Abstract
DNA topoisomerase II (EC 5.99.1.3) is necessary for chromosome condensation and disjunction in yeast but not for other functions. In mammalian cells, it has been reported to be necessary for progression toward mitosis but not for transit through mitosis. We have found, on the contrary, that specific inhibition of topoisomerase II (but not of topoisomerase I) interferes with mammalian mitotic progression. Metaphase is prolonged, and anaphase separation of chromatids is completely inhibited, in cells given high concentrations of topoisomerase II inhibitors; nevertheless these cells attempt cleavage, sometimes generating nucleate and anucleate daughters. Lower concentrations of inhibitors interfere with anaphase and produce abnormalities of segregation. DNA topoisomerase II activity is therefore necessary for mammalian chromatid separation, but it is not tightly coupled to the control of other mitotic events.
Full text
PDF

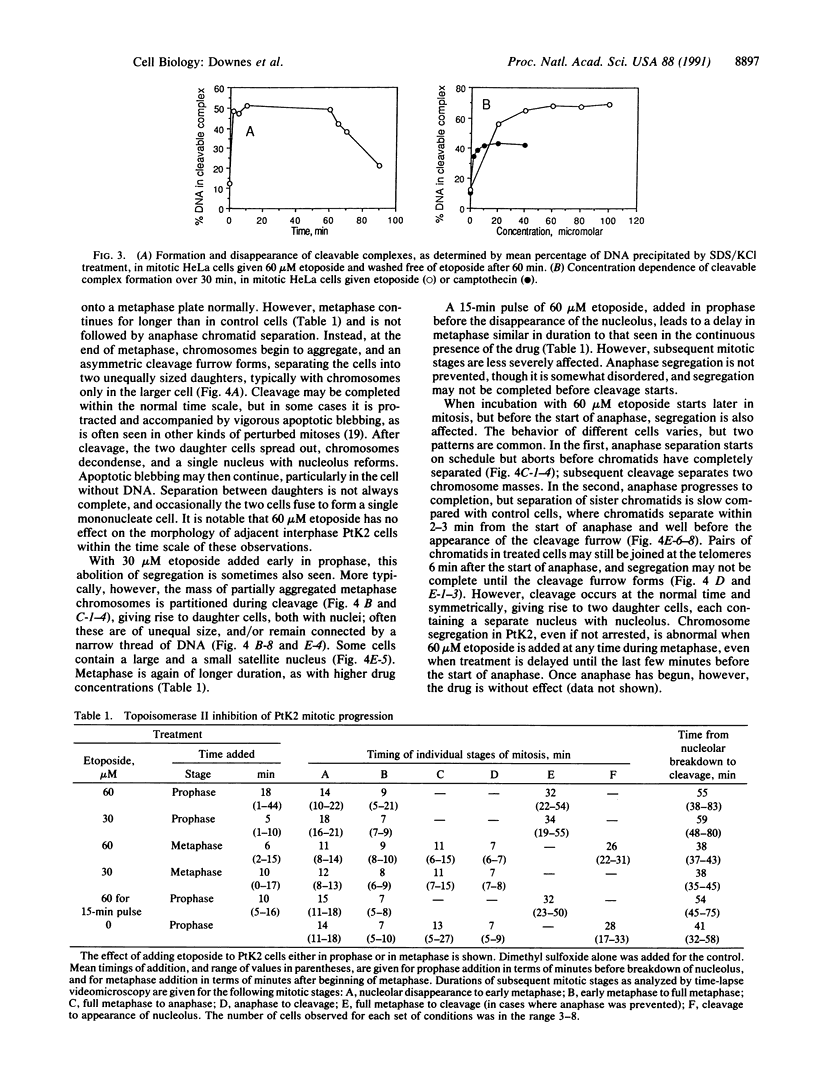


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Käs E., Laemmli U. K. Preferential, cooperative binding of DNA topoisomerase II to scaffold-associated regions. EMBO J. 1989 Dec 20;8(13):3997–4006. doi: 10.1002/j.1460-2075.1989.tb08582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J. G. Chinese hamster ovary cell mitosis and its response to ionizing radiation: a morphological analysis of the living cell. Radiat Res. 1989 May;118(2):311–323. [PubMed] [Google Scholar]
- Charron M., Hancock R. DNA topoisomerase II is required for formation of mitotic chromosomes in Chinese hamster ovary cells: studies using the inhibitor 4'-demethylepipodophyllotoxin 9-(4,6-O-thenylidene-beta-D-glucopyranoside). Biochemistry. 1990 Oct 16;29(41):9531–9537. doi: 10.1021/bi00493a006. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. S., Johnson R. T. DNA topoisomerases and DNA repair. Bioessays. 1988 Jun;8(6):179–184. doi: 10.1002/bies.950080602. [DOI] [PubMed] [Google Scholar]
- Downes C. S., Unwin D. M., Northfield R. G., Berry M. J. Automatic nitrous oxide synchronization of mitotic human cell cultures. Anal Biochem. 1987 Aug 15;165(1):56–58. doi: 10.1016/0003-2697(87)90200-4. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Heck M. M. Localization of topoisomerase II in mitotic chromosomes. J Cell Biol. 1985 May;100(5):1716–1725. doi: 10.1083/jcb.100.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulden M. E. Hypothesis: some mutagens directly alter specific chromosomal proteins (DNA topoisomerase II and peripheral proteins) to produce chromosome stickiness, which causes chromosome aberrations. Mutagenesis. 1987 Sep;2(5):357–365. doi: 10.1093/mutage/2.5.357. [DOI] [PubMed] [Google Scholar]
- Holm C., Stearns T., Botstein D. DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage. Mol Cell Biol. 1989 Jan;9(1):159–168. doi: 10.1128/mcb.9.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J. R., Pocklington M. J., Orr E. The F1 ATP synthetase beta-subunit: a major yeast novobiocin binding protein. J Cell Sci. 1990 Aug;96(Pt 4):675–682. doi: 10.1242/jcs.96.4.675. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Mullinger A. M., Skaer R. J. Perturbation of mammalian cell division: human mini segregants derived from mitotic cells. Proc R Soc Lond B Biol Sci. 1975 Jun 17;189(1097):591–602. doi: 10.1098/rspb.1975.0074. [DOI] [PubMed] [Google Scholar]
- Kalwinsky D. K., Look A. T., Ducore J., Fridland A. Effects of the epipodophyllotoxin VP-16-213 on cell cycle traverse, DNA synthesis, and DNA strand size in cultures of human leukemic lymphoblasts. Cancer Res. 1983 Apr;43(4):1592–1597. [PubMed] [Google Scholar]
- Kato J., Nishimura Y., Imamura R., Niki H., Hiraga S., Suzuki H. New topoisomerase essential for chromosome segregation in E. coli. Cell. 1990 Oct 19;63(2):393–404. doi: 10.1016/0092-8674(90)90172-b. [DOI] [PubMed] [Google Scholar]
- Koshland D., Hartwell L. H. The structure of sister minichromosome DNA before anaphase in Saccharomyces cerevisiae. Science. 1987 Dec 18;238(4834):1713–1716. doi: 10.1126/science.3317838. [DOI] [PubMed] [Google Scholar]
- Lock R. B., Ross W. E. Inhibition of p34cdc2 kinase activity by etoposide or irradiation as a mechanism of G2 arrest in Chinese hamster ovary cells. Cancer Res. 1990 Jun 15;50(12):3761–3766. [PubMed] [Google Scholar]
- Mazia D. The chromosome cycle and the centrosome cycle in the mitotic cycle. Int Rev Cytol. 1987;100:49–92. doi: 10.1016/s0074-7696(08)61698-8. [DOI] [PubMed] [Google Scholar]
- Pillidge L., Downes C. S., Johnson R. T. Defective post-replication recovery and u.v. sensitivity in a simian virus 40-transformed Indian muntjac cell line. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Jul;50(1):119–136. doi: 10.1080/09553008614550501. [DOI] [PubMed] [Google Scholar]
- Richter A., Strausfeld U., Knippers R. Effects of VM26 (teniposide), a specific inhibitor of type II DNA topoisomerase, on SV40 DNA replication in vivo. Nucleic Acids Res. 1987 Apr 24;15(8):3455–3468. doi: 10.1093/nar/15.8.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge M., Th'ng J., Hamaguchi J., Bradbury E. M. The topoisomerase II inhibitor VM-26 induces marked changes in histone H1 kinase activity, histones H1 and H3 phosphorylation, and chromosome condensation in G2 phase and mitotic BHK cells. J Cell Biol. 1990 Nov;111(5 Pt 1):1753–1762. doi: 10.1083/jcb.111.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D., Thomas W., Holm C. Segregation of recombined chromosomes in meiosis I requires DNA topoisomerase II. Cell. 1990 Mar 23;60(6):1009–1017. doi: 10.1016/0092-8674(90)90349-j. [DOI] [PubMed] [Google Scholar]
- Rowley R., Kort L. Novobiocin, nalidixic acid, etoposide, and 4'-(9-acridinylamino)methanesulfon-m-anisidide effects on G2 and mitotic Chinese hamster ovary cell progression. Cancer Res. 1989 Sep 1;49(17):4752–4757. [PubMed] [Google Scholar]
- Sheinin R. Preliminary characterization of the temperature-sensitive defect in DNA replication in a mutant mouse L cell. Cell. 1976 Jan;7(1):49–57. doi: 10.1016/0092-8674(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Arrest of segregation leads to accumulation of highly intertwined catenated dimers: dissection of the final stages of SV40 DNA replication. Cell. 1981 Sep;25(3):659–669. doi: 10.1016/0092-8674(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Oishi N., Crissman H. A. Cell cycle synchronization: reversible induction of G2 synchrony in cultured rodent and human diploid fibroblasts. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5104–5108. doi: 10.1073/pnas.87.13.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Ohkura H., Adachi Y., Morino K., Shiozaki K., Yanagida M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell. 1987 Sep 11;50(6):917–925. doi: 10.1016/0092-8674(87)90518-6. [DOI] [PubMed] [Google Scholar]
- Uemura T., Tanagida M. Mitotic spindle pulls but fails to separate chromosomes in type II DNA topoisomerase mutants: uncoordinated mitosis. EMBO J. 1986 May;5(5):1003–1010. doi: 10.1002/j.1460-2075.1986.tb04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. R., Earnshaw W. C. Mitotic chromatin condensation in vitro using somatic cell extracts and nuclei with variable levels of endogenous topoisomerase II. J Cell Biol. 1990 Dec;111(6 Pt 2):2839–2850. doi: 10.1083/jcb.111.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacksenhaus E., Sheinin R. Molecular cloning, primary structure and expression of the human X linked A1S9 gene cDNA which complements the ts A1S9 mouse L cell defect in DNA replication. EMBO J. 1990 Sep;9(9):2923–2929. doi: 10.1002/j.1460-2075.1990.tb07483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





