Abstract
HAND2 is an ancestral regulator of heart development and one of four transcription factors that control the reprogramming of fibroblasts into cardiomyocytes1–4. Deletion of Hand2 in mice results in right ventricle hypoplasia and embryonic lethality1,5. Hand2 expression is tightly regulated by upstream enhancers6,7 that reside within a super-enhancer delineated by histone H3 acetyl Lys27 (H3K27ac) modifications8. Here we show that transcription of a Hand2-associated long non-coding RNA, which we named upperhand (Uph), is required to maintain the super-enhancer signature and elongation of RNA polymerase II through the Hand2 enhancer locus. Blockade of Uph transcription, but not knockdown of the mature transcript, abolished Hand2 expression, causing right ventricular hypoplasia and embryonic lethality in mice. Given the substantial number of uncharacterized promoter-associated long non-coding RNAs encoded by the mammalian genome9, the Uph–Hand2 regulatory partnership offers a mechanism by which divergent non-coding transcription can establish a permissive chromatin environment.
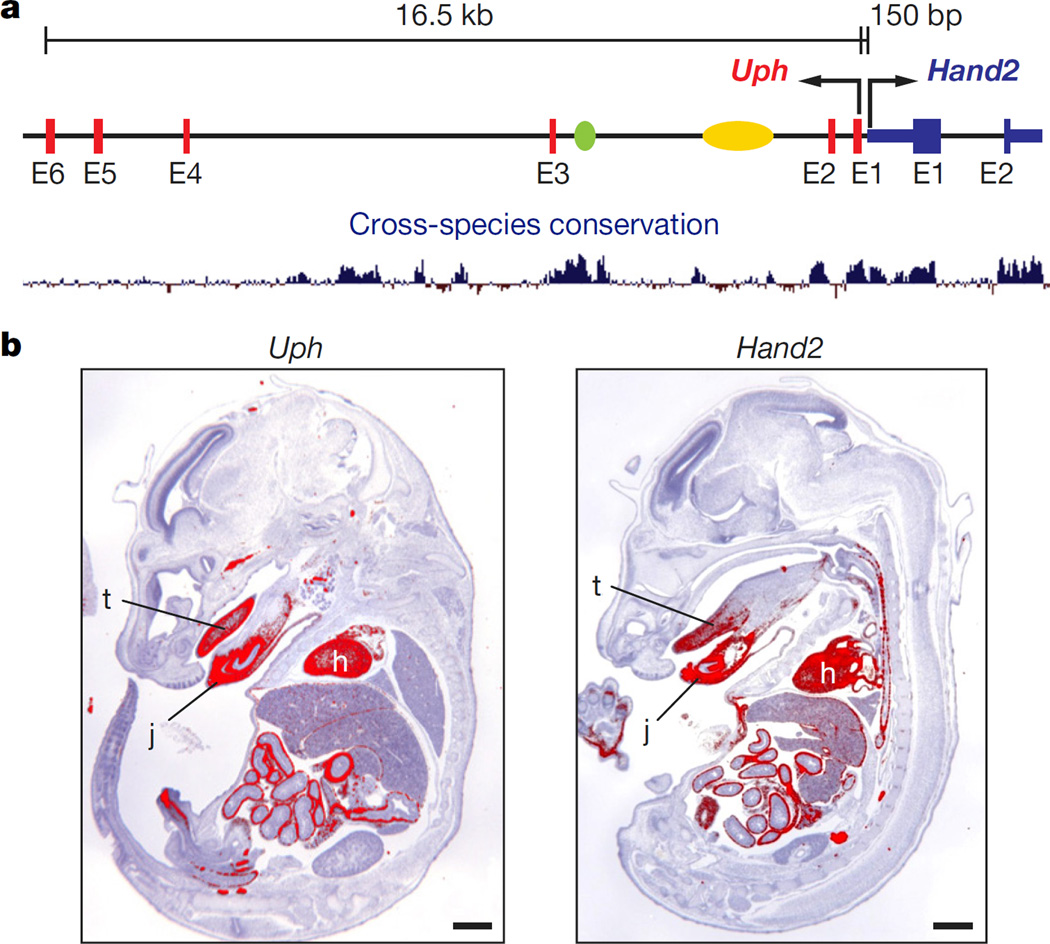
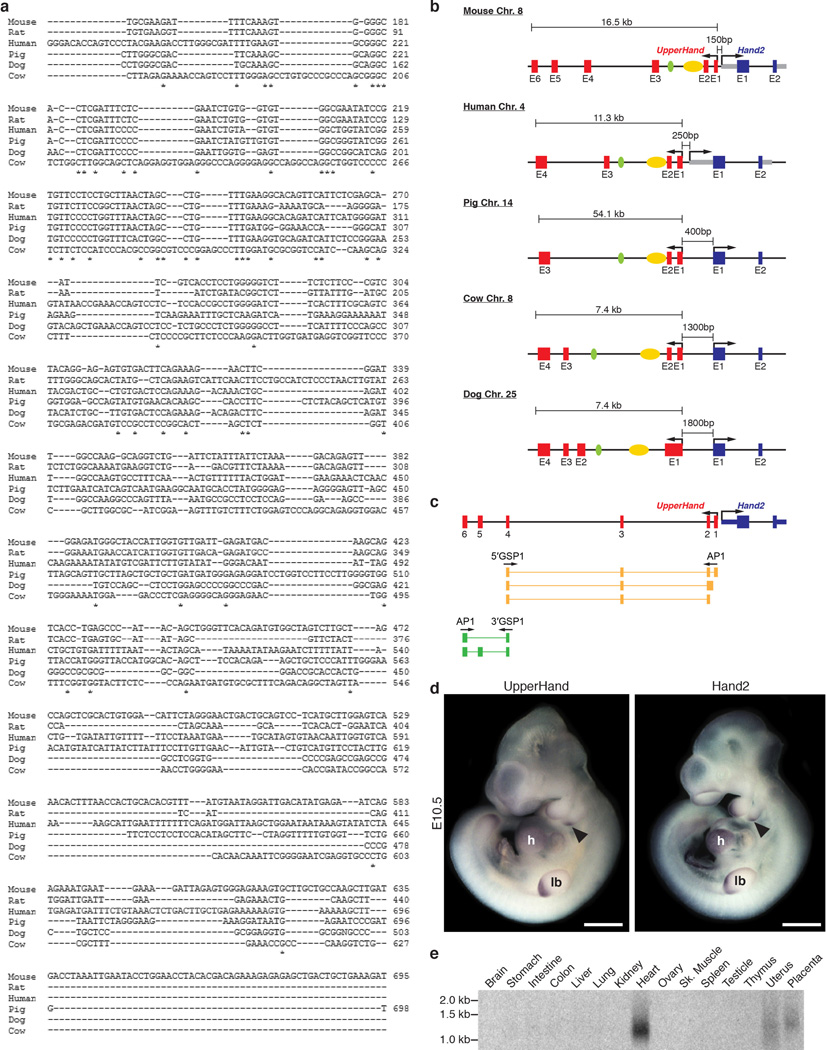
In all well-annotated mammalian species, long non-coding RNA (lncRNA) transcripts with a similar intron–exon organization are transcribed upstream of the Hand2 locus (Extended Data Fig. 1a, b). We refer to these transcripts as ‘upperhand’ (Uph), although the human transcripts have been referred to as DEIN (differentially expressed in neuroblastoma, also known as HAND2-AS1)10. Rapid amplification of cDNA ends (RACE) analysis of Uph revealed several alternatively spliced transcripts in the mouse heart (Extended Data Fig. 1c). The major mouse Uph transcript is 770 nucleotides long, contains six exons, a polyadenylation sequence and shares a bidirectional promoter with Hand2 (ref. 10; Fig. 1a). The mouse Uph locus encompasses ~16.5 kilobases and contains two enhancers that direct Hand2 expression in the heart and branchial arches6,7, as well as histone acetylation marks that delineate a cardiac super-enhancer in this locus8 (Fig. 1a).
Figure 1. Co-expression of upperhand (Uph) and Hand2.
a, The mouse Uph–Hand2 locus. Uph is transcribed 150 bp upstream of Hand2 and contains two Hand2 enhancers (branchial arch enhancer (green) and cardiac enhancer (yellow)) within one of its introns. Bottom, mammalian sequence conservation (blue). b, Section in situ hybridization of E15.5 mouse embryos for either Uph (left) or Hand2 (right). Expression was detected in heart (h), tongue (t) and jaw (j). Scale bars, 1 mm.
Similar to most characterized lncRNAs11, the Uph nucleotide sequence is not well-conserved, with only ~56% homology between mouse and human, and little conservation across other species (Extended Data Fig. 1a). However, Uph orthologues share a promoter and contain the conserved Hand2-associated cardiac and brachial arch enhancers within their second introns (Extended Data Fig. 1b).
Promoter-associated lncRNAs are often positively correlated with transcription of their protein-coding neighbour12,13. Indeed, whole-mount in situ hybridization for Uph in mouse embryos revealed expression in the heart, distal branchial arches and the developing limb bud at embryonic day (E)10.5, overlapping with Hand2 expression (Extended Data Fig. 1d). Uph expression was strong in the heart and continued to mirror Hand2 at later fetal stages (Fig. 1b). Northern blot analysis across several adult tissues revealed that Uph transcript levels were highest in the heart (Extended Data Fig. 1e).
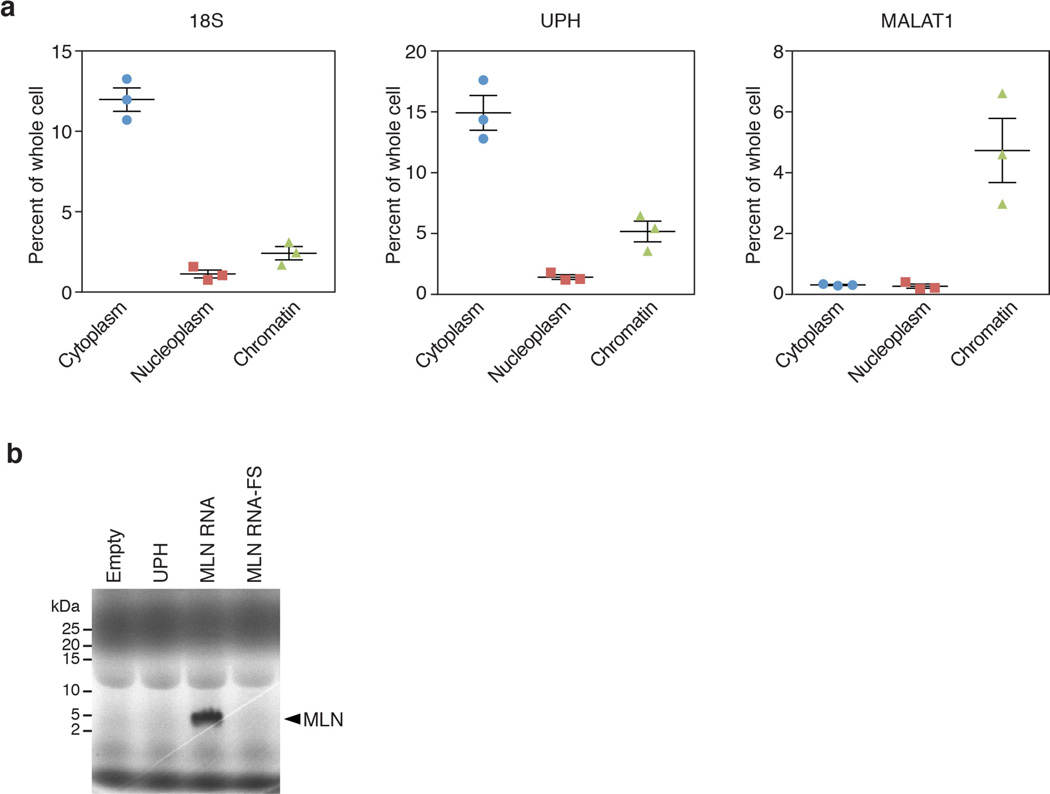
Cell fractionation of mouse neonatal cardiomyocytes showed Uph transcripts in all fractions but enrichment in the cytoplasm, similar to 18S RNA (Extended Data Fig. 2a). The distribution of Uph differed from nuclear lncRNAs such as Malat1, which associates with chromatin (Extended Data Fig. 2a). Previously, we reported that some annotated lncRNAs can encode micropeptides14,15. However, Uph does not contain a conserved open reading frame and in vitro transcription and translation of Uph did not produce any detectable peptides (Extended Data Fig. 2b). Together, these data reveal that Uph is a bona fide lncRNA that is co-expressed in a temporal and tissue-specific pattern with the essential cardiac transcription factor Hand2.
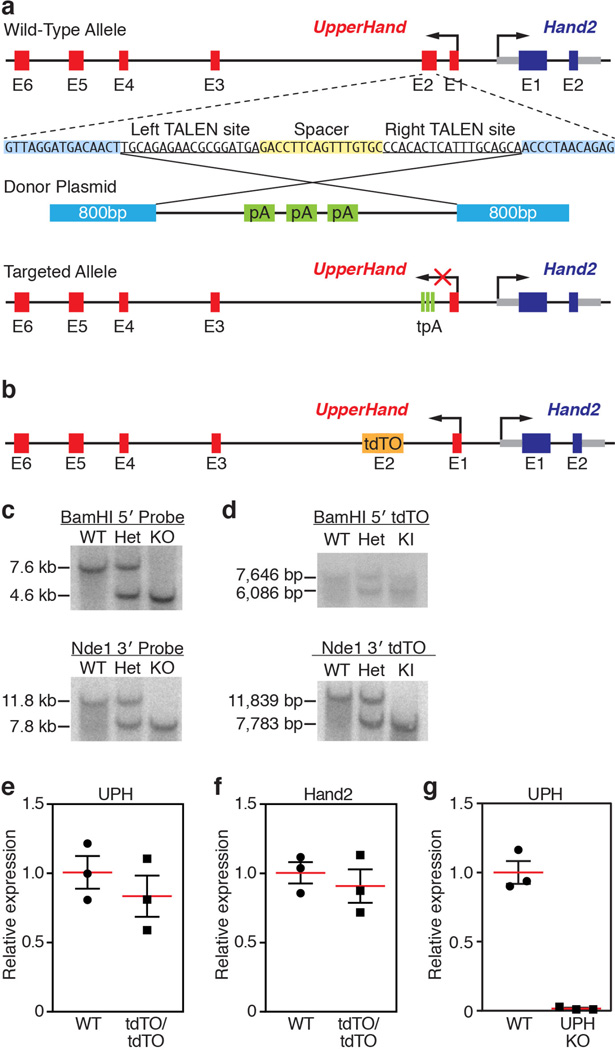
To examine Uph function in vivo, we used transcription activator-like effector nucleases (TALENs) to insert a triple polyadenylation sequence into exon 2 of the Uph locus in C57BL/6 mouse zygotes (Extended Data Fig. 3a). To avoid potential regulatory elements required for Hand2 expression, we inserted the triple polyadenylation cassette into a region with low sequence conservation. Furthermore, we generated a separate knock-in allele using a heterologous DNA sequence (tdTomato (tdTO) lacking a polyadenylation sequence) inserted into the same Uph locus (Extended Data Fig. 3b). Correct targeting of the triple polyadenylation and tdTO sequences was verified by Southern blot analysis (Extended Data Fig. 3c, d).
Mice homozygous for the Uph tdTO allele (UphtdTO/tdTO) were born at expected Mendelian ratios and showed no morphological defects or notable changes in Uph or Hand2 expression (Extended Data Fig. 3e, f). Thus, insertion of a heterologous DNA sequence into exon 2 of the Uph locus is not sufficient to disrupt normal Hand2 expression. However, from 31 pups born of heterozygous Uph (Uph+/−) intercrosses, no Uph-knockout (KO) offspring survived, suggesting that Uph transcription is required for embryonic survival.
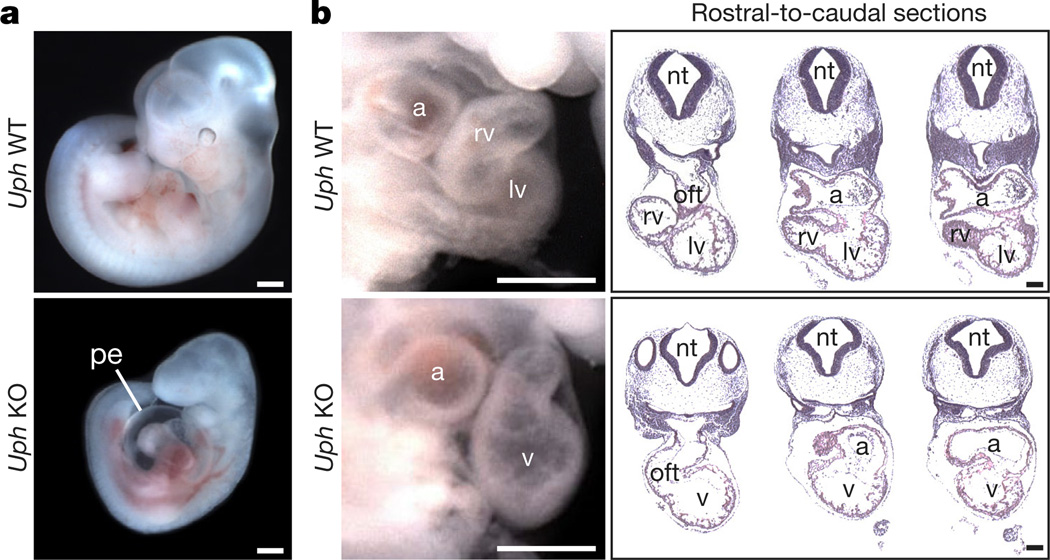
To pinpoint the embryonic death of Uph KO embryos, we collected embryos from timed pregnancies of Uph+/− matings. Uph KO embryos in a pure C57BL/6 background were present at approximate Mendelian ratios before E9.5 (20.6%; 13 out of 63 embryos collected), and showed no obvious size or morphological differences relative to wild-type littermates. However, by E10.5, the Uph KO embryos had a large pericardial effusion surrounding the heart and were growth restricted (Fig. 2a). Quantitative real-time PCR (qPCR) showed that Uph transcripts were reduced by 97% in Uph KO compared to wild-type hearts at E10.5, confirming successful termination of Uph transcription (Extended Data Fig. 3g). Histological analyses revealed that hearts from Uph KO embryos failed to develop a right ventricular chamber. Instead, the outflow tract and atrial chamber connected to a single left-sided ventricular chamber (Fig. 2b), a cardiac phenotype reminiscent of Hand2 KO embryos.
Figure 2. Cardiac defects of Uph KO embryos.
a, At E10.5, Uph KO embryos have pericardial effusion (pe) and appear smaller than wildtype (WT) littermate embryos. b, At E10.5, wild-type embryos (top) have distinct right and left ventricular (rv and lv, respectively) chambers, whereas Uph KO embryos (bottom) have a single ventricle (v). This abnormality is highlighted in rostral-to-caudal sections through the heart (left to right). a, atria; nt, neural tube; oft, outflow tract. Scale bars, 500 µ m (a) and 100 µ m (b).
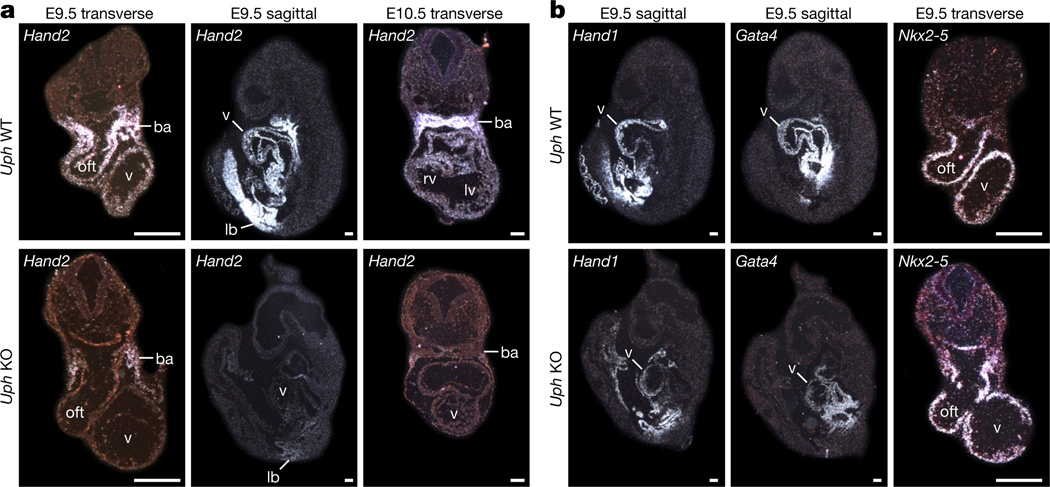
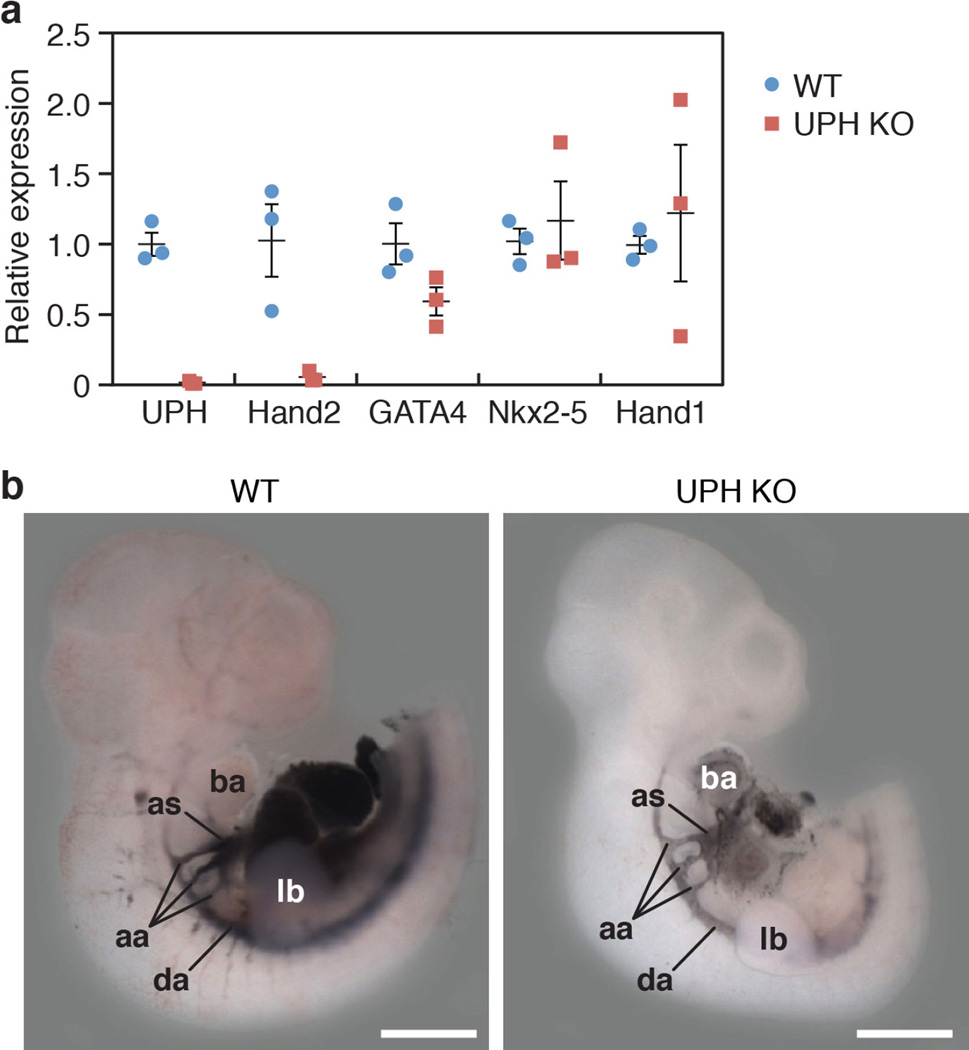
Because Uph KO embryos displayed a similar cardiac phenotype to Hand2 KO embryos, we analysed the expression of Hand2 and other cardiac transcription factors in Uph KO embryos using in situ hybridization. Notably, Hand2 expression was absent in the atria, ventricle and outflow tract of Uph KO embryos, but remained detectable, albeit at reduced levels, in the branchial arches and limb buds (Fig. 3a, Extended Data Fig. 4a). Consistent with the persistent expression of Hand2 in the branchial arches, Uph KO embryos lacked the vascular defects in the aortic arch arteries and the aortic sac typically seen in Hand2 mutant embryos1,5 (Extended Data Fig. 4b). Genes encoding other cardiac transcription factors essential for cardiac morphogenesis, namely Nkx2-5, Gata4 and the related Hand1, were not considerably changed in Uph KO embryos (Fig. 3b, Extended Data Fig. 4a), demonstrating the specificity of Uph transcription in regulating Hand2 expression.
Figure 3. Loss of cardiac Hand2 expression in Uph KO embryos.
a, In situ hybridizations of wild-type and Uph KO embryos at E9.5 (transverse and sagittal sections) and E10.5 (transverse) show loss of Hand2 expression in the heart of Uph KO embryos, with detectable expression in the branchial arches (ba) and limb bud (lb). b, In situ hybridizations of wild-type and Uph KO embryos at E9.5 (transverse and sagittal sections) using Hand1, Gata4 or Nkx2-5 probes. Scale bars, 100 µ.
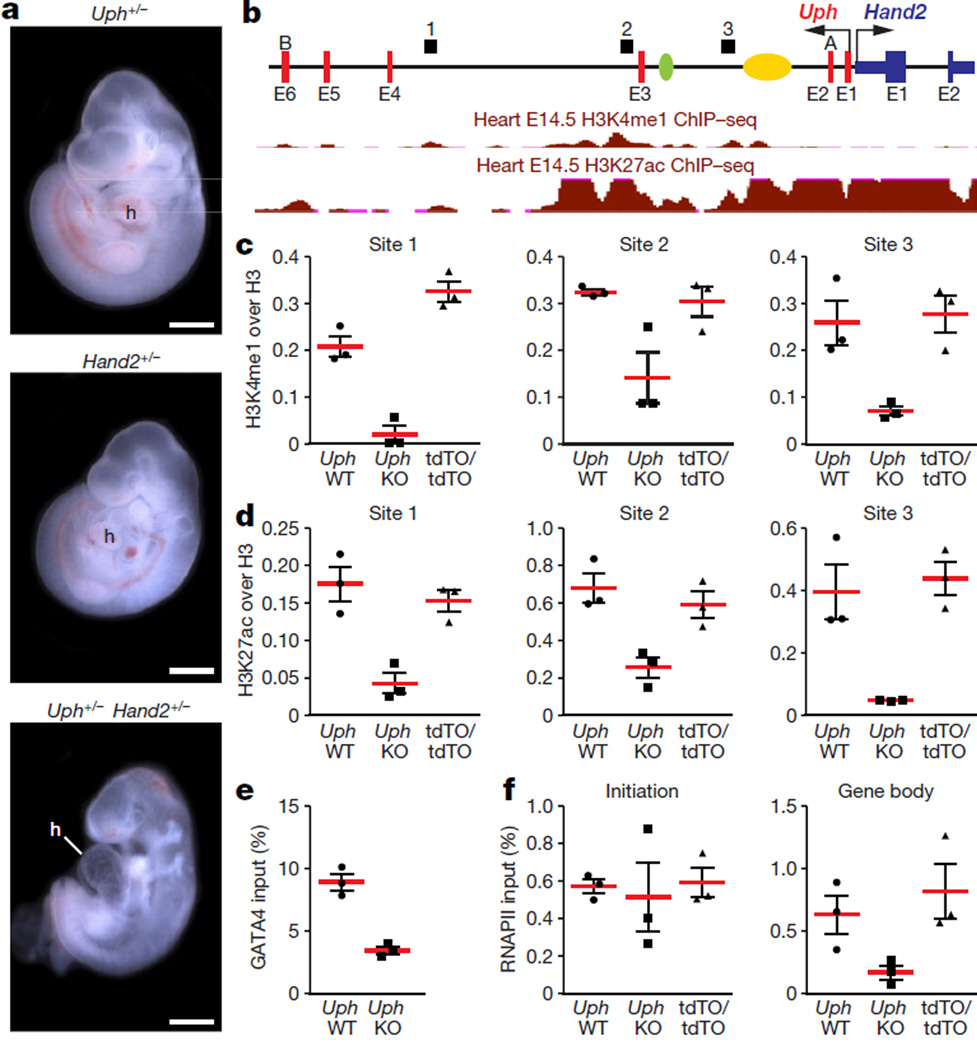
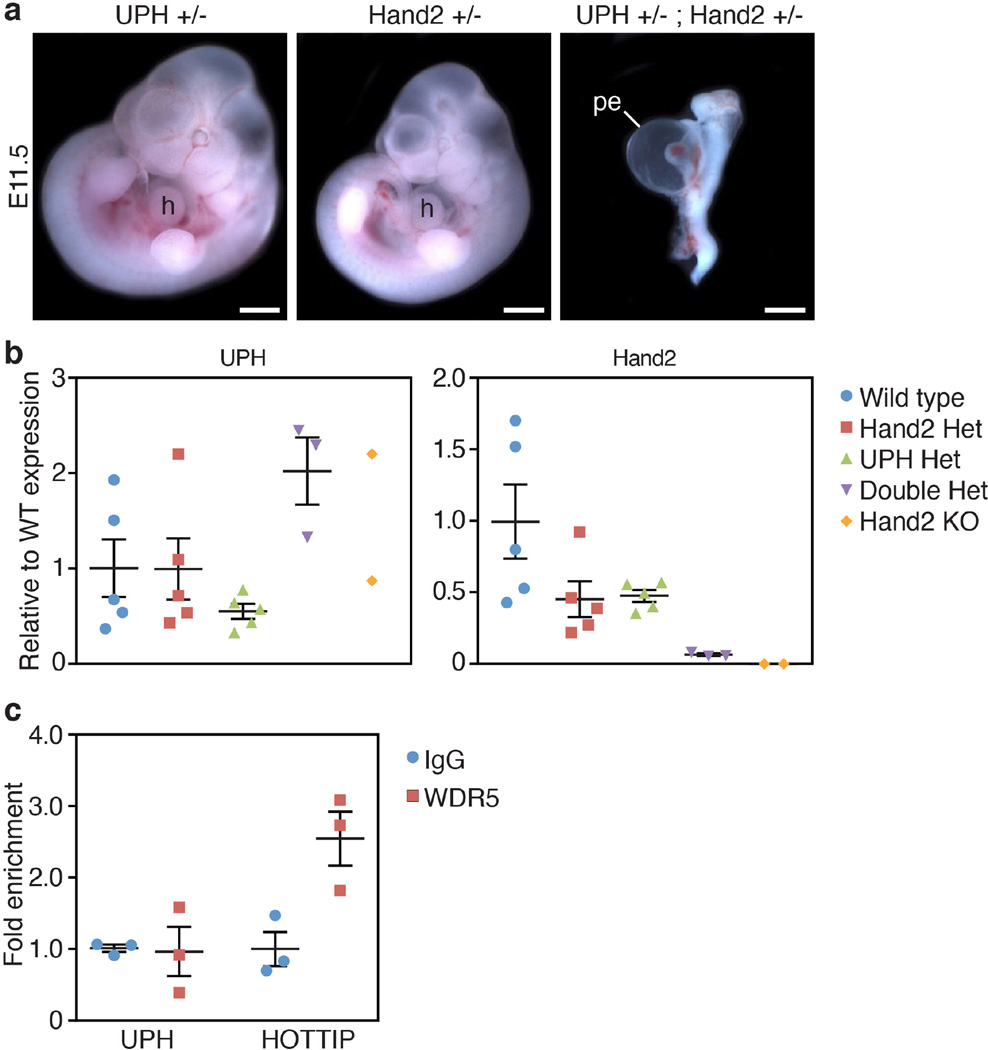
To determine whether Uph functions in cis or trans to regulate Hand2, we intercrossed mice carrying a Hand2 KO allele with mice carrying the Uph KO allele, to generate compound heterozygous (Uph+/− Hand2+/−) embryos. Notably, Uph+/− Hand2+/− embryos recapitulated the Uph KO phenotype, displaying pericardial effusion and embryonic lethality by E10.5 (Fig. 4a, Extended Data Fig. 5a). While Uph expression was not reduced in compound heterozygotes, Hand2 expression was decreased by greater than 90% (Extended Data Fig. 5b). These findings demonstrate that expression of Hand2 in the heart is dependent on transcription of Uph in cis, which cannot be compensated for by Uph transcripts generated on the sister allele.
Figure 4. Uph transcription in cis is required for active enhancer marks in the Uph locus and RNAPII elongation at the Hand2 gene.
a, E10.5 embryos heterozygous for either Uph (top) or Hand2 (middle) appear normal in size and morphology. By contrast, Uph+/− Hand2+/− double heterozygote embryos developed a single ventricle. Scale bars, 1 mm. b, Uph–Hand2 locus with letters (A or B) indicating GapmeR target sites, and numbers (1–3) indicating the regions analysed by qPCR following ChIP. The Hand2 branchial arch enhancer (green) and cardiac enhancer (yellow) are indicated. Shown in red are the ENCODE/LICR ChIP followed be sequencing (ChIP–seq) tracks for H3K4me1 and H3K27ac active enhancer markers. E1–E6, exons 1–6. See Methods for source data. c, d, H3K4me1 (c) and H3K27ac (d) modifications present at the Uph locus in E10.0 wild-type hearts are reduced in Uph KO hearts, and unchanged in UphtdTO/tdTO homozygous hearts. Values are normalized to total histone H3. e, GATA4 binding to the cardiac enhancer is reduced in E10.0 knockout hearts relative to wild type. f, ChIP using RNAPII (phosphoS2) antibody at the Hand2 transcriptional start site or gene body in wild-type, Uph KO and UphtdTO/tdTO homozygous hearts. Values normalized as a percentage of total input chromatin. Each point is one of three technical replicates of five pooled hearts for each genotype in each ChIP experiment, from one of two independent experiments; data are mean ± s.e.m.
Several lncRNAs have been proposed to function by recruiting the trithorax chromatin-modifying complex to gene loci16,17. To determine whether Uph interacts with the trithorax complex, we performed RNA immunoprecipitation using an antibody against the RNA-binding adaptor of the MLL-1 trithorax protein, WDR5. While the lncRNA HOTTIP formed a stable complex with WDR5 in the mouse HL-1 cardiomyocyte-like cell line18, we could not detect an interaction between MLL-1 and Uph (Extended Data Fig. 5c).
To determine whether the mature Uph RNA transcript is required for Hand2 expression, we performed knockdown of Uph using GapmeR antisense oligonucleotides19 in HL-1 cells and in the neuroblastoma cell line Neuro2a, both of which express Uph and Hand2 (Extended Data Fig. 6a). Knockdown of mature Uph transcripts by more than 90% using two different GapmeR oligonucleotides in both cell lines did not alter Hand2 expression (Extended Data Fig. 6b, c). Because Uph is alternatively spliced, we performed qPCR across all Uph exon junctions (Extended Data Fig. 6d). We further verified that efficient knockdown was attained in the nuclear fraction of HL-1 cells (Extended Data Fig. 6e). Additionally, overexpression of the full-length Uph transcript in HL-1 cells using transient transfection had no effect on the levels of Hand2 mRNA (Extended Data Fig. 6f). Together, these data suggest that, in contrast to premature transcriptional termination of Uph in vivo, the mature Uph transcript is not required for Hand2 expression.
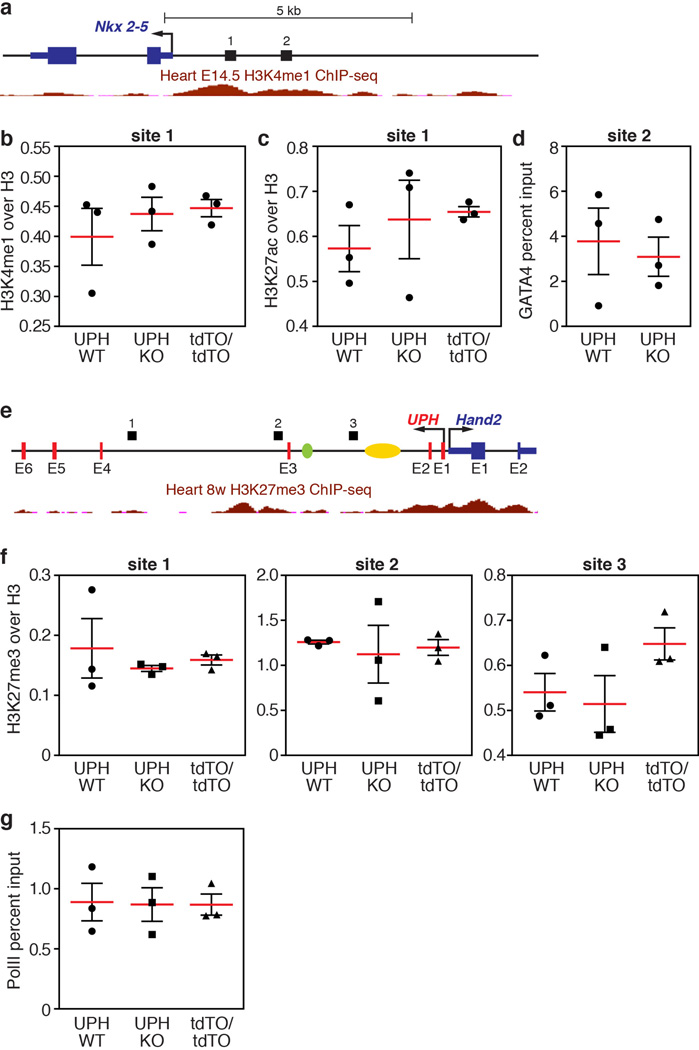
DNA loci with high regulatory capacity such as enhancers20,21, polycomb response elements22,23 or locus control regions24 are often associated with non-coding transcription and can function to maintain a permissive histone signature. In the developing heart, the super-enhancer at the Hand2 locus is defined by H3K27ac (Fig. 4b). To test whether Uph transcription is required to maintain the enhancer signature in the Uph–Hand2 locus, we performed chromatin immunoprecipitation (ChIP) on Uph KO and wild-type E10.0 hearts, using H3K4me1 and H3K27ac active enhancer marker antibodies. We found that the H3K4me1 and H3K27ac peaks normally established at the Uph–Hand2 locus were reduced in Uph KO hearts (Fig. 4c, d).
The GATA4 transcription factor is required for activation of the HAND2 cardiac enhancer in the Uph locus, and participates in establishing active H3K27ac marks7,25. We observed a reduction in GATA4 binding to the Hand2 cardiac enhancer in Uph KO hearts, a finding consistent with the reduced H3K27ac enhancer marks in the Uph locus (Fig. 4e). We observed no differences in H3K4me1 or H3K27ac levels at an active enhancer in the promoter region of Nkx2-5, no differences in GATA4 binding to the Nkx2-5 promoter, and no differences in H3K27me3 repressive marks between wild-type and Uph KO embryos at the Uph–Hand2 locus (Extended Data Fig. 7b–f).
Enhancer-associated histone acetylation is necessary for gene expression by promoting RNA polymerase II (RNAPII) elongation at gene promoters26. To measure the levels of RNAPII at the Hand2 locus, we performed ChIP on wild-type and Uph KO E10.0 hearts using the Ser2-phosphorylated RNAPII antibody, a marker of RNAPII elongation, at the Hand2 locus. Although there was no difference in RNAPII recruitment to the Hand2 transcriptional start site, RNAPII binding to the body of the Hand2 locus was decreased in Uph KO hearts (Fig. 4f). We found no differences in Ser2-phosphorylated RNAPII binding to the Nkx2-5 gene body between genotypes (Extended Data Fig. 7g). These findings suggest that the loss of GATA4 and histone acetylation marks in the Uph–Hand2 locus of Uph KO hearts prevents RNAPII elongation within the Hand2 locus.
Here we define the in vivo function of Uph, a cardiac-enriched lncRNA co-transcribed bi-directionally with the cardiac transcription factor Hand2. Termination of Uph transcription, but not of the mature transcript, resulted in the loss of Hand2 expression in the heart and partially phenocopied the lethal cardiac defects observed in Hand2 KO embryos. Compound Uph+/− Hand2+/− heterozygosity causes embryonic lethality, suggesting that transcription of Uph is required in cis to promote Hand2 expression. Termination of Uph transcription in vivo reduced GATA4 binding and the Uph–Hand2 super-enhancer signature, resulting in RNAPII pausing and loss of Hand2 expression in the heart. We predict that transcription of Uph governs Hand2 expression in all tissues for which the expression of Hand2 depends on enhancers located within the Uph locus. Future studies are needed to determine the role of Uph in the regulation of Hand2 in non-cardiac tissues, which are currently precluded owing to early embryonic lethality of the global Uph KO. Given the importance of Hand2 in the development of the palate, tongue, limbs, and sympathetic nervous system27–30, Uph provides a potentially important non-coding regulatory mechanism for the temporal and spatial control of Hand2 expression.
METHODS
Animal models
All experimental procedures involving animals in this study were reviewed and approved by the University of Texas Southwestern Medical Center’s Institutional Animal Care and Use Committee. Animals/embryos were allocated to experimental groups based on genotype and we did not use exclusion, randomization or blinding approaches. The sex of embryos used in these studies was not determined. In general, sample size was chosen to use the least number of animals/embryos to achieve statistical significance and no statistical methods were used to predetermine sample size.
In situ hybridization
Whole mount in situ hybridization was performed using digoxigenin-labelled antisense RNA probes specific to Uph and Hand2, using methods previously described31. Radioisotopic in situ hybridization studies on sections were performed as previously described32. Antisense RNA probe templates for Uph or Hand2 were generated using mouse heart cDNA and subcloned into pCRII TOPO (Life Technologies). Primer sequences are listed in Extended Data Table 1.
Subcellular fractionation of mouse neonatal cardiomyocytes
Neonatal cardiomyocytes were collected from ~50 post-natal day 1 (P1) C57BL/6 mice using the Neomyt kit (Cellutron, nc-6031) and fractionated as previously described33.
TALEN-mediated homologous recombination in mice
A TALEN pair specific for the Uph locus was designed using the ZiFiT Targeter Program (http://zifit.partners. org/ZiFiT/Introduction.aspx). Individual TALEN modules and donor vectors were constructed and assembled as previously described14. TALEN mRNAs and circular DNA donor plasmids were diluted to 25 ng µ l−1 and 3 ng µ l−1, respectively, and co-injected into the nucleus and cytoplasm of one-cell stage C57BL/6 zygotes and transferred into pseudopregnant mice. Cloning and genotyping primer sequences are listed in Extended Data Table 1.
Histology
Embryos were fixed in 4% formaldehyde in PBS and processed for paraffin histology, sectioned and stained with haematoxylin and eosin using routine procedures. At least 5 embryos of each genotype were sectioned for all experiments and data reported are representative of all samples collected.
ChIP
ChIP was performed using the SimpleChIP Kit (Cell Signaling, 9004) with the following adjustments to the standard protocol. In brief, embryonic hearts were dissected from E10.0 embryos and flash frozen in liquid nitrogen. On average, 5 hearts from identical genotypes were pooled in a 1.5 ml tube before formaldehyde cross-linking. Sonication was performed using a Bioruptor (Diagenode). The following antibodies were used: 10 µ l H3K4me1 (Abcam, ab8895), 10 µ l histone H3 (Cell Signaling), 10 µ l H3K27ac (Active Motif, 39133), 10 µ l H3K27me3 (Active Motif, 39155), 5 µ l RNA polymerase II CTD repeat YSPTSPS (phospho S2) (Abcam, ab5095), 5 µ l of GATA4 (sc-1237X). qPCR primer sequences are listed in Extended Data Table 1.
GapmeR antisense oligonucleotide knockdown of Uph in HL-1 and Neuro2a cells
The mouse neuroblastoma cell line Neuro2a was purchased from ATCC and authenticated by morphology. The cardiomyocyte-like cell line HL-1 was donated by the laboratory of W. Claycomb. Contamination from mycoplasma was not tested for in these cell lines. LNA longRNA GapmeR oligonucleotides specific for Uph were designed and purchased from Exiqon. HL-1 and Neuro2a cells were co-transfected with a plasmid encoding eGFP (CS2GFP, 0.3 ng µ l−1 final) and 50 nM GapmeR antisense oligonucleotides using Lipofectamine2000 (Life Technologies), according to the manufacturer’s recommended protocol. Two days after transfection, GFP-positive cells were FACS sorted and processed using TRIzol (Life Technologies) for downstream gene expression analyses. GapmeR antisense oligonucleotide sequences are listed in Extended Data Table 1.
RACE PCR
The sequences of Uph RNA transcripts expressed in the mouse heart were determined using 5′ and 3′ RACE PCR using commercially available Marathon-Ready cDNA (Clontech). RACE PCR was performed according to manufacturer’s recommended protocol, using primers specific to exon 4 of Uph and the marathon adaptor primer 1 (AP1). 5′ and 3′ Uph primer sequences are listed in Extended Data Table 1.
RNA immunoprecipitation
RNA–protein complexes from formaldehydecross-linked HL-1 cell lysates were immunoprecipitated using 2 µ l IgG (Cell Signaling) or 2 µ l WDR5 (Cell Signaling) antibodies. RNA purification and expression analyses were performed as previously described34.
Statistics
Unless otherwise indicated in the figure legends, error bars represent s.e.m. of at least 3 biological replicates of each genotype or sample.
Data availability
Expressed sequence tag (EST) sequences for alignment of Uph transcripts were downloaded from GenBank: rat (BF567084), pig (DN117952), dog (DN434464) and cow (DN282558), or from RefSeq: human (NR_003679.2). Previously published ChIP–seq data used in Fig. 4b and Extended Data Fig. 7a, b are available under accession code GSE31039 (ref. 35). Source data for Extended Data Figs 1e, 2b, 3c, d are available in Supplementary Fig. 1, and all other data that support the findings of this study are available from the corresponding author on request.
Extended Data
Extended Data Figure 1. Sequence alignment of several mammalian Uph transcripts.
a, Sequence alignment of several mammalian Uph transcripts performed using ClustalW. See Methods for source data. b, Diagram of the Hand2 locus in mammals showing the genomic organization and orientation of Uph. The Hand2 branchial arch enhancer (green) and cardiac enhancer (yellow) are shown. c, Diagram of the Uph transcripts expressed in the mouse heart, determined using 5′ and 3′ RACE using primers specific to exon 4 of Uph. AP1, marathon adaptor primer; 3′ GSP, 3′ Uph-specific primer from exon 4; 5′ GSP, 5′ Uph-specific primer from exon 4. d, Whole mount in situ hybridization of E10.5 mouse embryos. Expression was detected in heart, branchial arches (arrowhead), and limb bud. Scale bars, 1 mm. e, Northern blot analysis of total RNA from adult mouse tissues using a probe specific to the major Uph transcript. For gel source data, see Supplementary Fig. 1.
Extended Data Figure 2. Uph is a cytoplasmic lncRNA.
a, Subcellular fractionation of 18S, Uph and Malat1 lncRNA in mouse neonatal cardiomyocytes (n = 3 biological replicates from 1 of 5 independent experiments; mean ± s.e.m.). b, In vitro transcription and translation of a plasmid encoding the major Uph RNA. A plasmid encoding the myoregulin (MLN) micropeptide was used as a positive control, and myoregulin with a frameshift mutation (MLN RNA-FS) was used as a negative control. In contrast to myoregulin, Uph and the negative control (MLN RNA-FS) did not produce any detectable proteins, indicating that Uph is a bona fide lncRNA. For gel source data, see Supplementary Fig. 1.
Extended Data Figure 3. Targeting strategy for insertion of transcriptional termination or heterologous sequence into exon 2 of Uph.
a, Uph KO targeting strategy. Transcription activator-like effector nucleases (TALENs) were used to insert a triple polyadenylation (tpA) termination sequence into exon 2 (E2) of Uph. b, Using the same TALEN pair as in a, we introduced the coding sequence of tdTO, lacking a polyadenylation sequence, into exon 2 of the Uph locus. Exon 2, which includes the tdTO coding sequence, was spliced out of the mature Uph transcript, preventing expression of tdTO in these mice. c, Southern blot analysis of wild-type, heterozygous Uph+/− (Het) and Uph KO genomic DNA. BamHI-digested DNA hybridized with a 5′ -specific probe and NdeI-digested DNA hybridized with a 3′ -specific probe. For gel source data, see Supplementary Fig. 1. d, Southern blot analysis of wild-type, UphtdTO/+ heterozygous and UphtdTO/tdTO knock-in (KI) genomic DNA verified the correct targeting of the tdTO sequence into the Uph locus. DNA was digested with BamHI and hybridized with a 5′ -specific probe or digested with NdeI and hybridized with a 3′ -specific probe. For gel source data, see Supplementary Fig. 1. e, f, Expression of Uph (e) and Hand2 (f) in wild-type and UphtdTO/tdTO homozygous mice at E10.0 was not changed by the insertion of the tdTO sequence into exon 2 of the Uph locus (n = 3, representative of 3 independent experiments; mean ± s.e.m.). g, qPCR shows Uph transcripts decreased by ~97% in E10.5 hearts (n = 3 mice of each genotype from 1 of 3 independent experiments; mean ± s.e.m.).
Extended Data Figure 4. Aortic arch arteries are normal in Uph KO embryos.
a, qPCR quantification of gene expression at E10.0 showed robust downregulation of Uph and Hand2 expression in Uph KO hearts, with normal expression of other cardiac transcription factors (n = 3 mice of each genotype from 1 of 3 independent experiments; mean ± s.e.m.). b, India ink was injected into either the left ventricle of wild-type embryos or the single ventricle of Uph KO embryos at E10.5, to visualize the aortic arch arteries and circulation, which appeared normal in Uph KO embryos. aa, aortic arch arteries; as, aortic sac; da, dorsal aorta. Scale bars, 1 mm.
Extended Data Figure 5. Uph+/− Hand2+/− compound heterozygotes recapitulate Uph KO phenotype.
a, Uph+/− Hand2+/− double heterozygote embryos developed a single ventricle, pericardial effusion, and were severely growth restricted by E11.5. Scale bars, 1 mm. b, Uph expression is normal in double heterozygotes, whereas Hand2 was reduced by ~90%, relative to wild-type embryos (n = 5 mice for wild type, Hand2 het and Uph het, n = 3 for double het, n = 2 for Hand2 KO, from 1 of 2 independent experiments; mean ± s.e.m.). c, Immunoprecipitation of RNA using either IgG or WDR5 in HL-1 cells followed by qPCR for Uph revealed no binding to WDR5. The WDR5-interacting lncRNA HOTTIP was used as a positive control (n = 3 biological replicates from 1 of 3 independent experiments; mean ± s.e.m.).
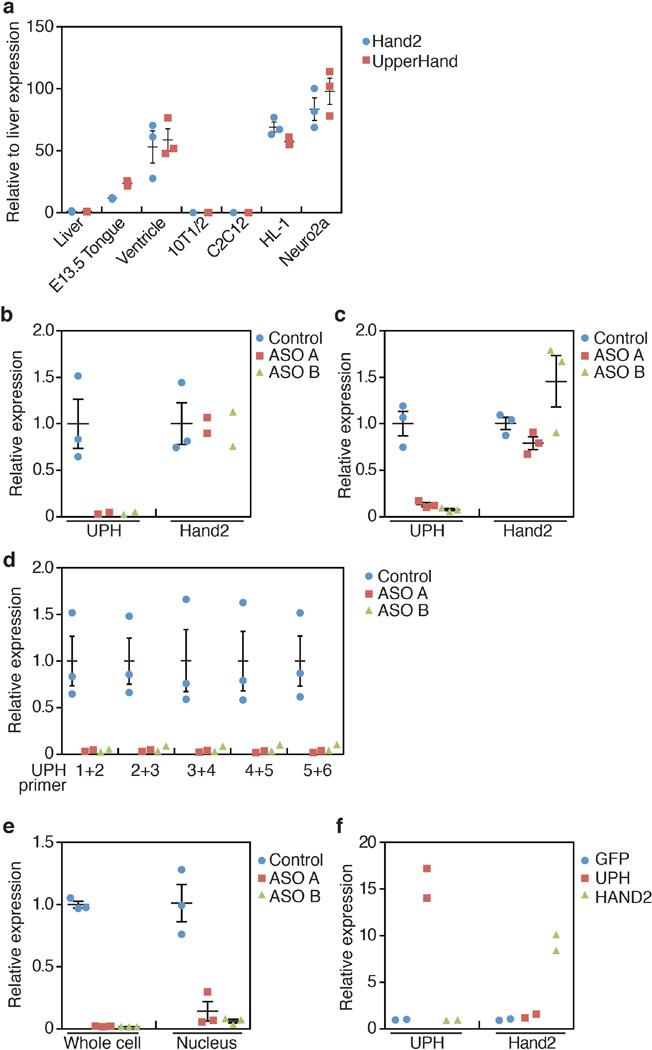
Extended Data Figure 6. Knockdown of mature Uph transcripts in HL-1 or Neuro2a cells does not affect Hand2 expression.
a, Expression of Uph (red) and Hand2 (blue) in various tissues and cell lines. Uph and Hand2 are robustly expressed in the heart, and the HL-1 and Neuro2a cell lines. Uph and Hand2 transcripts are not expressed in the liver, 10T1/2 fibroblasts or skeletal muscle C2C12 myotubes (n = 3 technical replicates; mean ± s.e.m.). b, c, Uph transcripts were reduced by ~90% in HL-1 (b) and Neuro2a (c) cells when transfected with two independent GapmeR antisense oligonucleotide probes (ASO A and B) against Uph. Expression of Hand2 was not changed (n = 2 for ASO A and B, n = 3 for control, from 1 of 2 independent experiments; mean ± s.e.m.). d, Uph transcripts were similarly downregulated across each exon–exon junction, measured using qPCR (n = 2 for ASO A and B, n = 3 for control, from 1 of 2 independent experiments; mean ± s.e.m.). e, Fractionation of HL-1 cells transfected with control or Uph-specific ASOs. The nuclear fraction of antisenseoligonucleotide-treated HL-1 cells showed a similar downregulation of Uph transcripts (n = 3 biological replicates from 1 of 3 independent experiments; mean ± s.e.m.). f, Overexpression of enhanced green fluorescent protein (eGFP; blue), the major Uph RNA (red) or Hand2 RNA (green) in HL-1 cells revealed that the mature Uph transcript did not alter Hand2 expression relative to wild type, and that Hand2 does not influence the expression of Uph in these cells (n = 2 biological replicates from 1 of 2 independent experiments; mean ± s.e.m.).
Extended Data Figure 7. ChIP and qPCR analyses of active cardiac enhancers regulating Nkx2-5 expression.
a, Diagram of the Nkx2-5 locus with numbers (1 and 2) indicating the region analysed by qPCR following ChIP. Shown in red are the ENCODE/LICR H3K4me1 active enhancer marks in the heart at E14.5. See Methods for source data. b, ChIP coupled with qPCR analysis of H3K4me1 marks, normalized to total histone H3, showed that the H3K4me1 marks bound to the Nkx2-5 promoter region are unchanged between wild-type, Uph KO and UphtdTO/tdTO homozygous hearts at E10.0. c, ChIP coupled with qPCR analysis of H3K27ac marks, normalized to total histone H3, showed that the H3K27ac marks bound to the Nkx2-5 promoter region are unchanged between wild-type, Uph KO and UphtdTO/tdTO homozygous hearts at E10.0. d, GATA4 binding to a GATA4 site in the Nkx2-5 promoter is unchanged between wild-type and Uph KO hearts at E10.0. e, Diagram of the Uph–Hand2 locus, with black bars indicating the regions analysed by qPCR following ChIP. Shown in red is the ENCODE/LICR H3K27me3 track for mouse heart. See Methods for source data. The branchial arch enhancer (green) and cardiac enhancer (yellow) are shown. f, ChIP coupled with qPCR analysis of the polycomb repressive marker H3K27me3, normalized to total histone H3, showed no differences between genotypes at each locus (1–3) indicated in the diagram in e. g, qPCR revealed no difference in the levels of Ser2-phosphorylated RNAPII between genotypes at the Nkx2-5 gene body. Each point is one of 3 technical replicates of 5 pooled hearts for each genotype in each ChIP experiment, from 1 of 2 independent experiments; mean ± s.e.m.
Extended Data Table 1.
Relevant sequences
| SEQUENCE NAME | FORWARD | REVERSE |
|---|---|---|
| Full Length UPH sequence | CACTCATAACCATAAGATAATTAAAACGG | TTTAAAAAATAATTTTTAATATACTATGTGCATGGTTGGATAGGT |
| UPH qPCR primers | CATTCTCGAGCAATTCGTCA | TGGTAGCCCATCTCCAACTC |
| UPH ISH probe | GATGAGACCTTCAGTTTGTGCC | ATACTATGTGCATGGTTGGATAGGT |
| Hand2 ISH probe | ATGAGTCTGGTGGGGGGC | TCACTGCTTGAGCTCCAGG |
| UPH targeting 5 ' arm | TATCGGAGCTCGCACCTCGGAGCTGGGAA | GATACGCGGCCGCGGATCCAGTTGTCATCCTAACTTGGGTCA |
| UPH targeting 3 ' arm | TATCGGCTAGCCATATGACCCTAACAGAGATTGCGAAGA | GATACATCGATCAGGGCAGTTAGGTCTCAGC |
| UPH 5 ' WT genotyping | CTCCTCTCCGGACAAGAATC | TGCTGCAAATGAGTGTGG |
| UPH 5 ' KO genotyping | CTCCTCTCCGGACAAGAATC | GGTTCCGGATCCACTAGTTCT |
| UPH 3 ' WT genotyping | AGAGAACGCGGATGAGACCT | CCCTTGCAAACAGAAGAAAGG |
| UPH 3 ' KO genotyping | GACCTGCAGCCCAAGCTA | CCCTTGCAAACAGAAGAAAGG |
| tdTO 5 ' WT genotyping | CTCCTCTCCGGACAAGAATC | TGCTGCAAATGAGTGTGG |
| tdTO 5 ' KO genotyping | CTCCTCTCCGGACAAGAATC | ATGACCTCCTCGCCCTTG |
| tdTO 3 ' WT genotyping | AGAGAACGCGGATGAGACCT | CCCTTGCAAACAGAAGAAAGG |
| tdTO 3 ' KO genotyping | GACCTGCAGCCCAAGCTA | CCCTTGCAAACAGAAGAAAGG |
| Southern BamHI probe | TGGTTTTCTTGTCGTTGCTG | CTGACTGGGTCCTTGAGCAT |
| Southern NdeI probe | TCCTGGGAAGGCACTATGTC | ACCTTCTCCTGCCCTTCATT |
| Hand1 ISH probe | CCATCATCACCACTCACACC | GCGCCCTTTAATCCTCTTCT |
| Nkx2-5 ISH probe | TATGGCTACAACGCCTACCC | GTGTGGAATCCGTCGAAAGT |
| ASO negative control | AACACGTCTATACGC | |
| ASO UPH A | GCTAGTTAAGCAGGA | |
| ASO UPH B | ATTCAATTTAGGTCAT | |
| 5GSP1 | CAGCTGTATGGGCTCAGGTGACTGC | |
| 3GSP1 | TGGAGATGGGCTACCATTGGTGTTGA | |
| AP1 | CCATCCTAATACGACTCACTATAGGGC | |
| Hand2 - Gata site | TTTACCCACTGGTCCCCTCT | TGGACAACATGGGACAGAAA |
| Nkx2-5 - Gata site | CTGCAACTATCACCCGGAAT | AGAAACCCCCATCTGTTTCC |
| UPH ChIP site 1 | TCACCTCCCCATGTCTTTTC | GAGGAACCTGCATTGCTTTC |
| UPH ChIP site 2 | ACCTCGGGCTTTCGATCTTA | GCTTGGGAAGGTAAGCCTTT |
| UPH ChIP site 3 | GAAACTAGCCTTGCCCCTTC | GGGTGCCTAGGGAGGAATAC |
| Nkx2-5 ChIP - site 1 | ACTGTGAAGCCCAATTCCAG | AACCAGAAATTGTGGCAAGG |
Supplementary Material
Acknowledgments
We thank D. Tennison for technical assistance and J. Cabrera for graphics. We thank the Histopathology Core Facility at University of Texas Southwestern for histology. This work was supported by grants from the National Institutes of Health (HL-077439, AR-067294, HL-130253, DK-099653 and U01-HL-100401), Fondation Leducq Networks of Excellence, Cancer Prevention and Research Institute of Texas and the Robert A. Welch Foundation (grant 1-0025 to E.N.O.), a pre-doctoral fellowship from the American Heart Association (14PRE19830031) to K.M.A. and a Muscular Dystrophy Association Development Grant (MDA377340) to D.M.A.
Footnotes
Online Content Methods, along with any additional Extended Data display items and Source Data, are available in the online version of the paper; references unique to these sections appear only in the online paper.
Author Contributions K.M.A., D.M.A. and E.N.O. designed experiments and analysed data. J.R.M. generated mutant mice from constructs designed and created by K.M.A. and D.M.A. K.M.A. performed qPCR, phenotypic analysis, dissections, ChIP, RNA immunoprecipitation, cardiomyocyte fractionation, Southern blot, and India ink injections. D.M.A performed northern blot, RACE, in vitro transcription/translation, and GapmeR transfections. J.M.S. and K.M.A. performed in situ hybridization and generated images. K.M.A., D.M.A, E.N.O. and R.B.D. wrote and edited the manuscript.
The authors declare no competing financial interests.
References
- 1.Srivastava D, et al. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat. Genet. 1997;16:154–160. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- 2.Han Z, Yi P, Li X, Olson EN. Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development. 2006;133:1175–1182. doi: 10.1242/dev.02285. [DOI] [PubMed] [Google Scholar]
- 3.Song K, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conway SJ, Firulli B, Firulli AB. A bHLH code for cardiac morphogenesis. Pediatr. Cardiol. 2010;31:318–324. doi: 10.1007/s00246-009-9608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamagishi H, Olson EN, Srivastava D. The basic helix-loop-helix transcription factor, dHAND, is required for vascular development. J. Clin. Invest. 2000;105:261–270. doi: 10.1172/JCI8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charité J, et al. Role of Dlx6 in regulation of an endothelin-1-dependent, dHAND branchial arch enhancer. Genes Dev. 2001;15:3039–3049. doi: 10.1101/gad.931701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFadden DG, et al. A GATA-dependent right ventricular enhancer controls dHAND transcription in the developing heart. Development. 2000;127:5331–5341. doi: 10.1242/dev.127.24.5331. [DOI] [PubMed] [Google Scholar]
- 8.Hnisz D, et al. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepoivre C, et al. Divergent transcription is associated with promoters of transcriptional regulators. BMC Genomics. 2013;14:914. doi: 10.1186/1471-2164-14-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voth H, et al. Co-regulated expression of HAND2 and DEIN by a bidirectional promoter with asymmetrical activity in neuroblastoma. BMC Mol. Biol. 2009;10:28. doi: 10.1186/1471-2199-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chodroff RA, et al. Long noncoding RNA genes: conservation of sequence and brain expression among diverse amniotes. Genome Biol. 2010;11:R72. doi: 10.1186/gb-2010-11-7-r72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kambara H, et al. Regulation of interferon-stimulated gene BST2 by a lncRNA transcribed from a shared bidirectional promoter. Front. Immunol. 2015;5:676. doi: 10.3389/fimmu.2014.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uesaka M, et al. Bidirectional promoters are the major source of gene activation-associated non-coding RNAs in mammals. BMC Genomics. 2014;15:35. doi: 10.1186/1471-2164-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson DM, et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160:595–606. doi: 10.1016/j.cell.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson BR, et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351:271–275. doi: 10.1126/science.aad4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grote P, et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell. 2013;24:206–214. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang KC, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YW, et al. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. eLife. 2014;3:e02046. doi: 10.7554/eLife.02046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein CA, et al. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 2010;38:e3. doi: 10.1093/nar/gkp841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaikkonen MU, et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol. Cell. 2013;51:310–325. doi: 10.1016/j.molcel.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TK, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt S, Prestel M, Paro R. Intergenic transcription through a polycomb group response element counteracts silencing. Genes Dev. 2005;19:697–708. doi: 10.1101/gad.326205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzog VA, et al. A strand-specific switch in noncoding transcription switches the function of a Polycomb/Trithorax response element. Nat. Genet. 2014;46:973–981. doi: 10.1038/ng.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho Y, Elefant F, Liebhaber SA, Cooke NE. Locus control region transcription plays an active role in long-range gene activation. Mol. Cell. 2006;23:365–375. doi: 10.1016/j.molcel.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 25.He A, et al. Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat. Commun. 2014;5:4907. doi: 10.1038/ncomms5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonkers I, Lis JT. Getting up to speed with transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 2015;16:167–177. doi: 10.1038/nrm3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanagisawa H, Clouthier DE, Richardson JA, Charité J, Olson EN. Targeted deletion of a branchial arch-specific enhancer reveals a role of dHAND in craniofacial development. Development. 2003;130:1069–1078. doi: 10.1242/dev.00337. [DOI] [PubMed] [Google Scholar]
- 28.Barron F, et al. Downregulation of Dlx5 and Dlx6 expression by Hand2 is essential for initiation of tongue morphogenesis. Development. 2011;138:2249–2259. doi: 10.1242/dev.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charité J, McFadden DG, Olson EN. The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development. 2000;127:2461–2470. doi: 10.1242/dev.127.11.2461. [DOI] [PubMed] [Google Scholar]
- 30.Lucas ME, Müller F, Rüdiger R, Henion PD, Rohrer H. The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development. 2006;133:4015–4024. doi: 10.1242/dev.02574. [DOI] [PubMed] [Google Scholar]
- 31.Anderson DM, et al. Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev. Dyn. 2006;235:792–801. doi: 10.1002/dvdy.20671. [DOI] [PubMed] [Google Scholar]
- 32.Shelton JM, Lee MH, Richardson JA, Patel SB. Microsomal triglyceride transfer protein expression during mouse development. J. Lipid Res. 2000;41:532–537. [PubMed] [Google Scholar]
- 33.Gagnon KT, Li L, Janowski BA, Corey DR. Analysis of nuclear RNA interference in human cells by subcellular fractionation and Argonaute loading. Nat. Protocols. 2014;9:2045–2060. doi: 10.1038/nprot.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conrad NK. Chapter 15. Co-immunoprecipitation techniques for assessing RNA-protein interactions in vivo. Methods Enzymol. 2008;449:317–342. doi: 10.1016/S0076-6879(08)02415-4. [DOI] [PubMed] [Google Scholar]
- 35.ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Expressed sequence tag (EST) sequences for alignment of Uph transcripts were downloaded from GenBank: rat (BF567084), pig (DN117952), dog (DN434464) and cow (DN282558), or from RefSeq: human (NR_003679.2). Previously published ChIP–seq data used in Fig. 4b and Extended Data Fig. 7a, b are available under accession code GSE31039 (ref. 35). Source data for Extended Data Figs 1e, 2b, 3c, d are available in Supplementary Fig. 1, and all other data that support the findings of this study are available from the corresponding author on request.