and mortality ratios of all participants who were receiving HAART with observation time divided into two-year periods. For each time period, participants were required to have started HAART at least 3 months before the end of the period to be incorporated in the analysis. We used the Cochran-Armitage trend test to examine trends in two-year mortality rates.
We also investigated the timing of HAART initiation on mortality among those who started treatment during the study period (again with participants divided into two-year periods of when they initiated treatment). Socio-demographic and clinical characteristics of individuals initiating HAART in each period were compared using Chi-square and Kruskal-Wallis tests. Cox proportional hazards models were used to determine the association between the two-year period of HAART initiation and the time to death. We included age, gender, presence of AIDS-defining illness (ADI), history of injection drug use, and baseline VL and CD4 count as potential covariates in the model, The final multivariate model was constructed using a backward stepwise procedure, with era of HAART initiation forced into the model. All analyses were conducted using SAS version 9.3 (SAS, Cary, North Carolina, United States).
RESULTS
A total of 8,185 DTP participants were receiving HAART at some point during the study period. The number of the DTP participants increased 56% from 4,002 patients in 2001–2002 to 6,244 in 2011–2012. All-cause mortality rates among all DTP participants remained constant or slightly increased for the first six years of the study (3.88, 4.16, and 4.24 per 100 person-years [PYRs], respectively) before declining from 2007–08 onwards. Overall mortality decreased 45% from 3.88 per 100 PYRs in 2001–2002 to 2.15 per 100 PYRs in 2011–2012 (p=0.02 for trend; Table 1 and Figure 1A). Kaplan-Meier curves for survival grouped by the year of initiation of ART is available in the Supplemental Material (Supplemental Figure 1).
Table 1.
Causes of death and two-year mortality rates among all DTP participants 2001 – 2012 (irrespective of the year in which they started ART)
| Time Period | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | Total | p-value† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=4002 | N=4268 | N=4577 | N=5064 | N=5669 | N=6244 | N=8185 | |||||||||
| n (%) | MR | n (%) | MR | n (%) | MR | n (%) | MR | n (%) | MR | n (%) | MR | n (%) | MR | ||
| Any death | 257 (6.42) | 3.88 | 294 (6.89) | 4.16 | 318 (6.95) | 4.24 | 240 (4.74) | 2.94 | 228 (4.02) | 2.46 | 224 (3.59) | 2.15 | 1561 (19.07) | 4.84 | 0.02 |
| HIV related | 155 (3.87) | 2.34 | 175 (4.10) | 2.47 | 208 (4.54) | 2.77 | 136 (2.69) | 1.67 | 93 (1.64) | 1.00 | 58 (0.93) | 0.56 | 825 (10.08) | 2.56 | 0.02 |
| Non-HIV related | 102 (2.55) | 1.54 | 119 (2.79) | 1.68 | 110 (2.41) | 1.47 | 104 (2.05) | 1.28 | 135 (2.38) | 1.46 | 166 (2.66) | 1.60 | 736 (0.99) | 2.28 | 0.69 |
| Specific Causes of Death (non-HIV) | |||||||||||||||
| Other infectious and parasitic disease | 4 (0.1) | 0.06 | 9 (0.21) | 0.13 | 5 (0.11) | 0.07 | 6 (0.12) | 0.07 | 3 (0.05) | 0.03 | 3 (0.05) | 0.03 | 30 (0.37) | 0.09 | 0.16 |
| Cancer (non-AIDS) | 12 (0.3) | 0.18 | 20 (0.47) | 0.28 | 18 (0.39) | 0.24 | 18 (0.36) | 0.22 | 36 (0.64) | 0.39 | 21 (0.34) | 0.20 | 125 (1.53) | 0.39 | 0.58 |
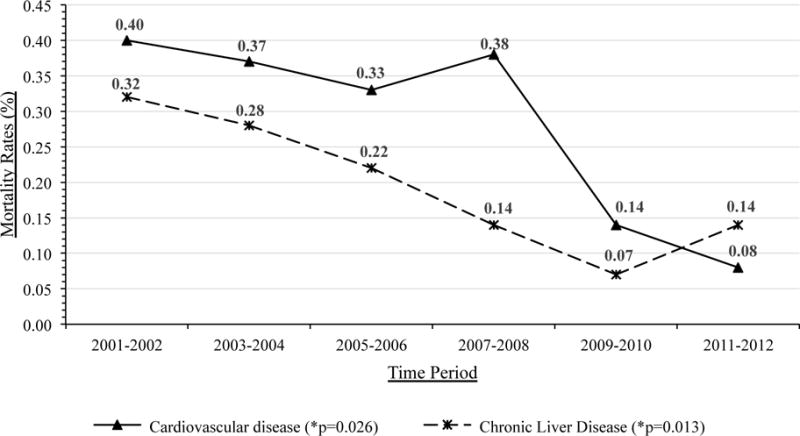
| Cardiovascular disease | 16 (0.4) | 0.24 | 16 (0.37) | 0.23 | 15 (0.33) | 0.20 | 19 (0.38) | 0.23 | 8 (0.14) | 0.09 | 5 (0.08) | 0.05 | 79 (0.97) | 0.25 | 0.03 |
| Chronic respiratory diseases | 3 (0.07) | 0.05 | 1 (0.02) | 0.01 | 3 (0.07) | 0.04 | 5 (0.1) | 0.06 | 5 (0.09) | 0.05 | 4 (0.06) | 0.04 | 21 (0.26) | 0.07 | 0.50 |
| Chronic Liver Disease | 13 (0.32) | 0.20 | 12 (0.28) | 0.17 | 10 (0.22) | 0.13 | 7 (0.14) | 0.09 | 4 (0.07) | 0.04 | 9 (0.14) | 0.09 | 55 (0.67) | 0.17 | 0.01 |
| Unintentional Injuries | 6 (0.15) | 0.09 | 9 (0.21) | 0.13 | 2 (0.04) | 0.03 | 5 (0.1) | 0.06 | 9 (0.16) | 0.10 | 0 (0) | 0 | 31 (0.38) | 0.10 | 0.23 |
| Suicide | 31 (0.77) | 0.47 | 25 (0.59) | 0.35 | 21 (0.46) | 0.28 | 25 (0.49) | 0.31 | 13 (0.23) | 0.14 | 0 (0) | 0 | 115 (1.41) | 0.36 | 0.003 |
| Other causes of death | 17 (0.42) | 0.26 | 27 (0.63) | 0.38 | 36 (0.79) | 0.48 | 19 (0.38) | 0.23 | 57 (1.01) | 0.61 | 124 (1.99) | 1.19 | 280 (3.42) | 0.87 | 0.07 |
Note: For each time period, for calculation of proportion (%), DTP participants were required to start ARV at least 3 months before the end of that period (e.g. 2001–2002, require participants start ARV ≤ Sep 30, 2002; a death is counted if it happens between Jan 01 2001 and Dec 31 2002); for calculation of mortality ratio (MR), no restriction was applied.
p-values were calculated for trend analysis based on the MR of every two year period
Figure 1.
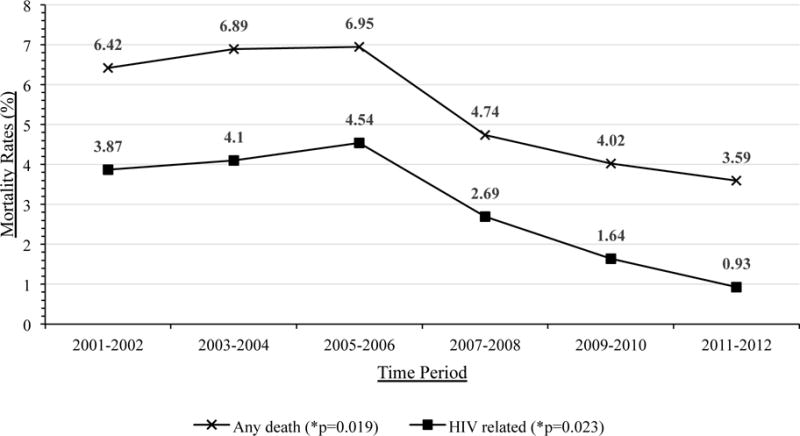
A. All-Cause and HIV-Related Mortality Rates for DTP participants receiving HAART
B. Cardiovascular and Chronic Liver Disease Mortality Rates for DTP participants receiving HAART
HIV-related mortality decreased 60% from 2.34 per 100 PYRs in 2001–2002 to 0.93 per 100 PYRs in 2011–2012 (p=0.02 for trend), as did the mortality from cardiovascular disease, chronic liver disease, and suicides (all p<0.05 for trend; Table 1 and Figure 1B). However the overall mortality rate from these causes were much lower (all less than 0.5 per 100 PYRs) than HIV-related mortality. There were no significant changes in overall non-HIV related mortality rates, nor in those due to non-AIDS cancers, chronic respiratory diseases, infectious and parasitic disease, unintentional injuries, and other causes of death (all p>0.05; Table 1).
There were 4,790 new antiretroviral naïve patients enrolled on HAART from January 2001 to December 2012. The number of individuals newly initiating treatment increased from 555 and 575 in 2001–02 and 2003–04, respectively to 659, 875, 901 and 847 in each of the subsequent two-year periods or on average by 9.6% every two years (Table 2). There were significant differences in baseline characteristics (e.g. Aboriginal ancestry, baseline CD4, baseline VL) and outcomes (e.g. virologic suppression, AIDS defining illness, death) when compared across time periods. These results are detailed in Table 2.
Table 2.
Comparison of Patient Demographics Among Individuals Initiating ART in each two-year period N=4412 people started ARV 2001–2012 and have >=12 months follow up
| Variable | Year of Initiation of ART | p-value | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 2001–2002 (N=555) |
2003–2004 (N=575) |
2005–2006 (N=659) |
2007–2008 (N=875) |
2009–2010 (N=901) |
2011–2012 (N=847) |
||
|
| |||||||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
|
| |||||||
| Sex | 0.27 | ||||||
| Female | 130 (23) | 126 (22) | 143 (22) | 172 (20) | 180 (20) | 158 (19) | |
|
| |||||||
| Aboriginal ancestry | <0.001 | ||||||
| No | 269 (48) | 287 (50) | 243 (37) | 293 (33) | 233 (26) | 167 (20) | |
| Yes | 93 (17) | 69 (12) | 83 (13) | 108 (12) | 101 (11) | 96 (11) | |
| Unknown | 193 (35) | 219 (38) | 333 (51) | 474 (54) | 567 (63) | 584 (69) | |
|
| |||||||
| Age at HAART Initiation, median (IQR)† | 39 (33–46) | 41 (35–48) | 42 (36–49) | 42 (35–49) | 42 (34–49) | 41 (33–49) | <0.001 |
|
| |||||||
| AIDS defining illness before ART | <0.001 | ||||||
| Yes | 91 (16) | 114 (20) | 111 (17) | 109 (12) | 105 (12) | 67 (8) | |
|
| |||||||
| Baseline CD4 | <0.001 | ||||||
| < 50 | 92 (17) | 94 (17) | 95 (15) | 82 (10) | 76 (8) | 51 (6) | |
| 50–199 | 236 (43) | 263 (46) | 278 (43) | 289 (34) | 190 (21) | 122 (15) | |
| 200–349 | 142 (26) | 153 (27) | 196 (30) | 340 (40) | 330 (37) | 201 (24) | |
| >= 350 | 82 (15) | 59 (10) | 80 (12) | 147 (17) | 300 (33) | 467 (56) | |
|
| |||||||
| Baseline CD4, median (IQR)† | 170 (80–275) | 160 (80–230) | 180 (100–250) | 220 (140–290) | 280 (170–390) | 370 (230–530) | <0.001 |
|
| |||||||
| Baseline VL (c/mL) | <0.001 | ||||||
| <100000 | 252 (46) | 269 (48) | 324 (51) | 492 (57) | 617 (69) | 585 (70) | |
|
| |||||||
| HCV | <0.001 | ||||||
| Always negative | 245 (44) | 287 (50) | 355 (54) | 485 (55) | 543 (60) | 542 (64) | |
| Ever positive | 256 (46) | 257 (45) | 262 (40) | 332 (38) | 296 (33) | 217 (26) | |
| Unknown | 54 (10) | 31 (5) | 42 (6) | 58 (7) | 62 (7) | 88 (10) | |
|
| |||||||
| HIV transmission risk group | <0.001 | ||||||
| Unknown or No information | 160 (29) | 166 (29) | 227 (34) | 293 (33) | 395 (44) | 441 (52) | |
| IDU | 211(38) | 213 (37) | 229 (35) | 276 (32) | 266 (30) | 195 (23) | |
| MSM | 128 (23) | 107 (19) | 112 (17) | 177 (20) | 141 (16) | 144 (17) | |
| Heterosexual | 33 (6) | 54 (9) | 52 (8) | 82 (9) | 66 (7) | 53 (6) | |
| IDU and MSM | 23 (4) | 35 (6) | 39 (6) | 47 (5) | 33 (4) | 14 (2) | |
|
| |||||||
| Virologic suppression (first year on ART) | <0.001 | ||||||
| Yes | 403 (73) | 450 (78) | 554 (84) | 767 (88) | 823 (91) | 765 (90) | |
|
| |||||||
| AIDS defining illness after HAART | <0.001 | ||||||
| Yes | 62 (11) | 53 (9) | 46 (7) | 49 (6) | 21 (2) | 14 (2) | |
|
| |||||||
| Adherence to therapy (first year of ART) | <0.001 | ||||||
| >= 95% | 303 (55) | 355 (62) | 454 (69) | 586 (67) | 634 (70) | 580 (68) | |
|
| |||||||
| Third drug class of baseline therapy | <0.001 | ||||||
| Unboosted PI | 62 (12) | 20 (4) | 41 (6) | 18 (2) | 2 (0) | 0 (0) | |
| Boosted PI | 157 (31) | 275 (49) | 401 (61) | 495 (57) | 420 (47) | 398 (47) | |
| NNRTI | 275 (54) | 254 (45) | 213 (32) | 345 (40) | 464 (51) | 399 (47) | |
| Other | 19 (4) | 12 (2) | 1 (0) | 14 (2) | 15 (2) | 50 (6) | |
|
| |||||||
| Physician experience, median (IQR)† | 129 (28–362) | 86 (18–275) | 104 (37–255) | 124 (33–312) | 159 (37–331) | 157 (40–330) | <0.001 |
|
| |||||||
| Deceased | <0.001 | ||||||
| All-cause death | 132 (24) | 106 (18) | 83 (13) | 77 (9) | 40 (4) | 10 (1) | |
|
| |||||||
| Underlying cause of death | NA | ||||||
| HIV-related disease | 67 (12) | 54 (9) | 30 (5) | 21 (2) | 7 (1) | 3 (0) | |
| Other infectious and/or parasitic diseases | 5 (1) | 0 (0) | 3 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Cancer (non-AIDS) | 10 (2) | 8 (1) | 8 (1) | 10 (1) | 3 (0) | 0 (0) | |
| Cardiovascular Disease | 7 (1) | 7 (1) | 5 (1) | 3 (0) | 2 (0) | 1 (0) | |
| Chronic Respiratory Diseases | 1 (0) | 2 (0) | 2 (0) | 1 (0) | 1 (0) | 0 (0) | |
| Chronic Liver Disease | 1 (0) | 3(1) | 6 (1) | 1 (0) | 2 (0) | 0 (0) | |
| Unintentional Injuries | 0 (0) | 1 (0) | 3 (0) | 3 (0) | 0 (0) | 0 (0) | |
| Suicides | 11 (2) | 3 (1) | 4 (1) | 1 (0) | 0 (0) | 0 (0) | |
| Other Causes of Death | 30 (5) | 28 (5) | 22 (3) | 37 (4) | 25 (3) | 6 (1) | |
denotes a continuous variable; data is expressed as median and 25th–75th percentile according.
Physician experience = the number of patients for whom the individuals’ treating physician has previously prescribed ART.
A total 4,589 antiretroviral naïve patients were included in the Cox proportional hazards model for death for individuals newly initiating HAART in each two-year time-period (Table 3). Enrollment into the DTP during the later periods was associated with reduced hazard ratios, including 2003–2004 (adjusted hazard ratio [aHR] 0.53), 2005–2006 (aHR 0.69), 2007–2008 (aHR 0.59), 2009–2010 (aHR 0.45), and 2011–2012 (aHR 0.52), even after adjustment for other covariates (p<0.001). Additional factors associated with death in the adjusted model included age of HAART initiation, female sex, AIDS-defining illness before ART, baseline CD4 count, and history of injection drug use. These results are detailed in Table 3.
Table 3.
Cox Proportional Hazards Model for Death among DTP Participants who initiated HAART in 2001–2012 (N=4589)
| Variable | Unadjusted | Adjusted | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
|
| ||||
| Year of initiation of ART | ||||
| 2001–2002 | 1.00 (−) | <0.001 | 1.00 (−) | <0.001 |
| 2003–2004 | 0.84(0.66, 1.06) | 0.75(0.59, 0.95) | ||
| 2005–2006 | 0.78(0.61, 1) | 0.69(0.54, 0.88) | ||
| 2007–2008 | 0.59(0.46, 0.76) | 0.59(0.46, 0.76) | ||
| 2009–2010 | 0.39(0.28, 0.53) | 0.45(0.32, 0.61) | ||
| 2011–2012 | 0.4(0.27, 0.58) | 0.52(0.36, 0.77) | ||
|
| ||||
| Age at ART Initiation | 1.04(1.03, 1.04) | <0.001 | 1.04(1.04, 1.05) | <0.001 |
|
| ||||
| Sex | ||||
| Female | 1.22(1.01, 1.46) | 0.04 | 1.41(1.16, 1.71) | <0.001 |
|
| ||||
| AIDS defining illness before ART | ||||
| Yes | 1.66(1.37, 2.01) | <0.001 | 1.29(1.05, 1.58) | 0.02 |
|
| ||||
| Baseline VL (log10 c/mL) | 1.5(1.28, 1.77) | <0.001 | NA† | |
|
| ||||
| Baseline CD4 (per 100 cells) | 0.73(0.69, 0.78) | <0.001 | 0.79(0.74, 0.84) | <0.001 |
|
| ||||
| Third drug of baseline therapy | ||||
| Unboosted PI | 1.00 (−) | 0.26 | NA† | |
| Boosted PI | 1.22(0.81, 1.85) | |||
| NNRTI | 1.04(0.69, 1.59) | |||
| Other | 1.27(0.67, 2.43) | |||
|
| ||||
| History of injection drug use | ||||
| No | 1.00 (−) | <0.001 | 1.00 (−) | <0.001 |
| Yes | 2.65(2.19, 3.21) | 2.74(2.25, 3.34) | ||
| Unknown | 2.38(1.88, 3.01) | 2.98(2.35, 3.78) | ||
NA denotes variables that were not included in the adjusted model.
DISCUSSION
From 2001–02 to 2011–12, there was a 45% reduction in all-cause mortality rates among all HIV-positive individuals receiving HAART in British Columbia. In addition to this overall reduction in mortality, there were reductions in the rates of deaths from HIV-related causes, and certain non-HIV related causes of death including cardiovascular diseases, chronic liver diseases, and suicides. Furthermore, individuals initiating HAART in 2011–12 were half as likely to die as those who initiated in 2001–02, irrespective of age, sex, history of injection drug use, baseline CD4 cell count, and HAART adherence. These findings coincided with a large expansion in HAART coverage in BC in 2006, and suggest that there may be broader benefits to HAART expansion apart from reduced mortality from HIV-related causes.
While much of the recent attention on expanding HIV treatment in British Columbia has focused on reductions in new HIV diagnoses,17 it is clear that there remain important clinical benefits for individuals already infected with HIV as well. Our findings highlight the mortality benefits of increasingly liberal treatment guidelines for HAART initiation, which now allow for treatment at higher CD4 cell counts. Despite the increase in median CD4 cell count during our study period, we demonstrated that these reductions in all-cause mortality and HIV-related mortality are independent of baseline CD4 count. These findings were similar to those published previously by the Antiretroviral Therapy Cohort Collaboration (ART-CC) from a slightly earlier time period. The ART-CC study included 43,355 patients enrolled across Europe and North America between 1996 and 2005, and reported a nearly 40% reduction in crude mortality rates from 1996–1999 to 2003–2005.2 The authors reported a life-expectancy of nearly two-thirds that of the general population, with the greatest mortality attributed to AIDS-defining illnesses. Furthermore, in a subsequent study, the authors reported greatly increased mortality hazard ratios attributable to AIDS-defining illnesses, including non-Hodgkin’s lymphoma, progressive multifocal leukoencephalopathy, and others.4
That HAART can influence clinical outcomes related to common chronic diseases was first suggested by The Strategies for Management of Antiretroviral Therapy (SMART).19 In addition to death from opportunistic diseases, patients without virologic suppression had a 60% increased hazard ratio for major cardiovascular, renal, and hepatic disease.19 The authors suggested that these events were associated with the level of immunodeficiency, and that continuous virologic suppression could improve outcomes through modulating immune function. Our results support these observations in that we observed reductions in rates of death from cardiovascular disease and chronic liver disease in our cohort during this era. Furthermore, the reductions in cardiovascular disease, chronic liver disease, and suicide in our cohort were persistent after adjustment for age, sex, and baseline CD4 cell count. Rates of death due to suicide were also significantly reduced in later time periods, however, it is unclear as to how earlier HIV treatment may have caused this. One possibility is that by engaging participants into HIV care earlier in the course of their illness, this may have fostered earlier and better access to mental health care for HIV-infected individuals.
There was a significant increase in median CD4 cell count in individuals enrolled in the BC DTP over the study period, from 170 to 370 cells/mm3 in the final time period. This coincided with changes in therapeutic guidelines to prescribe HAART for patients at increasingly higher CD4 cell counts, and unexpectedly also reduced the absolute number of individuals initiating therapy with very low CD4 counts. In 2001–02, HAART was recommended to be initiated before CD4 cell counts were <200 cells/mm3, with 60% of our cohort initiating therapy below this threshold.20 In contrast, only 20% of DTP participants initiated therapy in 2011–12 with CD4 cell counts <200 cells/mm3. Since 2012, international guidelines have recommended treatment of all HIV-infected individuals, irrespective of CD4 cell count.15 Significant reductions in HIV-related mortality attest to the efficacy of broadening HAART enrollment. However despite initiating more people on treatment with increasingly greater CD4 cell counts, HIV-related mortality still accounted for 25% of all-cause mortality in the later time periods. This finding has been reproduced in other populations and suggests that further improvements in clinical outcomes can be attained.16
Readers must be cautious when interpreting our results. Firstly, our database only includes only HIV-infected individuals who have accessed HAART in BC and does not include all HIV-infected individuals in the province. A previous study of mortality in the BC DTP between 1997 and 2005, found that, 40% of HIV and AIDS-related deaths in BC occurred in individuals who had never accessed treatment.21 Our study also only included individuals who were residing in BC and whom the program was not aware had moved to other provinces, and therefore individuals who started ART in BC but passed away in other provinces would not be captured in our analyses. However, the number of such individuals are likely quite small. We found that the proportion of the HIV deaths which occurred among DTP participants reflected almost 80% of the total HIV-related deaths in British Columbia in 2009–2010 (vital statistics data for the complete 2011–2012 time period was not available).22 This suggests that more HIV-infected individuals are accessing the DTP than in previous years. Furthermore, our analyses are performed by separating patients based on year of initiation of ART in two-year time periods. Differential results with adopting a longer time period are possible, although this would significantly reduce the ability to detect trends in our analyses. A further limitation is that our death data was recorded using ICD-10 coding based on physician assessments recorded on death certificates, which may be inherently limited by accuracy. In particular, we noted an increase in the number of deaths attributed to “other causes” of in 2009–10 and 2011–12. This increase was primarily due to increases in ill-defined or unknown causes of mortality and we do not have an explanation as to why this increase occurred for these time periods. Even though this increase was not statistically significant, it may have affected the trends for other diseases for which there was a known cause of death. Finally, since this is an observational study, we can only demonstrate an association between HAART expansion and subsequent reductions in mortality, but we cannot definitively prove causality. It is possible that other confounding factors may have caused the mortality reductions that we observed.
In conclusion, HAART expansion in BC over the past decade has resulted in a significant decrease in new HIV diagnoses and mortality, while increasing life expectancy.9,17 In this study, we clearly demonstrated a decrease in both all-cause and cause-specific mortality in HIV-infected individuals participating in the BC DTP over the past decade. Apart from reductions in HIV-related mortality, deaths from cardiovascular disease, chronic liver disease, and suicide have also significantly reduced. Our work suggest that a secondary benefit of increasing HAART coverage in BC is a reduction of non-HIV-related mortality, irrespective of baseline health status.
Supplementary Material
Appendix 1. ICD-10 Codes for Specific Causes of Death.
Acknowledgments
We thank the participants in the BC HIV/AIDS Drug Treatment Program and the physicians, nurses, social workers and volunteers who support them. We would like to thank the British Columbia Ministry of Health Services and Ministry of Healthy Living and Sport for their generous funding and support. DMM and VL are supported by Scholar Awards from the Michael Smith Foundation for Health Research.
Funding: Ministry of Health Services and Ministry of Healthy Living and Sport, British Columbia.
Footnotes
Conflict of Interests: None
Prior Presentations: Conference on Retroviruses and Opportunistic Infections 2014
References
- 1.Lima VD, Lepik KJ, Zhang W, Muldoon KA, Hogg RS, Montaner JS. Regional and temporal changes in HIV-related mortality in British Columbia, 1987–2006. Can J Public Health. 2010 Sep-Oct;101(5):415–419. doi: 10.1007/BF03404864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antiretroviral Therapy Cohort C. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008 Jul 26;372(9635):293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antiretroviral Therapy Cohort C. Mocroft A, Sterne JA, et al. Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009 Apr 15;48(8):1138–1151. doi: 10.1086/597468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belvedere LM, Miller CL, Hogg RS. Shifting sands: changing regional and gender-specific patterns of HIV/AIDS mortality in Canada, 1987 to 2008. Can J Public Health. 2012 May-Jun;103(3):202–206. doi: 10.1007/BF03403813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchacz K, Baker RK, Palella FJ, Jr, et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS. 2010 Jun 19;24(10):1549–1559. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 7.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003 Jul 5;362(9377):22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 8.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA: the journal of the American Medical Association. 2001 Nov 28;286(20):2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 9.Lima VD, Hogg RS, Harrigan PR, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007 Mar 30;21(6):685–692. doi: 10.1097/QAD.0b013e32802ef30c. [DOI] [PubMed] [Google Scholar]
- 10.Strategies for Management of Antiretroviral Therapy Study G. Emery S, Neuhaus JA, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. The Journal of infectious diseases. 2008 Apr 15;197(8):1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 11.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. The New England journal of medicine. 2009 Apr 30;360(18):1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA: the journal of the American Medical Association. 2006 Aug 16;296(7):827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 13.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA: the journal of the American Medical Association. 2008 Aug 6;300(5):555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 14.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA: the journal of the American Medical Association. 2010 Jul 21;304(3):321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 15.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA: the journal of the American Medical Association. 2012 Jul 25;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 16.Ehren K, Hertenstein C, Kummerle T, et al. Causes of death in HIV-infected patients from the Cologne-Bonn cohort. Infection. 2014 Feb;42(1):135–140. doi: 10.1007/s15010-013-0535-7. [DOI] [PubMed] [Google Scholar]
- 17.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010 Aug 14;376(9740):532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson S, Cescon A, Samji H, et al. Cohort Profile: HAART Observational Medical Evaluation and Research (HOMER) cohort. International journal of epidemiology. 2014 Mar 17; doi: 10.1093/ije/dyu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strategies for Management of Antiretroviral Therapy Study G. El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. The New England journal of medicine. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter CC, Cooper DA, Fischl MA, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA: the journal of the American Medical Association. 2000 Jan 19;283(3):381–390. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 21.Joy R, Druyts EF, Brandson EK, et al. Impact of neighborhood-level socioeconomic status on HIV disease progression in a universal health care setting. Journal of acquired immune deficiency syndromes. 2008 Apr 1;47(4):500–505. doi: 10.1097/QAI.0b013e3181648dfd. [DOI] [PubMed] [Google Scholar]
- 22.Vital Statistics Agency B. BC Annual Report (2001–2011) 2011 Available from: http://www.vs.gov.bc.ca/stats/annual/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. ICD-10 Codes for Specific Causes of Death.


