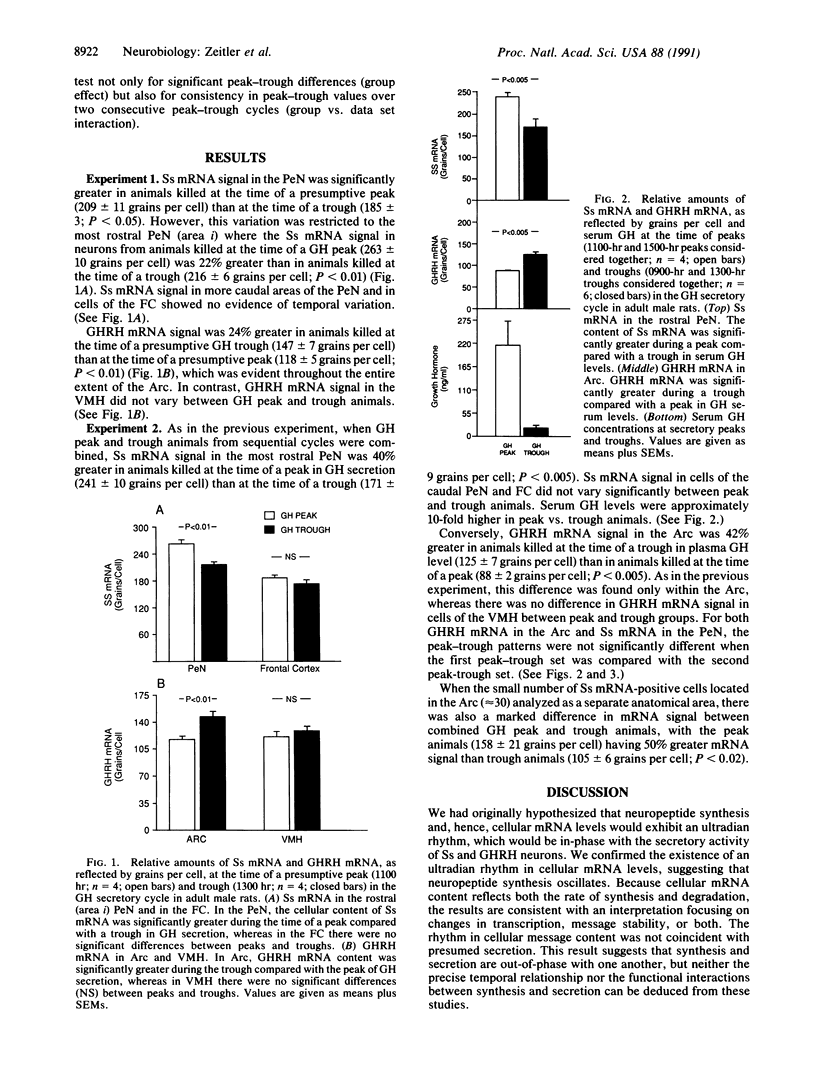
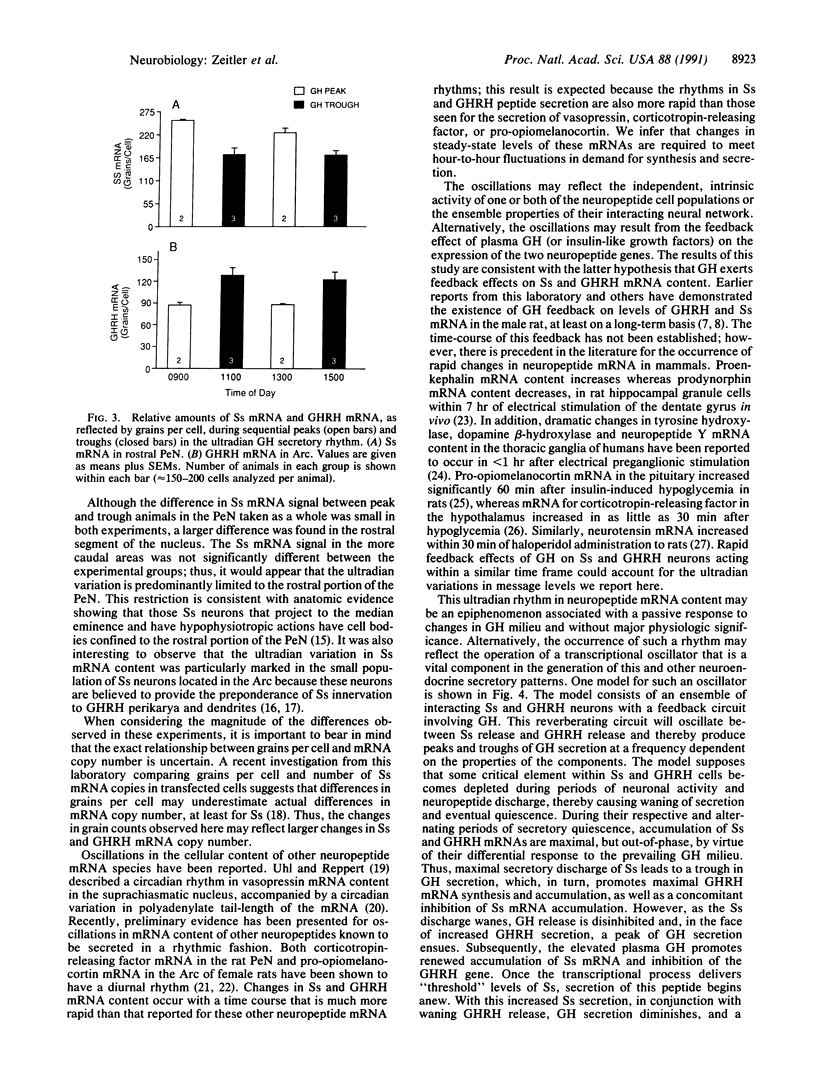
Abstract
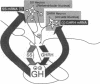
In the adult male rat, growth hormone (GH) secretion is characterized by an ultradian rhythm generated by the rhythmic interplay of the stimulatory effects of GH-releasing hormone (GHRH) and the inhibitory effects of somatostatin (Ss). Although considerable evidence indicates that GHRH and Ss are secreted in reciprocal 3- to 4-hr rhythms, the mechanism underlying the rhythmic secretion of these two neuropeptides is unknown. We tested the hypothesis that the rhythmic and reciprocal oscillations in secretion of Ss and GHRH are associated with parallel changes in synthesis and that this would be reflected by coincident oscillations in levels of the respective mRNAs. In the first experiment, Ss mRNA was significantly greater in the periventricular nucleus of animals sacrificed at the time of a presumed peak in the GH rhythm than in animals sacrificed at the time of a presumed trough; this variation was limited to the anterior third of this nucleus. Conversely, GHRH mRNA content throughout the arcuate nucleus was significantly greater at the time of a GH trough. In the second experiment, groups of animals were sacrificed during two consecutive cycles. In this set of animals, Ss mRNA content was 40% greater (P less than 0.005) during peak GH concentrations, whereas GHRH mRNA content was 42% greater (P less than 0.005) during the GH trough. This difference persisted when the two cycles were analyzed separately. The findings that the cellular mRNA content for Ss and GHRH varies in a reciprocal manner with the presumed secretion of these neuropeptides suggest that, like secretion, the synthesis of Ss and GHRH also varies rhythmically. The occurrence of this rhythm suggests a model for a transcriptional oscillator that may subserve the generation of this and possibly other neuroendocrine rhythms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argente J., Chowen-Breed J. A., Steiner R. A., Clifton D. K. Somatostatin messenger RNA in hypothalamic neurons is increased by testosterone through activation of androgen receptors and not by aromatization to estradiol. Neuroendocrinology. 1990 Oct;52(4):342–349. doi: 10.1159/000125618. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Downs T. R., Frohman L. A. Feedback regulation of growth hormone (GH)-releasing hormone gene expression by GH in rat hypothalamus. Mol Endocrinol. 1988 Mar;2(3):236–241. doi: 10.1210/mend-2-3-236. [DOI] [PubMed] [Google Scholar]
- Chowen-Breed J. A., Steiner R. A., Clifton D. K. Sexual dimorphism and testosterone-dependent regulation of somatostatin gene expression in the periventricular nucleus of the rat brain. Endocrinology. 1989 Jul;125(1):357–362. doi: 10.1210/endo-125-1-357. [DOI] [PubMed] [Google Scholar]
- Daikoku S., Hisano S., Kawano H., Chikamori-Aoyama M., Kagotani Y., Zhang R. J., Chihara K. Ultrastructural evidence for neuronal regulation of growth hormone secretion. Neuroendocrinology. 1988 May;47(5):405–415. doi: 10.1159/000124955. [DOI] [PubMed] [Google Scholar]
- Horváth S., Palkovits M., Görcs T., Arimura A. Electron microscopic immunocytochemical evidence for the existence of bidirectional synaptic connections between growth hormone-releasing hormone- and somatostatin-containing neurons in the hypothalamus of the rat. Brain Res. 1989 Feb 27;481(1):8–15. doi: 10.1016/0006-8993(89)90479-4. [DOI] [PubMed] [Google Scholar]
- Kawano H., Daikoku S., Saito S. Immunohistochemical studies of intrahypothalamic somatostatin-containing neurons in rat. Brain Res. 1982 Jun 24;242(2):227–232. doi: 10.1016/0006-8993(82)90304-3. [DOI] [PubMed] [Google Scholar]
- Low M. J., Hammer R. E., Goodman R. H., Habener J. F., Palmiter R. D., Brinster R. L. Tissue-specific posttranslational processing of pre-prosomatostatin encoded by a metallothionein-somatostatin fusion gene in transgenic mice. Cell. 1985 May;41(1):211–219. doi: 10.1016/0092-8674(85)90075-3. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Cerelli G. M., Rosenfeld M. G., Evans R. M. Characterization of cDNA and genomic clones encoding the precursor to rat hypothalamic growth hormone-releasing factor. Nature. 1985 Apr 4;314(6010):464–467. doi: 10.1038/314464a0. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I., Setalo G., Csontos C., Petrusz P., Flerko B., Negro-Vilar A. Combined retrograde tracing and immunocytochemical identification of luteinizing hormone-releasing hormone- and somatostatin-containing neurons projecting to the median eminence of the rat. Endocrinology. 1989 Dec;125(6):2812–2821. doi: 10.1210/endo-125-6-2812. [DOI] [PubMed] [Google Scholar]
- Morris B. J., Feasey K. J., ten Bruggencate G., Herz A., Höllt V. Electrical stimulation in vivo increases the expression of proenkephalin mRNA and decreases the expression of prodynorphin mRNA in rat hippocampal granule cells. Proc Natl Acad Sci U S A. 1988 May;85(9):3226–3230. doi: 10.1073/pnas.85.9.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky P. M., Vale W. Patterns of growth hormone-releasing factor and somatostatin secretion into the hypophysial-portal circulation of the rat. Science. 1985 Oct 25;230(4724):461–463. doi: 10.1126/science.2864742. [DOI] [PubMed] [Google Scholar]
- Robinson B. G., Frim D. M., Schwartz W. J., Majzoub J. A. Vasopressin mRNA in the suprachiasmatic nuclei: daily regulation of polyadenylate tail length. Science. 1988 Jul 15;241(4863):342–344. doi: 10.1126/science.3388044. [DOI] [PubMed] [Google Scholar]
- Rogers K. V., Vician L., Steiner R. A., Clifton D. K. The effect of hypophysectomy and growth hormone administration on pre-prosomatostatin messenger ribonucleic acid in the periventricular nucleus of the rat hypothalamus. Endocrinology. 1988 Feb;122(2):586–591. doi: 10.1210/endo-122-2-586. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Rivier J., Vale W. W. The distribution of growth-hormone-releasing factor (GRF) immunoreactivity in the central nervous system of the rat: an immunohistochemical study using antisera directed against rat hypothalamic GRF. J Comp Neurol. 1985 Jul 1;237(1):100–115. doi: 10.1002/cne.902370108. [DOI] [PubMed] [Google Scholar]
- Schalling M., Stieg P. E., Lindquist C., Goldstein M., Hökfelt T. Rapid increase in enzyme and peptide mRNA in sympathetic ganglia after electrical stimulation in humans. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4302–4305. doi: 10.1073/pnas.86.11.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Tozawa F., Yamada M., Ushiyama T., Tomori N., Sumitomo T., Nakagami Y., Demura H., Shizume K. Insulin-induced hypoglycemia increases corticotropin-releasing factor messenger ribonucleic acid levels in rat hypothalamus. Endocrinology. 1988 Sep;123(3):1371–1375. doi: 10.1210/endo-123-3-1371. [DOI] [PubMed] [Google Scholar]
- Tannenbaum G. S., Ling N. The interrelationship of growth hormone (GH)-releasing factor and somatostatin in generation of the ultradian rhythm of GH secretion. Endocrinology. 1984 Nov;115(5):1952–1957. doi: 10.1210/endo-115-5-1952. [DOI] [PubMed] [Google Scholar]
- Tannenbaum G. S., Martin J. B. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology. 1976 Mar;98(3):562–570. doi: 10.1210/endo-98-3-562. [DOI] [PubMed] [Google Scholar]
- Tozawa F., Suda T., Yamada M., Ushiyama T., Tomori N., Sumitomo T., Nakagami Y., Demura H., Shizume K. Insulin-induced hypoglycemia increases proopiomelanocortin messenger ribonucleic acid levels in rat anterior pituitary gland. Endocrinology. 1988 Apr;122(4):1231–1235. doi: 10.1210/endo-122-4-1231. [DOI] [PubMed] [Google Scholar]
- Uhl G. R., Reppert S. M. Suprachiasmatic nucleus vasopressin messenger RNA: circadian variation in normal and Brattleboro rats. Science. 1986 Apr 18;232(4748):390–393. doi: 10.1126/science.3961487. [DOI] [PubMed] [Google Scholar]
- Urman S., Kaler L., Critchlow V. Effects of hypothalamic periventricular lesions on pulsatile growth hormone secretion. Neuroendocrinology. 1985 Nov;41(5):357–362. doi: 10.1159/000124202. [DOI] [PubMed] [Google Scholar]
- Willoughby J. O., Brogan M., Kapoor R. Hypothalamic interconnections of somatostatin and growth hormone releasing factor neurons. Neuroendocrinology. 1989 Nov;50(5):584–591. doi: 10.1159/000125285. [DOI] [PubMed] [Google Scholar]
- Wise P. M., Scarbrough K., Weiland N. G., Larson G. H. Diurnal pattern of proopiomelanocortin gene expression in the arcuate nucleus of proestrous, ovariectomized, and steroid-treated rats: a possible role in cyclic luteinizing hormone secretion. Mol Endocrinol. 1990 Jun;4(6):886–892. doi: 10.1210/mend-4-6-886. [DOI] [PubMed] [Google Scholar]
- Zeitler P., Argente J., Chowen-Breed J. A., Clifton D. K., Steiner R. A. Growth hormone-releasing hormone messenger ribonucleic acid in the hypothalamus of the adult male rat is increased by testosterone. Endocrinology. 1990 Sep;127(3):1362–1368. doi: 10.1210/endo-127-3-1362. [DOI] [PubMed] [Google Scholar]