Abstract
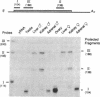
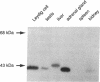
Observations of patients deficient in the steroidogenic enzyme 3 beta-hydroxy-delta 5-steroid dehydrogenase/isomerase (3 beta HSD) have suggested the presence of distinct 3 beta HSD structural gene(s) that are expressed at peripheral sites, possibly the liver. We now report the isolation of cDNA clones representing three forms of 3 beta HSD from mouse Leydig cell and liver libraries. The three forms share significant identify but differ from each other by 5-10% within their coding regions. RNA that hybridizes to radiolabeled 3 beta HSD probes is present in the gonads, adrenal glands, liver, and kidneys of both sexes. Ribonuclease protection analysis using antisense probes derived from each of the three forms demonstrates that one form, 3 beta HSD I, is restricted to steroidogenic tissues. Two other forms, 3 beta HSD II and III, are expressed in liver and kidney but are not detected in steroidogenic tissues. A polyclonal antibody raised against the human placental form of 3 beta HSD recognizes a 42-kDa protein in gonadal and adrenal tissue and a 45-kDa protein in liver. The antibody recognizes a 42-kDa protein in kidney only weakly. 3 beta HSD enzyme activity is present in testicular, adrenal, hepatic, and renal tissue, with adrenal tissue possessing the highest specific activity. When expressed as total 3 beta HSD activity for whole organ mass, activity is greatest in the liver. The results demonstrate that the mouse liver is a significant site of 3 beta HSD activity and demonstrate the existence of multiple 3 beta HSD structural genes in the mouse.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer H. C., Bauer H. Micromethod for the determination of 3-beta-HSD activity in cultured cells. J Steroid Biochem. 1989 Oct;33(4A):643–646. doi: 10.1016/0022-4731(89)90054-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Devine P. L., Kelly N. S., Adams J. B. 3 beta-hydroxysteroid isomerase dehydrogenase in guinea-pig kidney: possible involvement in 11-deoxycorticosterone formation in situ. J Steroid Biochem. 1986 Aug;25(2):265–270. doi: 10.1016/0022-4731(86)90427-9. [DOI] [PubMed] [Google Scholar]
- Doody K. M., Carr B. R., Rainey W. E., Byrd W., Murry B. A., Strickler R. C., Thomas J. L., Mason J. I. 3 beta-hydroxysteroid dehydrogenase/isomerase in the fetal zone and neocortex of the human fetal adrenal gland. Endocrinology. 1990 May;126(5):2487–2492. doi: 10.1210/endo-126-5-2487. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Hales D. B., Payne A. H. Glucocorticoid-mediated repression of P450scc mRNA and de novo synthesis in cultured Leydig cells. Endocrinology. 1989 May;124(5):2099–2104. doi: 10.1210/endo-124-5-2099. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I., Hu Z. Y., Baulieu E. E., Robel P. Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology. 1989 Oct;125(4):2083–2091. doi: 10.1210/endo-125-4-2083. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H. M., Pène J. J. Optimal conditions for supercoil DNA sequencing with the Escherichia coli DNA polymerase I large fragment. Gene Anal Tech. 1988 Mar-Apr;5(2):32–39. doi: 10.1016/0735-0651(88)90024-6. [DOI] [PubMed] [Google Scholar]
- Lorence M. C., Corbin C. J., Kamimura N., Mahendroo M. S., Mason J. I. Structural analysis of the gene encoding human 3 beta-hydroxysteroid dehydrogenase/delta 5----4-isomerase. Mol Endocrinol. 1990 Dec;4(12):1850–1855. doi: 10.1210/mend-4-12-1850. [DOI] [PubMed] [Google Scholar]
- Lorence M. C., Murry B. A., Trant J. M., Mason J. I. Human 3 beta-hydroxysteroid dehydrogenase/delta 5----4isomerase from placenta: expression in nonsteroidogenic cells of a protein that catalyzes the dehydrogenation/isomerization of C21 and C19 steroids. Endocrinology. 1990 May;126(5):2493–2498. doi: 10.1210/endo-126-5-2493. [DOI] [PubMed] [Google Scholar]
- Luu The V., Lachance Y., Labrie C., Leblanc G., Thomas J. L., Strickler R. C., Labrie F. Full length cDNA structure and deduced amino acid sequence of human 3 beta-hydroxy-5-ene steroid dehydrogenase. Mol Endocrinol. 1989 Aug;3(8):1310–1312. doi: 10.1210/mend-3-8-1310. [DOI] [PubMed] [Google Scholar]
- Milewich L., Shaw C. B., Sontheimer R. D. Steroid metabolism by epidermal keratinocytes. Ann N Y Acad Sci. 1988;548:66–89. doi: 10.1111/j.1749-6632.1988.tb18793.x. [DOI] [PubMed] [Google Scholar]
- Mori H., Shimizu D., Fukunishi R., Christensen A. K. Morphometric analysis of testicular Leydig cells in normal adult mice. Anat Rec. 1982 Dec;204(4):333–339. doi: 10.1002/ar.1092040406. [DOI] [PubMed] [Google Scholar]
- Nolan C. J., Payne A. H. Genotype at the P450scc locus determines differences in the amount of P450scc protein and maximal testosterone production in mouse Leydig cells. Mol Endocrinol. 1990 Oct;4(10):1459–1464. doi: 10.1210/mend-4-10-1459. [DOI] [PubMed] [Google Scholar]
- Pang S., Levine L. S., Stoner E., Opitz J. M., Pollack M. S., Dupont B., New M. I. Nonsalt-losing congenital adrenal hyperplasia due to 3 beta-hydroxysteroid dehydrogenase deficiency with normal glomerulosa function. J Clin Endocrinol Metab. 1983 Apr;56(4):808–818. doi: 10.1210/jcem-56-4-808. [DOI] [PubMed] [Google Scholar]
- Perkins L. M., Payne A. H. Quantification of P450scc, P450(17) alpha, and iron sulfur protein reductase in Leydig cells and adrenals of inbred strains of mice. Endocrinology. 1988 Dec;123(6):2675–2682. doi: 10.1210/endo-123-6-2675. [DOI] [PubMed] [Google Scholar]
- Quinn P. G., Payne A. H. Oxygen-mediated damage of microsomal cytochrome P-450 enzymes in cultured leydig cells. Role in steroidogenic desensitization. J Biol Chem. 1984 Apr 10;259(7):4130–4135. [PubMed] [Google Scholar]
- Rosenfield R. L., Rich B. H., Wolfsdorf J. I., Cassorla F., Parks J. S., Bongiovanni A. M., Wu C. H., Shackleton C. H. Pubertal presentation of congenital delta 5-3 beta-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 1980 Aug;51(2):345–353. doi: 10.1210/jcem-51-2-345. [DOI] [PubMed] [Google Scholar]
- Stalvey J. R., Payne A. H. Maximal testosterone production in Leydig cells from inbred mice relates to the activity of 3 beta-hydroxysteroid dehydrogenase-isomerase. Endocrinology. 1984 Oct;115(4):1500–1505. doi: 10.1210/endo-115-4-1500. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. L., Berko E. A., Faustino A., Myers R. P., Strickler R. C. Human placental 3 beta-hydroxy-5-ene-steroid dehydrogenase and steroid 5----4-ene-isomerase: purification from microsomes, substrate kinetics, and inhibition by product steroids. J Steroid Biochem. 1988 Nov;31(5):785–793. doi: 10.1016/0022-4731(88)90287-7. [DOI] [PubMed] [Google Scholar]
- Thomas J. L., Myers R. P., Strickler R. C. Human placental 3 beta-hydroxy-5-ene-steroid dehydrogenase and steroid 5----4-ene-isomerase: purification from mitochondria and kinetic profiles, biophysical characterization of the purified mitochondrial and microsomal enzymes. J Steroid Biochem. 1989 Aug;33(2):209–217. doi: 10.1016/0022-4731(89)90296-3. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J., Siegel R. A., Chowers I. In vitro conversion of pregnenolone to progesterone by discrete brain areas of the male rat. J Steroid Biochem. 1980 Aug;13(8):961–963. doi: 10.1016/0022-4731(80)90171-5. [DOI] [PubMed] [Google Scholar]
- Zhao H. F., Labrie C., Simard J., de Launoit Y., Trudel C., Martel C., Rhéaume E., Dupont E., Luu-The V., Pelletier G. Characterization of rat 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase cDNAs and differential tissue-specific expression of the corresponding mRNAs in steroidogenic and peripheral tissues. J Biol Chem. 1991 Jan 5;266(1):583–593. [PubMed] [Google Scholar]
- Zhao H. F., Rheáume E., Trudel C., Couët J., Labrie F., Simard J. Structure and sexual dimorphic expression of a liver-specific rat 3 beta-hydroxysteroid dehydrogenase/isomerase. Endocrinology. 1990 Dec;127(6):3237–3239. doi: 10.1210/endo-127-6-3237. [DOI] [PubMed] [Google Scholar]
- Zhao H. F., Simard J., Labrie C., Breton N., Rhéaume E., Luu-The V., Labrie F. Molecular cloning, cDNA structure and predicted amino acid sequence of bovine 3 beta-hydroxy-5-ene steroid dehydrogenase/delta 5-delta 4 isomerase. FEBS Lett. 1989 Dec 18;259(1):153–157. doi: 10.1016/0014-5793(89)81516-9. [DOI] [PubMed] [Google Scholar]