Abstract
Background
Right ventricular (RV) morphology has been associated with drivers of atrial fibrillation (AF) risk including left ventricular (LV) and pulmonary pathology, systemic inflammation and neurohormonal activation. The aim of this study was to investigate the association between RV morphology and risk of incident AF.
Methods and Results
We interpreted cardiac magnetic resonance (CMR) imaging in 4,204 participants free of clinical cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis (MESA). Incident AF was determined using hospital discharge records, study electrocardiograms, and Medicare claims data. The study sample (n=3,819) was 61±10 years old and 47% male with 47.2% current/former smokers. Following adjustment for demographics and clinical factors including incident heart failure, higher RV ejection fraction (RVEF; hazard ratio [HR]: 1.16 per standard deviation [SD], 95% CI: 1.03–1.32, p=0.02) and greater RV mass (HR: 1.25 per SD, 95% CI: 1.08–1.44, p=0.002) were significantly associated with incident AF. After additional adjustment for the respective LV parameter, higher RVEF remained significantly associated with incident AF (HR: 1.15 per SD, 95% CI: 1.01–1.32, p = 0.04) whereas the association was attenuated for RV mass (HR 1.16 per SD, 95% CI: 0.99–1.35, p = 0.07). In a subset of patients with available spirometry (n=2,540), higher RV ejection fraction and mass remained significantly associated with incident AF after additional adjustment for lung function (p=0.02 for both).
Conclusions
Higher RV ejection fraction and greater RV mass were associated with an increased risk of atrial fibrillation in a multi-ethnic population free of clinical cardiovascular disease at baseline.
Keywords: atrial fibrillation, heart ventricles, magnetic resonance imaging
Introduction
Atrial fibrillation (AF) is a major public health burden associated with stroke, heart failure, and mortality.1, 2 Improved diagnostic surveillance and increased survival following diagnosis frame the rising prevalence of AF which is estimated to affect nearly 12 million people in the United States by 2050.3, 4 Improved AF risk prediction, particularly in those without cardiovascular disease, is necessary for timely deployment of therapies and preventative strategies.5 To this end, standard clinical risk factors (eg. diabetes, hypertension, obesity, and smoking) account for only half of the risk of AF in contemporary community-based cohorts.6 These observations suggest that more refined markers of AF risk that directly reflect the underlying pathophysiology of disease development may be useful.
There is an increasing recognition of the prognostic and clinical importance of the right ventricle (RV) in cardiovascular disease.7–10 RV morphology may reflect pulmonary pathology11 and subclinical LV disease12, 13 or may directly contribute to cardiovascular risk.7 To date, the relationship between cardiovascular structure and incident AF has centered on the left heart,14–16 whereas the relationship between RV morphology and incident AF remains unknown. RV morphology may serve as a structural indicator of established clinical risk factors (eg. obesity, sleep apnea)17, 18 or biologically relevant pathways (eg. inflammation, neurohormonal activation)19, 20 implicated in the pathogenesis of AF.
To examine the relationship between RV morphology and AF risk, we studied participants in the Multi-Ethnic Study of Atherosclerosis (MESA) – a prospective, multi-ethnic cohort free of cardiovascular disease at baseline – with detailed cardiac magnetic resonance (CMR) imaging of RV structure and function.
Methods
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective, multi-center cohort study whose methods and objectives have been previously detailed.21 In brief, 6,814 men and women aged 45 to 84 years without clinical cardiovascular disease (including stroke, myocardial infarction, heart failure, AF, or coronary heart disease) were enrolled between 2000 and 2002 in 6 US communities (Forsyth County, North Carolina; Northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; Los Angeles, CA). 5,098 participants underwent CMR imaging and 5,004 had scans interpretable for LV structure and function. The MESA-Right Ventricle (MESA-RV) Study was an ancillary study which selected 4,634 of the available 5,004 scans (without regard to age, sex, or race) and attempted to read 4,484 of which 4,204 were available and interpretable for RV morphology (Figure 1).7 For this study, participants were excluded if baseline characteristics including electrocardiographic (ECG) data were missing. Given the established relationship between LV systolic dysfunction and AF, participants with baseline CMR LVEF <50% were additionally excluded.
Figure 1.
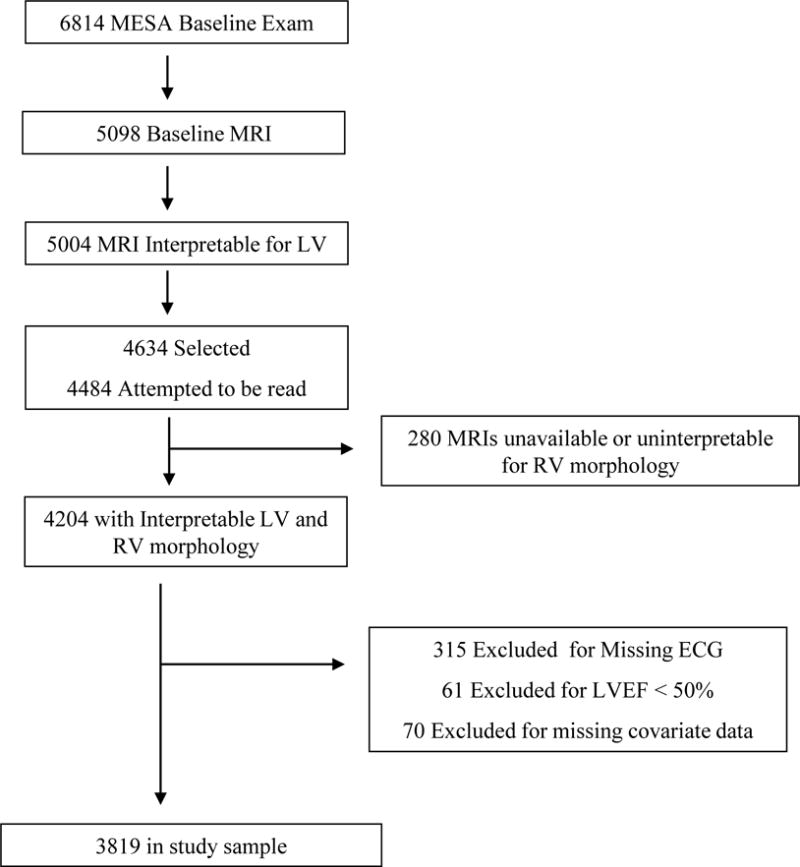
Study Sample
The protocols of MESA were approved by the Institutional Review Boards of each collaborating institution and the National Heart, Lung, and Blood Institute (NHLBI). Participants provided written informed consent.
Cardiac MRI (CMR) Measures
The CMR protocol and interpretation including RV assessment have been previously described in detail.22, 23 In brief, CMR was performed using a 1.5-Tesla system with ECG gating and fast gradient echo cine images with temporal resolution of ≤50 milliseconds. CMR interpretation was performed at a core laboratory as previously described.7 RV assessment included manual trace of endocardial and epicardial borders of short-axis cine images at end-systole and end-diastole. Contours were modified at basal slices using the tricuspid valve to exclude the right atrium and thus avoid overestimation of RV volume assessment. RV volumes (end-diastolic, RVEDV; end-systolic, RVESV) were calculated using Simpson’s rule. RV volumes included the outflow tract, papillary muscles, and trabeculae. RV mass was determined at end-diastole as the difference between end-diastolic epicardial and endocardial volumes multiplied by the specific gravity of the myocardium (1.05 g/mL).23 The septum, papillary muscles and trabeculae were not included in the measurement of RV mass as previously described.7, 23 The original MESA CMR protocol did not measure left atrium (LA) size; however, LA volume was measured in a subset of participants with AF and interpretable CMR images along with a 1:1 matched (age, sex, and race) population as previously reported.14
Intra- and inter-reader intra-class correlation coefficients for RV mass, volume, and function have been previously reported in MESA-RV using blinded re-reads of CMR scans.7 Intra-reader correlation coefficients were 0.94 for RV mass, 0.99 for RVEDV, and 0.89 for RVEF. Inter-reader correlation coefficients were 0.89 for RV mass, 0.96 for RV EDV, and 0.80 for RVEF.
Covariates
Race/ethnicity was self-reported using 2000 US Census criteria for race (white, black, Chinese) and ethnicity (Hispanic or non-Hispanic). Participants self-identifying as Hispanic were categorized as Hispanic. Body mass index (BMI), self-reported intentional exercise, education level, hypertension, smoking status (never, former, or current), smoking pack years, diabetes mellitus, and medication use (angiotensin-converting enyzme inhibitor [ACEi], angiotensin receptor blocker [ARB], beta-blocker, anti-arrhythmic medications) were assessed using standardized methods.24 Spirometry was performed in a subset of participants in the MESA Lung ancillary study, as previously described.12
Endpoint Ascertainment
AF was determined using MESA-ascertained hospital-discharge International Classification of Disease (ICD) codes, MESA study ECG, or Medicare inpatient claims data.14 Per study protocol, participants were contacted by study coordinators at intervals of 9–12 months to solicit updated hospitalizations, outpatient diagnoses and procedures, and deaths.7, 21 Trained personnel abstracted all relevant hospital records, including ECGs, clinical history and procedures and transmitted these data to the coordinating center. Medicare claims data were used to identify AF diagnoses for inpatient encounters for participants ≥65 years enrolled in fee-for-service Medicare. ICD-9 codes for atrial fibrillation (427.31) and atrial flutter (427.32) were used. Incident AF was ascertained through December 31, 2011. For patients who did not have incident AF, time was set to (a) date of death (if patient died), (b) date of last MESA follow-up if prior to 12/31/2011, or (c) the end of the ascertainment window if follow-up was after 2011.
Statistical Analysis
Continuous data are presented as mean ± standard deviation. Categorical data are presented as frequency. Univariable and multivariable Cox proportional hazards models were used to examine the associations between RV morphologic measures and time to AF. The proportional hazards assumption was tested for all models using Schoenfeld residuals. We estimated univariate associations for RV mass (RVEDM), RV volumes (RVESV, RVEDV), and RV function (RVEF) per standard deviation increase in each measure. We then adjusted for age, sex, race/ethnicity, BMI, hypertension, diabetes, medication use (ACEi, ARB, beta-blocker, anti-arrhythmic), smoking (status, pack years), and education level. In a subset with available spirometry, adjustment was additionally performed for forced expiratory volume in 1 sec (FEV1). Anthropometrics, diabetes status, hypertension, smoking status, and medication use were assessed as time-varying covariates. Given the established association between CMR-derived LVH and incident AF in MESA,14 models for RV volumes (RVESV, RVEDV) and RV function (RVEF) were also initially adjusted for CMR-LVH (LV mass >95th percentile of healthy MESA population, as defined previously).14 Next, given the established relationship between RV morphology and incident heart failure,7 and the association of heart failure and atrial fibrillation,25 we further adjusted multivariable models for incident heart failure prior to or concurrent with AF (heart failure ascertainment in MESA previously described in detail).26 To more closely assess the relationship between the RV morphology and AF risk, we finally adjusted models for the respective LV parameter (eg. for RVEDM, adjustment for LVEDM). Finally, in a nested case:control subpopulation of MESA-RV participants with LA volume measures (138 pairs matched on age, sex, ethnicity), we examined the association between RV measures and incident AF. We then utilized a conditional shared frailty Cox proportional hazards model with incident AF as the outcome and RV measures as the independent variable. Multivariable adjustment include matching factors, to account for residual confounding, as well as anthropomorphic measures, medication use, clinical characteristics (smoking, diabetes mellitus, incident HF) and CMR-defined LVH as specified above, followed by the addition of LA volume. Freedom from AF stratified by the median value of significant RV measures (i.e. RVEDM, RVEF) was estimated using Cox proportional hazards models with multivariable-adjustment as specified above. SAS (version 9.3; SAS Institute, Cary, NC) and R (version 3.2.1; R Project of Statistical Computing) were used for all analyses. Values of P<0.05 were considered statistically significant.
Results
Baseline Characteristics
The total number of MESA participants with CMR assessment, LVEF ≥50% and complete baseline covariate and ECG assessment was 3,819 (Figure 1). The mean age of the study sample was 61±10 years and 46.5% were male (Table 1). The mean body-mass index was 27.9±5 kg/m2 and 47.2% were former/current smokers. The prevalence of hypertension was 42.3%, and 11.3% of participants had diabetes mellitus. Baseline CMR-derived biventricular structure and function measures are shown in Table 1. Spirometry was assessed in a subset of the study population under another ancillary study (n=2,540) and the mean FEV1 was 2413±732 mL. Compared to patients excluded, study participants were slightly younger and less likely to have hypertension or diabetes mellitus (Supplementary Table 1).
Table 1.
Baseline Characteristics of the Study Population
| Baseline Characteristic | Study Sample (N=3,819) |
|---|---|
|
| |
| Demographic and Clinical Variables | |
|
| |
| Age, year | 61±10 |
| Male gender, n (%) | 1,774 (47) |
| Race/ethnicity, n (%) | |
| Caucasian | 1,485 (39) |
| African American | 976 (26) |
| Hispanic | 859 (23) |
| Chinese | 499 (13) |
| Education level, n (%) | |
| Less than high school | 636 (16) |
| High school/GED | 690 (18) |
| Less than college (more than high school) | 1,100 (29) |
| Bachelor degree | 685 (18) |
| Graduate degree | 708 (19) |
| Body mass index, kg/m2 | 27.9±5.0 |
| Smoking status, n (%) | |
| Never | 2,017 (53) |
| Former | 1,343 (35) |
| Current | 459 (12) |
| Smoking pack-years | 10.7±23.0 |
| Forced expiratory volume in 1 second, ml* | 2413±732 |
| Diabetes mellitus, n (%) | 432 (11) |
| Hypertension, n (%) | 1,616 (42) |
| Medications, n (%) | |
| RAAS inhibitor | 632 (17) |
| β-blocker | 352 (9) |
| Anti-arrhythmic | 21 (<1) |
|
| |
| Cardiac Structure and Function | |
|
| |
| Right Ventricle | |
| End-diastolic mass, grams | 21±5 |
| End-diastolic volume, mL | 124±31 |
| End-systolic volume, mL | 37±14 |
| Ejection Fraction, % | 71±6 |
| Left Ventricle | |
| End-diastolic mass, grams | 144±38 |
| End-diastolic volume, mL | 126±31 |
| End-systolic volume, mL | 39±15 |
| Ejection Fraction, % | 70±7 |
Assessed in a subset of the study population (n=2,540). GED, general education development test; RAAS, renin angiotensin aldosterone system
RV Morphology and Incident Atrial Fibrillation
During the study period, there were 308 incident AF events during a median follow-up of 10.2 years [IQR: 9.6 – 10.7]. In unadjusted analysis, greater RVEF and lower RVESV were associated with incident AF (p<0.004 for both) (Table 2). In multivariable models with time-varying adjustment of clinical and lifestyle measures, both higher RVEF (hazard ratio [HR]: 1.21 per standard deviation [SD], 95% CI: 1.08–1.35, p=0.02) and greater RV mass (HR: 1.21 per SD, 95% CI: 1.04–1.40, p=0.002) were significantly associated with incident AF. In the subset of participants with baseline spirometry, there were significant associations between higher RVEF, RVEDM, and RVEDV and incident AF after additional adjustment for FEV1 (p<0.02 for all, Supplementary Table 2).
Table 2.
Proportional Hazards Models for Right Ventricular Structure and Function and Incident Atrial Fibrillation
| HR | 95% CI | P | |
|---|---|---|---|
|
| |||
| RV EF | |||
|
| |||
| Unadjusted | 1.21 | 1.08 – 1.35 | 0.001 |
| Model 1* | 1.16 | 1.03 – 1.32 | 0.02 |
| Model 2† | 1.16 | 1.03 – 1.32 | 0.02 |
| Model 2 + LV EF | 1.15 | 1.01 – 1.32 | 0.04 |
|
| |||
| RV EDM | |||
|
| |||
| Unadjusted | 0.93 | 0.83 – 1.05 | 0.23 |
| Model 1‡ | 1.25 | 1.09 – 1.44 | 0.002 |
| Model 2† | 1.25 | 1.08 – 1.44 | 0.002 |
| Model 2 + LV EDM | 1.16 | 0.99 – 1.35 | 0.07 |
|
| |||
| RV EDV | |||
|
| |||
| Unadjusted | 0.92 | 0.82 – 1.03 | 0.15 |
| Model 1* | 1.10 | 0.94 – 1.28 | 0.23 |
| Model 2† | 1.11 | 0.95 – 1.29 | 0.18 |
| Model 2 + LV EDV | 1.00 | 0.82 – 1.21 | 0.97 |
|
| |||
| RV ESV | |||
|
| |||
| Unadjusted | 0.84 | 0.75 – 0.94 | 0.004 |
| Model 1* | 0.91 | 0.79 – 1.06 | 0.24 |
| Model 2† | 0.92 | 0.79 – 1.07 | 0.28 |
| Model 2 + LV ESV | 0.89 | 0.76 – 1.05 | 0.17 |
Hazard ratios are per standard deviation (STDEV) increase in parameter (STDEV RV EF: 6.3%; RV EDM: 4.5 grams; RV EDV: 31.0 ml; RV ESV: 15.0 ml).
Model 1 includes adjustment for age, sex, race, body mass index, hypertension, diabetes, medication use (RAAS, β-blocker, anti-arrhythmic), smoking (status, pack-years), education level, and left ventricular hypertrophy.
Model 2 includes adjustment for prevalent or concurrent heart failure at time of AF diagnosis in addition to Model 1 covariates.
Model 1 for RVEDM does not include adjustment for left ventricular hypertrophy; adjustment for LV mass (LVEDM) is performed subsequently. HR, hazard ratio; CI, confidence interval; RV, right ventricular; LV, left ventricular; EF, ejection fraction; EDM, end-diastolic mass; EDV, end-diastolic volume; ESV, end-systolic volume.
To evaluate whether the association between RV measures and incident AF was mediated by intercurrent heart failure, multivariable models were further adjusted for prevalent heart failure at the time of AF diagnosis. Greater RVEF and RV mass remained associated with incident AF in heart failure-adjusted models (p ≤0.02 for both, Table 2). To further isolate the relationship between RV morphology and incident AF, associations were further adjusted for the respective LV parameter. After adjusting for LVEF, higher RVEF remained significantly associated with incident AF (HR: 1.15 per SD, 95% CI: 1.01–1.32, p=0.04), whereas the association between greater RV mass and incident AF was slightly weakened after adjustment for LV mass (HR: 1.16 per SD, 95% CI: 0.99–1.35, p=0.07). In participants with baseline spirometry, both greater RVEF (adjusted HR: 1.23 per SD, 95% CI: 1.03–1.46; p=0.02) and RV mass (adjusted HR: 1.26 per SD, 95% CI: 1.04–1.54; p=0.02) remained associated with incident AF after adjustment for demographic and clinical factors, FEV1, and respective LV parameter (Supplementary Table 2). Finally, in a nested case:control subpopulation of 276 patients (138 with incident AF, 138 controls matched on age, sex, ethnicity) with LA volume measurement, increasing RV EF was significantly associated with incident AF in multivariable-adjusted models (HR: 1.38 per SD [95% CI: 1.14–1.66, p<0.001) and remained significantly associated following additional adjustment for LA volume (HR 1.27 per SD [95% CI: 1.06–1.53], p=0.011) (Supplementary Table 3).
When stratified by median value, participants with RVEDM and RVEF above the median had a significantly greater incidence of AF over the study duration even after adjustment for demographic and clinical factors (Figure 2). In the dichotomized assessment of RV morphology, RVEDM above the median remained significantly associated with AF after further adjustment for LVEDM (p=0.03) whereas the association between RVEF and AF was attenuated after LVEF adjustment (p=0.07) (Figure 2).
Figure 2.
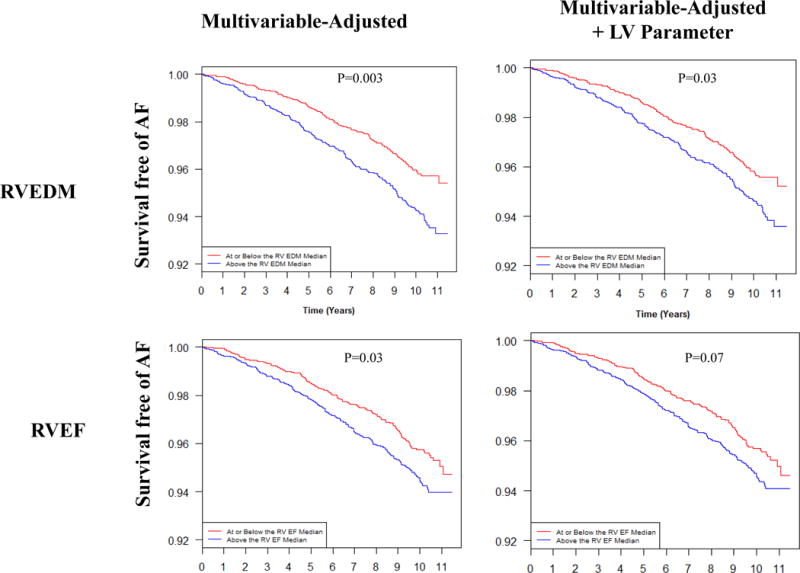
Survival Free of Atrial Fibrillation Stratified by Median Right Ventricular Mass and Function. Shown is estimated survival free of AF for study participants stratified by median RVEDM (top) and RVEF (bottom). Multivariable adjustment was for age, sex, race, body mass index, hypertension, diabetes, medication use (RAAS, β-blocker, anti-arrhythmic), smoking (status, pack-years), education level, and left ventricular hypertrophy. Adjustment was then additionally performed for respective LV parameter (i.e. LVEF for RVEF model; LVEDM for RVEDM model). Multivariable model for RVEDM does not include adjustment for left ventricular hypertrophy as adjustment for LV mass (i.e. LVEDM) is performed in the LV parameter-adjusted model. RV, right ventricular; LV, left ventricular; EF, ejection fraction; EDM, end-diastolic mass.
Discussion
In this study, we examined the association between RV morphology and risk of incident AF in a multi-ethnic cohort free of cardiovascular disease at baseline. We demonstrate that higher RVEF and greater RV mass are associated with incident AF even after accounting for baseline lung function, incident heart failure, and parameters of LV structure and function. These data raise the possibility of RV morphology as a structural biomarker of AF risk.
The prognostic role of RV structure and function in cardiopulmonary disease is well-established.7, 9, 27, 28 In patients free of cardiovascular disease at baseline, greater RV mass is associated with an increased risk of heart failure and cardiovascular mortality.7 In MESA participants, abnormal RV morphology has been linked to subclinical LV dysfunction,13 conditions associated with abnormal pulmonary vascular function (obesity, emphysema),17, 29 as well as biomarkers of inflammation and neurohormonal activation.19, 20, 30 In turn, each of these features – pulmonary pathology, left heart function, systemic inflammation and neurohormonal activation – has been implicated in the pathogenesis of AF.31, 32 Nevertheless, there has been no assessment of the relationship between RV morphology and incident AF in patients free of cardiovascular disease at baseline.
In this study we identified significant associations between greater RVEF and mass with incident AF. There are several possible explanations for these associations. First, RV morphology may serve as a structural surrogate for systemic processes implicated in AF pathogenesis including inflammation, neurohormonal activation, and sex hormone excess. For example, systemic markers of inflammation, including elevated levels of CRP and IL-6, have been associated with atrial electrical remodeling and increased risk of AF in healthy community cohorts.33–36 In MESA, the relationship between RV morphology and markers of inflammation has proven complex, although extreme levels of CRP and IL-6 were positively associated with RV mass.19 Likewise, greater RV mass has been associated with neurohormonal activation,20 which has been implicated as a risk factor for AF via its influence on myocardial fibrosis, atrial stretch, and modulation of ionic channel function.36, 37,38, 39 Finally, in a subset of the MESA cohort, higher RVEF was associated with higher levels of estradiol, postulated to reflect myocardial effects of estrogen receptor activation.39 Elevated estradiol has been associated with an increased risk of AF in healthy community cohorts,38 and increasing RVEF may reflect a structural signature of sex hormone levels.
Second, individuals with established risk factors for AF (eg. obesity, sleep disordered breathing) harbor significantly greater RV mass even after adjustment for LV mass.17 Sleep disordered breathing has been associated with both LV and RV hypertrophy40 and independently predicts incident AF.18, 41 Nevertheless, in our study, the association between RVEF and RV mass with AF persisted after adjustment for multiple measures reflective of pulmonary vascular function and RV afterload including body mass index, smoking status, hypertension, LVH, and FEV1 suggesting a potential unique importance for RV structure and function in AF pathogenesis independent of comorbidities. For example, a higher RV mass – and consequently higher RV end-diastolic pressure or increased RV elastance – may lead to the same deleterious consequences on atrial mechanics as higher LV mass (i.e. atrial stretch, neurohormonal activation, atrial fibrosis).14 Whether early changes in RV morphology (RVEF, RVEDM) serve as a more sensitive barometer of systemic drivers of AF (eg. inflammation, neurohormonal activation)19, 20 that precede atrial remodeling may warrant future investigation.
Clinical Implications
The RV is a unique morphological structure – sensitive to changes in the pulmonary and left heart circulation, with unique myofibril geometry, distinct developmental origins, and incompletely understood modes of remodeling.8, 42 As such, it represents a novel and potentially integrative measure of systemic processes, hemodynamic perturbation, and local myocardial changes (Figure 3).8, 42 Historically, the prognostic role of RV structure and function has been limited to patients with established cardiopulmonary disease, where a lower RVEF and more dilated RV is associated with morbidity and mortality.27, 28, 43, 44 In this study, we extend the prognostic relevance of the RV to individuals without prevalent cardiovascular disease, identifying a different phenotype (i.e. greater RV ejection fraction and mass) associated with AF risk. We would highlight that a similar phenotype has been associated with cardio-pulmonary pathology in the MESA population. For example, greater RV mass has been associated with increased heart failure and cardiovascular mortality,7 and smaller RV volumes have been associated with worsening lung function.11 To that end, this structural phenotype (i.e. increased RVEF, increased RV mass) may represent early, compensatory remodeling of the RV – a process which to date remains incompletely understood.42 Indeed, while similar compensatory remodeling has been well-described for the LV,45, 46 our findings suggest that a similar intermediate phenotype for the RV may have prognostic implications.
Figure 3.
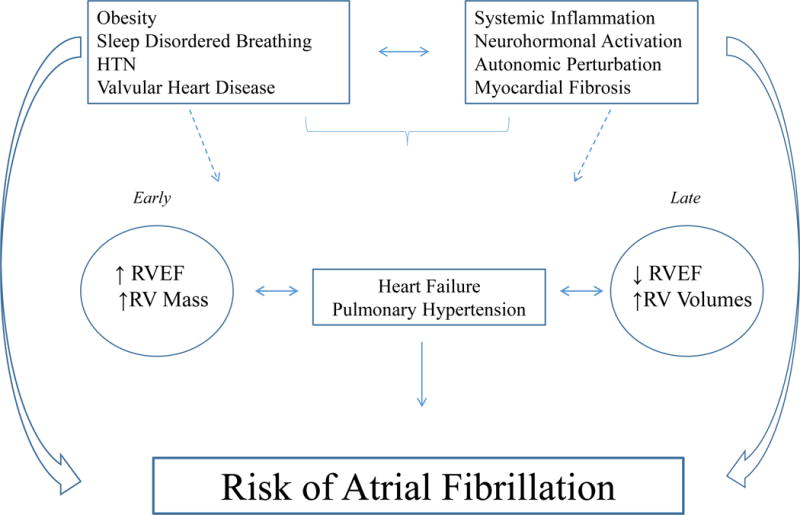
Schematic representation of the possible relationships between RV morphology and AF pathogenesis. Shown is a schematic reflecting the possible relationships between clinical phenotypes, systemic processes, and temporal changes in ‘at-risk’ RV morphology (early vs. late) in the context of AF pathogenesis. RV, right ventricle; EF, ejection fraction; HTN, hypertension, PH, pulmonary hypertension.
An improved understanding of the influence of systemic processes (inflammation, neurohormonal activation) and cardiopulmonary changes (pulmonary vascular function, LV morphology) on RV morphology may help refine our understanding of RV adaptation and its related impact on AF pathogenesis. Morphological and structural features of the left heart – including fibrosis and ventricular strain – have been associated with incident AF in healthy populations, as well as clinical outcomes following AF ablation (i.e. recurrence, recovery of LV function).47–49 Whether similar changes in RV morphology and function influence clinical outcomes in AF may warrant future exploration. Given its suggested integrative role, RV morphology may serve as a potent marker of incident cardiovascular risk, although its practical use remains untested.
Limitations
Our study has several strengths including its prospective design, large sample size of multi-ethnic participants, comprehensive evaluation of CMR-based cardiac structural measures, time-varying adjustment of clinical risk factors, and blinded adjudication of endpoints. There are some additional limitations. First, not all MESA participants tolerated CMR examination and not all CMR scans were interpretable. There were minor differences in age, sex, medication use, and smoking history in those included compared to those excluded (Supplementary Table 1), which may influence generalizability. Second, assessments of left atrium volumes were not included in the original MESA protocol and were only available for a subpopulation of study participants in which there was limited power to assess RV-AF associations. Within this subpopulation, we demonstrate that RV EF retained significant association with incident AF, even after adjustment for LA volumes. Whether the identified association between RV mass and incident AF is confounded by LA volume could not be assessed in this study, although we would note that the association persisted even with adjustment for LVH, a significant structural driver of LA enlargement.14 Third, RVEF is optimally assessed after accounting for tricuspid regurgitation which was not assessed in MESA although we would note that participants with a history of valve replacement or significant valvular heart disease at baseline were excluded from enrollment. While we cannot exclude the possibility of interval valvular heart disease after study enrollment, we do not believe the association between baseline RV measures and incident AF was meaningfully influenced by right or left sided heart disease in this healthy population. Participants with significant valvular heart disease were excluded from the MESA trial. Fourth, given the methodology of AF ascertainment, we were unable to detect asymptomatic AF or AF not associated with hospitalization. In addition, AF subtype was not assessed and may be differentially associated with abnormal RV morphology. Finally, given the design of the MESA study, we were unable to account for temporal changes in RV and LV function during study follow-up although all models included time-varying clinical covariates which could have influenced cardiac structure during the study follow-up period.
Conclusion
In conclusion, greater RV ejection fraction and mass were associated with an increased risk of AF in a multi-ethnic population free of clinical cardiovascular disease at baseline. Additional investigation of the relationship between RV morphology and AF may help to refine our understanding of RV morphological adaptation, its potential role as a structural biomarker of AF risk, and ultimately an improved understanding of AF pathogenesis.
Supplementary Material
WHAT IS KNOWN
Right ventricular (RV) structure and function provide prognostic information for patients with established cardiovascular disease.
RV morphology has been associated with known drivers of atrial fibrillation (AF) risk including systemic inflammation, neurohormonal activation, and pulmonary pathology.
The relationship between RV morphology and incident AF risk is uncertain.
WHAT THE STUDY ADDS
In 4204 individuals free of cardiovascular disease at baseline, higher RV ejection fraction and greater RV mass were associated with incident AF even after accounting for lung function, incident heart failure, and measures of left heart structure and function.
RV morphology may serve as an integrative barometer of AF risk, independent of left heart function. The clinical utility of RV morphology assessment in AF risk prediction and its potential influence on outcomes in AF warrants further investigation.
Acknowledgments
The authors would like to thank Drs. Jonathan Chrispin, Joao A.C. Lima, and Samian Nazarian for the assistance regarding left atrial volume measurements. In addition, the authors would like to thank the other investigators, staff, and participants of the MESA studies for their valuable contributions. This manuscript was reviewed by the MESA investigators for scientific content and consistency of data interpretation with previous MESA publications and significant comments were incorporated before submission for publication. A full list of participating MESA Investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding: Dr. Chatterjee was supported by NHLBI T-32 HL-007575 which does not represent a relevant disclosure but does support time for writing. This study was funded by National Institutes of Health R01-HL086719, R01-HL077612, K24-HL103844, and N01-HC95159 through N01-HC95169.
Footnotes
Journal Subject Terms: atrial fibrillation, epidemiology, magnetic resonance imaging
Disclosures: None
References
- 1.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menke J, Luthje L, Kastrup A, Larsen J. Thromboembolism in atrial fibrillation. Am J Cardiol. 2010;105:502–510. doi: 10.1016/j.amjcard.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 4.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Wagoner DR, Piccini JP, Albert CM, Anderson ME, Benjamin EJ, Brundel B, Califf RM, Calkins H, Chen PS, Chiamvimonvat N, Darbar D, Eckhardt LL, Ellinor PT, Exner DV, Fogel RI, Gillis AM, Healey J, Hohnloser SH, Kamel H, Lathrop DA, Lip GY, Mehra R, Narayan SM, Olgin J, Packer D, Peters NS, Roden DM, Ross HM, Sheldon R, Wehrens XH. Progress toward the prevention and treatment of atrial fibrillation: A summary of the Heart Rhythm Society Research Forum on the Treatment and Prevention of Atrial Fibrillation, Washington, DC, December 9–10, 2013. Heart Rhythm. 2015;12:e5–e29. doi: 10.1016/j.hrthm.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC, Heckbert SR. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25:71–76. doi: 10.1016/j.annepidem.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawut SM, Barr RG, Lima JA, Praestgaard A, Johnson WC, Chahal H, Ogunyankin KO, Bristow MR, Kizer JR, Tandri H, Bluemke DA. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi-Ethnic Study of Atherosclerosis (MESA)–right ventricle study. Circulation. 2012;126:1681–1688. doi: 10.1161/CIRCULATIONAHA.112.095216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120:992–1007. doi: 10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee NA, Steiner J, Lewis GD. It is time to look at heart failure with preserved ejection fraction from the right side. Circulation. 2014;130:2272–2277. doi: 10.1161/CIRCULATIONAHA.114.013536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, White M, Aban IB, Mujib M, Dell’Italia LJ, Ahmed A. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121:252–258. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawut SM, Poor HD, Parikh MA, Hueper K, Smith BM, Bluemke DA, Lima JA, Prince MR, Hoffman EA, Austin JH, Vogel-Claussen J, Barr RG. Cor pulmonale parvus in chronic obstructive pulmonary disease and emphysema: the MESA COPD study. J Am Coll Cardiol. 2014;64:2000–2009. doi: 10.1016/j.jacc.2014.07.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chahal H, Heckbert SR, Barr RG, Bluemke DA, Jain A, Habibi M, Alonso A, Kronmal R, Jacobs DR, Jr, Lima JA, Watson KE, Liu K, Smith LJ, Greenland P. Ability of Reduced Lung Function to Predict Development of Atrial Fibrillation in Persons Aged 45 to 84 Years (from the Multi-Ethnic Study of Atherosclerosis-Lung Study) Am J Cardiol. 2015;115:1700–1704. doi: 10.1016/j.amjcard.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dibble CT, Lima JA, Bluemke DA, Chirinos JA, Chahal H, Bristow MR, Kronmal RA, Barr RG, Ferrari VA, Propert KJ, Kawut SM. Regional left ventricular systolic function and the right ventricle: the multi-ethnic study of atherosclerosis right ventricle study. Chest. 2011;140:310–316. doi: 10.1378/chest.10-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chrispin J, Jain A, Soliman EZ, Guallar E, Alonso A, Heckbert SR, Bluemke DA, Lima JA, Nazarian S. Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014;63:2007–2013. doi: 10.1016/j.jacc.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, Roman MJ, Devereux RB. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS) Am Heart J. 2006;151:412–418. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 16.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 17.Chahal H, McClelland RL, Tandri H, Jain A, Turkbey EB, Hundley WG, Barr RG, Kizer J, Lima JA, Bluemke DA, Kawut SM. Obesity and right ventricular structure and function: the MESA-Right Ventricle Study. Chest. 2012;141:388–395. doi: 10.1378/chest.11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin GM, Colangelo LA, Lloyd-Jones DM, Redline S, Yeboah J, Heckbert SR, Nazarian S, Alonso A, Bluemke DA, Punjabi NM, Szklo M, Liu K. Association of Sleep Apnea and Snoring With Incident Atrial Fibrillation in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2015;182:49–57. doi: 10.1093/aje/kwv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harhay MO, Tracy RP, Bagiella E, Barr RG, Pinder D, Hundley WG, Bluemke DA, Kronmal RA, Lima JA, Kawut SM. Relationship of CRP, IL-6, and fibrinogen with right ventricular structure and function: the MESA-Right Ventricle Study. Int J Cardiol. 2013;168:3818–3824. doi: 10.1016/j.ijcard.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ventetuolo CE, Lima JA, Barr RG, Bristow MR, Bagiella E, Chahal H, Kizer JR, Lederer DJ, Bluemke DA, Kawut SM. The renin-angiotensin system and right ventricular structure and function: The MESA-Right Ventricle Study. Pulm Circ. 2012;2:379–386. doi: 10.4103/2045-8932.101657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 22.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. Am J Roent. 2006;186:S357–365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 23.Tandri H, Daya SK, Nasir K, Bomma C, Lima JA, Calkins H, Bluemke DA. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol. 2006;98:1660–1664. doi: 10.1016/j.amjcard.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 24.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45:683–692. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 25.Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J. 2015;36:3250–3257. doi: 10.1093/eurheartj/ehv513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chahal H, Bluemke DA, Wu CO, McClelland R, Liu K, Shea SJ, Burke G, Balfour P, Herrington D, Shi P, Post W, Olson J, Watson KE, Folsom AR, Lima JA. Heart failure risk prediction in the Multi-Ethnic Study of Atherosclerosis. Heart. 2015;101:58–64. doi: 10.1136/heartjnl-2014-305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourantas CV, Loh HP, Bragadeesh T, Rigby AS, Lukaschuk EI, Garg S, Tweddel AC, Alamgir FM, Nikitin NP, Clark AL, Cleland JG. Relationship between right ventricular volumes measured by cardiac magnetic resonance imaging and prognosis in patients with chronic heart failure. Eur J Heart Fail. 2011;13:52–60. doi: 10.1093/eurjhf/hfq161. [DOI] [PubMed] [Google Scholar]
- 28.Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grau M, Barr RG, Lima JA, Hoffman EA, Bluemke DA, Carr JJ, Chahal H, Enright PL, Jain A, Prince MR, Kawut SM. Percent emphysema and right ventricular structure and function: the Multi-Ethnic Study of Atherosclerosis-Lung and Multi-Ethnic Study of Atherosclerosis-Right Ventricle Studies. Chest. 2013;144:136–144. doi: 10.1378/chest.12-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawut SM, Barr RG, Johnson WC, Chahal H, Tandri H, Jain A, Bristow MR, Kizer JR, Bagiella E, Lima JA, Bluemke DA. Matrix metalloproteinase-9 and plasminogen activator inhibitor-1 are associated with right ventricular structure and function: the MESA-RV Study. Biomarkers. 2010;15:731–738. doi: 10.3109/1354750X.2010.516455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac Fibrosis in Patients With Atrial Fibrillation: Mechanisms and Clinical Implications. J Am Coll Cardiol. 2015;66:943–959. doi: 10.1016/j.jacc.2015.06.1313. [DOI] [PubMed] [Google Scholar]
- 32.Nishida K, Datino T, Macle L, Nattel S. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J Am Coll Cardiol. 2014;64:823–831. doi: 10.1016/j.jacc.2014.06.1172. [DOI] [PubMed] [Google Scholar]
- 33.Mitrokhin VM, Mladenov MI, Kamkin AG. Effects of interleukin-6 on the bio-electric activity of rat atrial tissue under normal conditions and during gradual stretching. Immunobiology. 2015;220:1107–1112. doi: 10.1016/j.imbio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Polovina MM, Ostojic MC, Potpara TS. Relation of Biomarkers of Inflammation and Oxidative Stress with Hypertension Occurrence in Lone Atrial Fibrillation. Mediators Inflamm. 2015;2015:653026. doi: 10.1155/2015/653026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 36.Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Vasan RS, Wang TJ, Agarwal SK, McManus DD, Franco OH, Yin X, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Astor BC, Ballantyne CM, Hoogeveen RC, Arai AE, Soliman EZ, Ellinor PT, Stricker BH, Gudnason V, Heckbert SR, Pencina MJ, Benjamin EJ, Alonso A. B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: the CHARGE-AF Consortium of community-based cohort studies. Europace. 2014;16:1426–1433. doi: 10.1093/europace/euu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrell NW, Morris KG, Stenmark KR. Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol. 1995;269:H1186–1194. doi: 10.1152/ajpheart.1995.269.4.H1186. [DOI] [PubMed] [Google Scholar]
- 38.Magnani JW, Moser CB, Murabito JM, Sullivan LM, Wang N, Ellinor PT, Vasan RS, Benjamin EJ, Coviello AD. Association of sex hormones, aging, and atrial fibrillation in men: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2014;7:307–312. doi: 10.1161/CIRCEP.113.001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, Kizer JR, Lima JA, Kawut SM. Sex hormones are associated with right ventricular structure and function: The MESA-right ventricle study. Am J Respir Crit Care Med. 2011;183:659–667. doi: 10.1164/rccm.201007-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noda A, Okada T, Yasuma F, Nakashima N, Yokota M. Cardiac hypertrophy in obstructive sleep apnea syndrome. Chest. 1995;107:1538–1544. doi: 10.1378/chest.107.6.1538. [DOI] [PubMed] [Google Scholar]
- 41.Kwon Y, Gharib SA, Biggs ML, Jacobs DR, Jr, Alonso A, Duprez D, Lima J, Lin GM, Soliman EZ, Mehra R, Redline S, Heckbert SR. Association of sleep characteristics with atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Thorax. 2015;70:873–879. doi: 10.1136/thoraxjnl-2014-206655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62:D22–33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Lewis GD, Murphy RM, Shah RV, Pappagianopoulos PP, Malhotra R, Bloch KD, Systrom DM, Semigran MJ. Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ Heart Fail. 2011;4:276–285. doi: 10.1161/CIRCHEARTFAILURE.110.959437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE, Vonk-Noordegraaf A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 45.Bajpai JK, A PS, A KA, A KD, Garg B, Goel A. Impact of prehypertension on left ventricular structure, function and geometry. J Clin Diagn Res. 2014;8:7–10. doi: 10.7860/JCDR/2014/8023.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Pei F, Shao L, Chen J, Sun K, Zhang X, Zhang C, Liu J, Xiao C, Hui R. Prevalence and risk factors of abnormal left ventricular geometrical patterns in untreated hypertensive patients. BMC Cardiovasc Disord. 2014;14:136–142. doi: 10.1186/1471-2261-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Addison D, Farhad H, Shah RV, Mayrhofer T, Abbasi SA, John RM, Michaud GF, Jerosch-Herold M, Hoffmann U, Stevenson WG, Kwong RY, Neilan TG. Effect of Late Gadolinium Enhancement on the Recovery of Left Ventricular Systolic Function After Pulmonary Vein Isolation. J Am Heart Assoc. 2016;5:e003570. doi: 10.1161/JAHA.116.003570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wijesurendra RS, Liu A, Eichhorn C, Ariga R, Levelt E, Clarke WT, Rodgers CT, Karamitsos TD, Bashir Y, Ginks M, Rajappan K, Betts T, Ferreira VM, Neubauer S, Casadei B. “Lone” Atrial Fibrillation Is Associated With Impaired Left Ventricular Energetics That Persist Despite Successful Catheter Ablation. Circulation. 2016;134:1068–1081. doi: 10.1161/CIRCULATIONAHA.116.022931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tops LF, Den Uijl DW, Delgado V, Marsan NA, Zeppenfeld K, Holman E, van der Wall EE, Schalij MJ, Bax JJ. Long-term improvement in left ventricular strain after successful catheter ablation for atrial fibrillation in patients with preserved left ventricular systolic function. Circ Arrhythm Electrophysiol. 2009;2:249–257. doi: 10.1161/CIRCEP.108.838748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


