Abstract
Background
Little is known about the effects of phosphorus additives on patients with kidney disease.
Study Design
Randomized, double-blind, cross-over trial
Setting & Participants
31 adults with early stages of presumed chronic kidney disease (estimated glomerular filtration rate [eGFR] ≥ 45 ml/min/1.73m2; urine albumin-creatinine ratio [ACR] sex-specific cut points: men ≥ 17 mg/g, women ≥ 25 mg/g).
Intervention
Higher vs lower phosphorus intake for 3 weeks. Higher phosphorus intake was achieved by addition of commercially available diet beverages and breakfast bars to diet.
Outcomes
Change in 24-hour urine albumin excretion and plasma fibroblast growth factor 23
Measurements
Two 24-hour urine collections and a single fasting blood draw at the end of each period.
Results
Mean baseline values of phosphorus intake, 24-hour urine phosphorus excretion, and eGFR were 1113 ± 549 (SD) mg/d, 688 ± 300 mg/d, and 74.6 ± 22.0 ml/min/1.73m2. Median albuminuria was 82.7 (IQR, 39.6–174.1) mg/d. While phosphorus intake from study products increased by 993 mg/d (p<0.001) during the higher compared to lower phosphorus additive period, background phosphorus intake decreased by 151 mg/d (p=0.004). Higher phosphorus additive consumption increased 24-hour urine phosphorus excretion by 505 (95% CI, 381–629) mg/d (p<0.001), but did not significantly increase albuminuria (higher vs lower: 14.3%; 95% CI, −2.5% to 34.0%; p=0.1) or fibroblast growth factor 23 (higher vs lower: 3.4%; 95% CI, −5.9% to 13.6%; p=0.4).
Limitations
Small sample size, short duration of intervention, changes in background diet during the intervention
Conclusions
A 3-week consumption of higher phosphorus food additives did not significantly increase albuminuria. Further studies are needed to confirm these results.
Keywords: phosphorus intake, phosphate, albuminuria, urine albumin excretion, proteinuria, fibroblast growth factor 23 (FGF-23), nutrition, chronic kidney disease (CKD), parathyroid hormone (PTH), diet, modifiable risk factor, randomized controlled trial
Phosphorus additives are commonly used by the food industry to decrease preparation time, improve taste, and extend the shelf-life of processed foods. 1 Phosphorus additives can be found in 40%–50% of top-selling grocery items, 2 are highly absorbable, 3 and may have greater physiologic effects than phosphorus from natural sources. 4 While these additives are deemed GRAS (generally regarded as safe) by the US Food and Drug Administration, recent studies have raised concerns that high phosphorus intake could have detrimental effects on health. 5–7
Phosphorus additives contribute significantly to total phosphorus intake. According to 2009–2010 NHANES (National Health and Nutrition Examination Survey) data, mean estimated phosphorus intake is 1655 mg/d in men and 1190 mg/d in women. 8 While these estimates of phosphorus intake are more than double the estimated average requirement of 580 mg/d, they remain far below the tolerable upper level (UL) (4000 mg/d), defined as the highest level of consumption likely to cause no adverse health effects. 9 In patients with chronic kidney disease (CKD), restriction of phosphorus intake is only advised in patients who develop hyperphosphatemia according to the KDIGO (Kidney Disease: Improving Global Outcomes) CKD guideline. 10
High phosphorus intake can stimulate secretion of fibroblast growth factor 23 (FGF- 23), 11,12 a phosphaturic hormone that has been linked to left ventricular hypertrophy and increased risk of heart failure and death. 13,14 Consuming a high phosphorus load can acutely raise serum phosphorus levels, and impair endothelial function in individuals with normal kidney function. 15 Other potential adverse health effects include raising parathyroid hormone (PTH) levels and disturbing bone mineral metabolism. 6,16 In animal models, high phosphorus intake can cause calcium deposition in the kidney, proximal tubular injury, and albuminuria. 17–19 Previously, we reported a direct association between changes in 24-hour urine phosphorus excretion and albuminuria that persisted even with adjustment for differences in protein intake. 20
Given that little is known about the effect of phosphorus intake on albuminuria in humans, we conducted a randomized, crossover trial to determine the effects of higher versus lower intake of phosphorus additives on urinary albumin excretion and FGF-23.
Methods
Overview
The Study of Dietary Additive Phosphorus on Proteinuria and FGF-23 (SODA-POP) was a single-center, randomized, double-blind, two-period cross-over study funded by the American Heart Association and the National Kidney Foundation. We recruited participants at the Johns Hopkins Prohealth Clinical Research Unit, a community-based research clinic in Baltimore, MD. The Johns Hopkins University institutional review board approved the protocol (NA_00082089). Each participant provided written, informed consent.
Participants
Trial participants were adults with early stages of presumed CKD (albuminuria and eGFR ≥ 45 ml/min/1.73m2 by the CKD-EPI creatinine equation). 21 We selected this cut point because current guidelines recommend phosphorus restriction in the setting of hyperphosphatemia, a complication which rarely occurs above an eGFR of 45 ml/min/1.73m2. 10,22 Albuminuria was defined using sex-specific cut points for albumin-creatinine ratio (ACR): men, ≥ 17 mg/g; women, ≥ 25 mg/g. 23 Main exclusion criteria included blood pressure ≥ 160/100 mm Hg; recent change in blood pressure medications; random glucose ≥ 250 mg/dL; urine ACR > 1000 mg/g; glomerulonephritis; primary hyperparathyroidism; systemic diseases affecting calcium or phosphorus levels; abnormal serum phosphorus or calcium concentrations; phenylketonuria; consumption of ≥ 14 alcoholic drinks per week; use of phosphorus binders, antacids (long-term), phosphorus supplements, or high dose vitamin D (> 5,000 U/d); and participation in another clinical trial influencing diet or blood pressure.
Primary recruitment strategies included mass mailing of study brochures to individuals with self-reported type 2 diabetes in neighboring zip codes and to past and current participants of other research studies. The first participants began the protocol in February 2014, and the last participant completed the study in September 2015.
Participant Flow
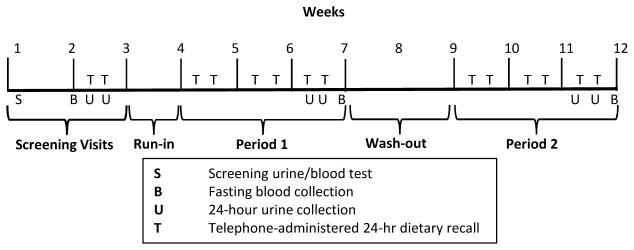
Participants underwent two study visits to determine eligibility as well as a 1-week run-in period to ensure they could tolerate and adhere to the provided diet beverages and breakfast bars (Figure 1). Participants who remained interested in continuing the study were then randomized to 1 of 2 intervention sequences. Randomization assignments were generated by a computer program, and an unblinded staff member opened a sealed, opaque envelope with the randomized intervention sequence. Participants, research staff in direct contact with participants, and investigators remained blinded throughout the study. Each intervention period lasted 3 weeks with a washout period of 2–4 weeks between feeding periods.
Figure 1.
Study Schedule
Higher and Lower Phosphorus Additive Periods
We advised all participants to avoid phosphorus additives in their diet in both study periods so that participants would not consume more than the UL of 4000 mg/d. To achieve a sufficient contrast in phosphorus intake between study periods, we provided participants with 1 commercially-available breakfast bar and 64 oz of orange-colored diet beverages per day (Table 1). Products were similar in appearance in both study arms, but differed by the presence or absence of phosphorus additives. Diet beverage powder mixes were repackaged into containers without labels; breakfast bars were given in their original packaging to preserve freshness.
Table 1.
Nutritional Content of Study Products
| Higher Phosphorus Period | Lower Phosphorus Period | Difference | |||||
|---|---|---|---|---|---|---|---|
| Cinnamon Toast Crunch Milk’n Cereal Bar* | Crystal Light Classic Orange** | Total | Rice Krispies Treat^ | Wyler’s Light Sunsplash Orange | Total | ||
| Total energy (kcal/d) | 180 | 40 | 220 | 90 | 40 | 130 | 90 |
| Carbohydrate (g/d) | 33 | 0 | 33 | 17 | 0 | 17 | 16 |
| Total fat (g/d) | 4 | 0 | 4 | 2 | 0 | 2 | 2 |
| Protein (g/d) | 3 | 0 | 3 | <1 | 0 | 0 | <3 |
| Sodium (mg/d) | 130 | 0 | 130 | 105 | 0 | 105 | 25 |
| Calcium (mg/d) | 250 | 800 | 1050 | 0 | 800 | 800 | 250 |
| Phosphorus* (mg/d) | 205 | 804 | 1009 | 11 | 0 | 11 | 998 |
| Ascorbic acid (mg/d) | 9 | 480 | 489 | 0 | 480 | 480 | 9 |
Note: Nutrient information is manufacturer reported with the exception of phosphorus. Phosphorus was measured at Medallion labs, Minneapolis, MN using 5 samples of Crystal Light Classic Orange and 3 samples of each of the other products.
Manufactured by General Mills
Manufactured by Kraft Foods
Manufactured by Kelloggs
We measured phosphorus content of the products by ashing samples at high temperature, digesting them in acid, and using inductively coupled plasma atomic emission spectrometry at Medallion Laboratories Inc (Minneapolis, MN). The mean daily amounts of phosphorus provided by the phosphorus additive-containing beverage and breakfast bar were 804 ± 99 mg/d and 205 ± 5 mg/d, respectively, compared to 0 ± 0 mg/d and 11 ± 2 mg/d for their respective phosphorus additive–free counterparts. This resulted in a difference in study product phosphorus content of 998 mg/d (Table 1). 24 Manufacturer-reported content of other macronutrients and micronutrients is shown in Table 1. Notably, study products with phosphorus additives contained 1050 mg/d of calcium whereas study products without phosphorus additives contained 800 mg/d of calcium. Both the products with and without phosphorus additives contained substantial amounts of ascorbic acid (489 and 480 mg/d, respectively).
Measurements
We masked participants and study staff involved in collection of outcome data to the randomization sequence. At baseline and at the end of each intervention period, we collected two 24-hour urine samples and one fasting blood sample. We asked participants to repeat 24-hour urine collections if they missed more than 1 void or collected < 500 ml of urine. Urine albumin was measured by a local laboratory (Quest Diagnostics) using an immunoturbidimetry assay. Blood samples were collected and centrifuged, with serum and plasma aliquots stored at −70°C. Fasting plasma samples were sent on dry ice to the University of Miami, where FGF-23 were measured using a second-generation carboxy-terminal assay (Immutopics, Santa Clara, CA), and PTH was measured using an intact PTH assay (Roche, Indianapolis, IN).
To assess changes in background diet during the study, the Johns Hopkins Institute for Clinical and Translational Research Nutrition Core administered 24-hour telephone dietary recalls twice to assess baseline intake, and then twice weekly during each intervention period (6 per period) using the US Department of Agriculture (USDA) Multiple Pass Method. 25 During these phone calls, we asked participants about their adherence to consumption of the breakfast bars and diet beverages.
Blood pressure was measured at 3 visits during the screening phase, and at the end of weeks 1, 2, and 3. At each visit, 3 readings were obtained in the seated position by trained and certified observers after 5 minutes of rest with an Omron HEM-907 device (Omron Healthcare Inc, Bannockburn, Ill) using a standardized protocol. Baseline and end-of-period blood pressure were the average of all available measurements taken during each period. Height and weight were measured using standardized protocols.
Analytic Considerations
Sample size for this study was calculated using the xsampsi module in STATA, based on a previous study with repeat 24-hour urine collections (standard deviation, 1.04). 20 At an α level of 0.05, we anticipated that a sample size of 30 participants with mean albuminuria of 100 mg/d would result in >80% power to detect a 13% difference in log- transformed albuminuria between the higher and lower phosphorus additive periods. Using data from a previous study with repeat carboxy-terminal FGF-23 measurements (standard deviation, 11 RU/ml), we anticipated that we would have >80% power to detect a 6 RU/ml difference in FGF-23. 26
Co-primary outcomes were comparison of end-of-period albuminuria (mean of two 24-hour urine collections) and end-of-period FGF-23. Other outcomes investigated included blood pressure and intact PTH. Distributions of outcome variables were evaluated for normality; albuminuria, intact PTH, and FGF-23 were log-transformed. Main analyses were intention-to-treat using mixed effects models allowing intercepts to vary for each individual. Carryover effects were examined by using treatment by assignment-order interaction terms. Pre-specified sensitivity analyses were conducted excluding patients who were non-compliant, prior to data analysis. In the first sensitivity analysis, we excluded patients who were deemed non-compliant, based on missing product pickups and follow-up visits; this was determined by blinded members of the research team. In the second sensitivity analysis, we excluded patients who were suspected to be poorly compliant based on 24-hour urine phosphorus excretion data (< 250 mg difference between the higher and lower period).
Pre-specified subgroups included above and below median values of eGFR, albuminuria, and 24-hour urine phosphorus, and serum phosphorus. For each outcome, a p value < 0.05 was considered statistically significant without adjustment for multiple comparisons. All analyses were performed using STATA version 13.1 (StataCorp LP, College Station, TX).
Results
Study Participants
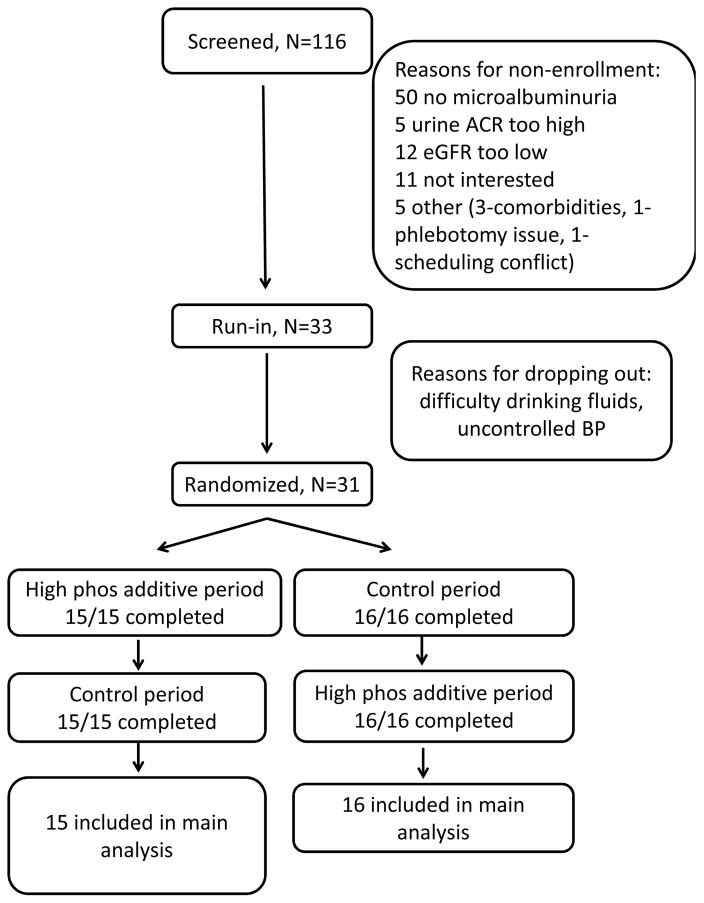
Out of 116 prescreened participants, 83 were ineligible, with the most common reason being no albuminuria; 33 patients were eligible and started the run-in period (Figure 2). During run-in, one patient withdrew because of difficulty drinking the prescribed diet beverage, and another patient withdrew due to high blood pressure; 31 participants were randomized.
Figure 2. Participant Flow.
Abbreviations: ACR (albumin/creatinine ratio), eGFR (estimated glomerular filtration rate)
Mean age was 66.0 years, 32% were female, 90% were black, 87% had hypertension, and 48% had diabetes (Table 2). Mean baseline values of eGFR, serum phosphorus, and calcium were 74.6 ± 22.0 (standard deviation) ml/min/1.73m2, 3.57 ± 0.45 mg/dL, and 9.48 ± 0.29 mg/dL, respectively. Median values of albuminuria, FGF-23, and intact PTH were 82.7 (IQR, 39.6–174.1) mg/d, 129.6 (IQR, 101.8–158.5) RU/ml, and 50.7 (IQR, 35.2–62.7) pg/ml. At baseline, mean dietary phosphorus intake was 1113 ± 549 mg/d and mean 24-hour urine phosphorus excretion was 688 ± 300 mg/d (Table 3).
Table 2.
Baseline Characteristics by Randomization Order
| Variable | Lower then Higher^ (n=16) | Higher then Lower^ (n=15) |
|---|---|---|
|
| ||
| Age (y) | 64.2 (9.9) | 68.0 (7.3) |
|
| ||
| Female sex | 4 (25%) | 6 (40%) |
|
| ||
| Black race | 14 (88%) | 14 (93%) |
|
| ||
| Smoking status | ||
|
| ||
| Current | 5 (31%) | 5 (33%) |
|
| ||
| Former | 6 (38%) | 4 (27%) |
|
| ||
| Never | 5 (31%) | 6 (40%) |
|
| ||
| Hypertension | 13 (81%) | 14 (93%) |
|
| ||
| Diabetes | 8 (50%) | 7 (47%) |
|
| ||
| Dyslipidemia | 8 (50%) | 10 (67%) |
|
| ||
| Coronary Artery Disease | 2 (13%) | 1 (7%) |
|
| ||
| Systolic BP (mmHg) | 130.0 (11.3) | 129.4 (15.9) |
|
| ||
| Diastolic BP (mmHg) | 73.7 (8.0) | 69.2 (10.1) |
|
| ||
| BMI (kg/m2) | 29.8 (5.5) | 31.9 (4.7) |
|
| ||
| eGFR (ml/min/1.73m2) | 79.0 (25.3) | 69.9 (17.6) |
|
| ||
| Serum Phosphorus (mg/dl) | 3.62 (0.41) | 3.52 (0.50) |
|
| ||
| Serum Calcium (mg/dl) | 9.51 (0.34) | 9.46 (0.25) |
|
| ||
| 25(OH)D (nmol/l) | 28.1 (10.2) | 39.1 (25.3) |
|
| ||
| 1,25(OH)2D (pg/ml) | 51.9 (14.8) | 47.8 (12.6) |
|
| ||
| FGF-23 (RU/ml) * | 108.6 [92.1–136.7] | 157.2 [120.8–207.1] |
|
| ||
| Intact PTH (pg/ml) | 44.0 [34.3–59.2] | 54.8 [35.2–72.3] |
|
| ||
| Albuminuria (mg/d) | 107.4 [51.5–226.2] | 67.1 [26.6–101.4] |
|
| ||
| Albuminuria category | ||
| < 30 mg/g | 1 (6%) | 4 (27%) |
| 30–299 mg/g | 12 (75%) | 11 (73%) |
| ≥ 300 mg/g | 3 (19%) | 0 (0%) |
Note: Values for categorical variables are given as count (proportion); values for continuous variables are given as mean ± standard deviation or median [interquartile range]. Conversion factors for units: calcium in mg/dL to mmol/L, ×0.2495; phosphorus in mg/dL to mmol/L, ×0.3229;
Refers to lower then higher and higher then lower phosphorus additive periods.
P value <0.05 for comparison between randomization order groups
Abbreviations: 25(OH)D, 1,25-dihydroxyvitamin D; 1,25(OH)2D, 25, hydroxyvitamin D; BMI, body mass index; BP, blood pressure; eGFR (estimated glomerular filtration rate), FGF-23 (fibroblast growth factor 23), PTH (parathyroid hormone)
Table 3.
Changes in Phosphorus and Calcium Excretion, Weight and Self-reported Diet During the Study
| Baseline | Lower phosphorus period | Higher phosphorus period | Change* | P value | |
|---|---|---|---|---|---|
| 24-h urine phosphorus (mg/d) | 688 (300) | 526 (294) | 1031 (376) | 505 (381, 629) | <0.001 |
| 24-h urine calcium (mg/d) | 97.5 (81.9) | 129.3 (94.7) | 105.6 (90.8) | −23.7 (−45.4, −2.0) | 0.03 |
| Weight (kg) | 88.9 (15.6) | 88.9 (15.6) | 88.7 (15.7) | −0.2 (−0.7, 0.3) | 0.4 |
| Diet1 | |||||
| Total energy (kcal/d) | 1877 (689) | 2051 (7300) | 1908 (717) | −143 (−292, 6) | 0.06 |
| Sodium intake (mg/d) | 3060 (1376) | 3436 (1371) | 3313 (1266) | −123 (−435, 189) | 0.4 |
| Calcium intake (mg/d) | 696 (378) | 873.5 (289.0) | 795 (273) | −79 (−176, 19) | 0.1 |
| Study product phosphorus intake | 0 | 9.0 (0.2) | 1002.1 (24.6) | 993.1 (984.6, 1001.6) | <0.001 |
| Background phosphorus intake (mg/d) | 1113 (549) | 1355 (498) | 1204 (429) | −151 (−252, −49) | 0.004 |
| Protein intake (g/d) | 78.6 (35.2) | 87.9 (33.0) | 79.9 (29.2) | −8.0 (−14.7,−1.2) | 0.02 |
Note: Values are given as mean ± standard deviation or change (95% confidence interval).
Background diet assessed by 24-hour telephone dietary recalls twice to assess baseline intake, and then twice weekly during each intervention period (6 per period) not including intervention products.
Value during higher phosphorus additive period less value during lower phosphorus additive period.
Changes in Phosphorus and Calcium Excretion During Study Periods
Compared to the baseline period, phosphorus excretion increased by 343 (95% confidence interval [CI], 227–458) mg/d (p<0.001) during the higher phosphorus additive period and decreased by 162 (95% CI, 88 to 237) mg/d (p<0.001) during the lower phosphorus additive period; the difference between higher and lower phosphorus additive periods was 505 (95% CI, 381–629) mg/d (p<0.001; Table 3). Calcium excretion tended to increase during both the higher phosphorus additive period (8.0 [95% CI, −4.9 to 20.9] mg/d; p=0.2) and the lower phosphorus additive period (31.8 [95% CI, 14.3 to 49.2] mg/d; p<0.001); calcium excretion was 23.7 (95% CI, −45.4 to −2.0) mg/d lower during the higher phosphorus additive period compared to the lower phosphorus additive period (P=0.03).
Self-reported Compliance, Background Diet, and Adverse Effects During Study Periods
Participants reported excellent adherence to use study products, resulting in an estimated 993 (95% CI, 985–1002) mg/d difference in phosphorus intake from the 2 sets of study products (P<0.001). However, 2 of the 31 patients were non-compliant, as evidenced by multiple missed visits to pick up food/beverage products. Among all participants, background phosphorus intake, assessed by multiple 24-hour dietary recalls, was lower during the higher phosphorus additive period compared to the lower phosphorus additive period (−151 [95% CI, −252 to −49] mg/d; p=0.004) as was total protein intake (−8.0 [95% CI, −14.7 to −1.2] g/d; p=0.02). Reported adverse effects were similar during the two periods with gastrointestinal complaints frequently reported in both the higher phosphorus additive period (61%) and the lower phosphorus additive period (68%).
Effect of Higher Phosphorus Additive Consumption on Study Outcomes
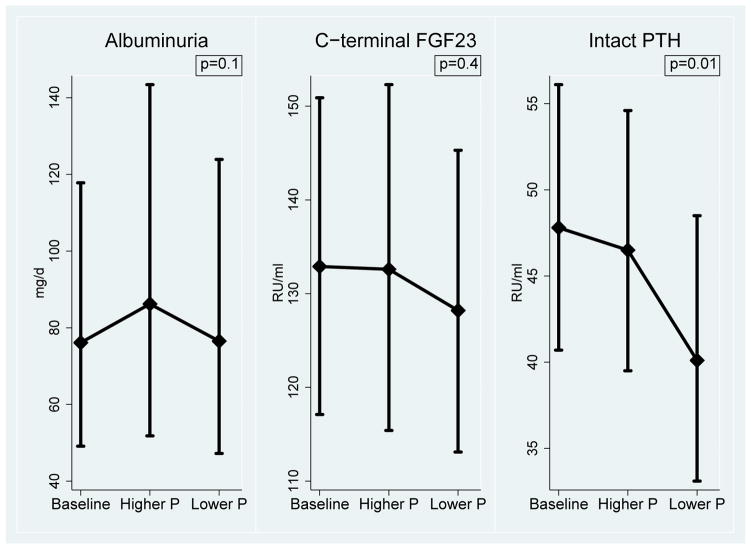
Albuminuria did not increase significantly with higher phosphorus additive consumption (change of 14.3%; 95% CI, −2.5% to 34.0%; p=0.1; Figure 3, Table 4). Higher phosphorus additive consumption also had no effect on FGF-23 (change of 3.4%; 95% CI, −5.9% to 13.6%; p=0.4), but did increase intact PTH by 15.9% (95% CI, 3.8%–29.5%; p=0.01; Figure 3, Table 4). Higher phosphorus additive consumption had no effect on serum phosphorus (change of −0.10 [95% CI, −0.29 to 0.09] mg/dL; p=0.3), systolic (−1.1 [95% CI, −4.1 to 1.9] mm Hg; p=0.5) or diastolic (−0.8 [95% CI, −2.7 to 1.0] mm Hg; p=0.4) blood pressure. We detected no significant carryover effect of phosphorus additive supplementation on albuminuria or other outcomes.
Figure 3. Changes in Outcomes during the study.
Graphs show geometric means with 95% confidence intervals. P values are for comparison between end of higher phosphorus additive period and end of lower phosphorus additive period. Abbreviations: FGF23 (fibroblast growth factor 23), PTH (parathyroid hormone), P (phosphorus)
Table 4.
Baseline, End-of-Period, and Change in Outcomes
| Baseline | End of lower phosphorus period | End of higher phosphorus period | Change* | P value | |
|---|---|---|---|---|---|
| Albuminuria1 (mg/d) | 74.4 (47.7, 116.0) | 76.4 (47.2,123.8) | 86.2 (51.8, 143.4) | 14.3% (−2.5%, 34.0%) | 0.1 |
| FGF-231 (RU/ml) | 132.9 (117.1, 150.9) | 128.2 (113.1, 145.3) | 132.6 (115.4, 152.3) | 3.4% (−5.9%, 13.6%) | 0.4 |
| Intact PTH1 (pg/ml) | 47.8 (40.7, 56.1) | 40.1 (33.1, 48.5) | 46.5 (39.5, 54.6) | 15.9% (3.8%, 29.5%) | 0.01 |
| Serum phosphorus (mg/dL) | 3.57 (0.45) | 3.46 (0.57) | 3.36 (0.51) | −0.10 (−0.29, 0.09) | 0.3 |
| Systolic BP (mmHg) | 128.0 (15.6) | 128.0 (16.1) | 126.9 (13.5) | −1.1 (−4.1, 1.9) | 0.5 |
| Diastolic BP (mmHg) | 69.6 (9.1) | 70.2 (11.4) | 69.3 (10.8) | −0.8 (−2.7, 1.0) | 0.4 |
Note: Data are given as mean ± standard deviation or value (95% CI). Conversion factor for phosphorus in mg/dL to mmol/L, ×0.3229.
Geometric mean (95% CI)
From lower phosphorus additive period to higher phosphorus additive period.
Abbreviations: CI, confidence interval; FGF-23 (fibroblast growth factor 23), PTH (parathyroid hormone), BP blood pressure
Sensitivity Analyses
In our first sensitivity analysis excluding 2 patients who were non-compliant with product pick-ups and follow-up visits, higher phosphorus additive consumption increased albuminuria by 17.3% (95% CI, 0.08%–37.5%), though this was of borderline significance (p=0.05). In our second sensitivity analysis excluding 6 patients who had < 250 mg/d difference in 24-hour urine phosphorus excretion between the higher and lower phosphorus periods, higher phosphorus additive consumption significantly increased albuminuria by 23.7% (95% CI, 4.6%–46.3%; p=0.01).
Findings were largely unchanged for FGF-23, intact PTH, serum phosphorus, and systolic and diastolic blood pressure in sensitivity analyses (Table S1, available as online supplementary material).
Tests for Interaction and Subgroup Analyses
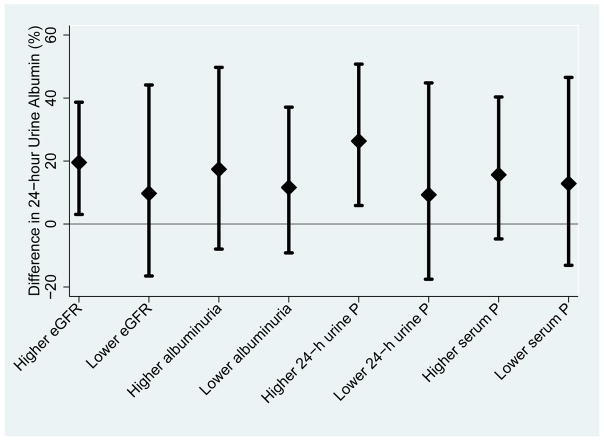
The effect of higher phosphorus additive consumption on albuminuria tended to be greater in patients with higher baseline 24-hour urine phosphorus (p for interaction=0.2). Patients with baseline 24-hour urine phosphorus excretion > 686 mg/d had an increase in albuminuria of 26.3% (95% CI, 5.9%–50.8%; p=0.01) whereas patients with baseline 24-hour urine phosphorus excretion < 686 mg/d had a non-significant increase in albuminuria of 9.3% (95% CI, −17.5% to 44.8%; p=0.5; Figure 4). Otherwise, findings were similar in patients with eGFR ≥ or < 69.4 ml/min/1.73m2, albuminuria ≥ or < 82.7 mg/d, and serum phosphorus ≥ or < 3.5 mg/dL. There was no effect of higher phosphorus additive consumption on FGF-23 in any subgroups tested and no significant interactions detected.
Figure 4. Subgroup Analyses Examining the Effect of Higher Phosphorus Additive Intake on Albuminuria.
Higher subgroups are at or above median values and lower subgroups are below median values of eGFR (69.4 ml/min/1.73m2), albuminuria (82.7 mg/d), phosphaturia (686 mg/d), and serum phosphorus (3.5 mg/d). The effect of higher phosphorus additive intake on albuminuria was nominally stronger in patients with higher baseline phosphorus excretion although this was not significant (p=0.2). Abbreviations: eGFR (estimated glomerular filtration rate), P (phosphorus)
Discussion
In this randomized clinical trial, higher phosphorus additive consumption did not significantly increase albuminuria in adults with early stages of CKD. Although the main analysis was not statistically significant for albuminuria, additional sensitivity analyses, excluding patients with suspected poor compliance, suggest that high phosphorus additive consumption may have modestly increased albuminuria.
To our knowledge, this is the first randomized trial in humans specifically designed to investigate the effect of higher phosphorus additive consumption on urine albumin excretion in patients with early stages of CKD. Various animal models have demonstrated that a high phosphorus diet can result in calcium phosphate deposition, resultant proximal tubular injury, and increased albuminuria, as rapidly as in 3 days to 2 weeks in rats. 17–19,27. Conversely, limiting phosphorus intake preserves kidney function, reduces vascular calcification, and lowers mortality in experimental models of kidney disease. 19,28,29 Unfortunately, most studies of the effects of phosphorus restriction on kidney function in humans have been confounded by concurrent restriction in protein intake. 30,31 A small study found no effect of high phosphorus additive intake on urine albumin excretion in 10 young healthy women without baseline albuminuria. 32
Surprisingly, we found no significant effect of higher phosphorus additive consumption on FGF-23. However, not all studies have shown a direct relationship between high phosphorus intake and FGF-23. 11,12,26,33–35 Prior studies that have shown an effect of high phosphorus intake on FGF-23 have required a comparison period of relatively low phosphorus intake (500–1100 mg/d), 11,12,33 or the combination of phosphorus restriction with phosphorus-binding medications. 26,36 By comparison, mean phosphorus intake in our study was 1346 mg/d during the lower phosphorus additive period, similar to a study that found no effect of a high phosphorus/high protein diet on FGF-23. 35 The change in 24-hour urine phosphorus from baseline to the higher phosphorus additive period was only 343 mg/d, which would suggest that roughly one third of the phosphorus administered was absorbed. We suspect this may have been due to calcium phosphate precipitation in the small intestine, 37 or unreported poor compliance. Thus, a larger contrast in absorbed phosphorus may have been needed to affect FGF-23 levels.
Calcium excretion increased relative to baseline for both periods, but more calcium was absorbed during the lower phosphorus period than the higher phosphorus additive period (23.7 mg/d difference between periods in 24-hour urine calcium). Higher calcium absorption during the lower phosphorus additive period would have suppressed PTH, which may have contributed to the significant difference in PTH between periods. However, the increased calcium load in both periods may have also affected FGF-23 levels, which we had not anticipated. In a randomized trial of 148 patients with moderate CKD, calcium-based binders increased FGF-23 levels whereas sevelamer decreased FGF-23 and lanthanum had no effect on FGF-23. 38 Calcium supplementation has also been shown to directly stimulate FGF-23 expression in mice. 39 Thus, it is possible that the added calcium load increased FGF-23 levels, making it more difficult to detect an effect of higher phosphorus intake on FGF-23.
Our findings add to a growing body of research that suggests potential harm with phosphorus intake at levels within recommended intake levels (ie, below the current tolerable upper limit of 4000 mg/d). 5–7 We previously found that phosphorus intake above 1400 mg/d was associated with increased risk of death in a cohort of apparently healthy US adults. 5 Several studies have found that phosphorus additive supplementation, achieving total phosphorus intake of 1660–2300 mg/d, can have adverse effects on markers of bone mineral metabolism in healthy individuals. 6,7,11,12 Furthermore, phosphorus additives are commonly complexed with sodium, 40 which would be expected to independently increase albuminuria and blood pressure. 41,42
For patients with CKD, current KDIGO guidelines recommend phosphorus restriction only in individuals who develop hyperphosphatemia. 10 There are no recommendations to restrict phosphorus intake for primary prevention due to a lack of clinical trial data. Avoidance of phosphorus additives may be an effective strategy that deserves further study in patients with CKD. In a randomized trial of patients with end-stage renal disease, providing education (and magnifying glasses) to identify phosphorus additives on food labels was effective in significantly lowering serum phosphorus levels. 43 However, meals without phosphorus additives are more expensive than similar meals with phosphorus additives. 2 Given the fact that individuals in poverty are more likely to live in food deserts and have albuminuria and hyperphosphatemia, engagement of policymakers and industry will be needed to improve access to affordable, unprocessed foods. 44–46
Our trial has limitations. First, to minimize expense and participant burden, we chose to supplement participants’ free-living diets with commercially-available beverages and breakfast bars rather than conducting a tightly-controlled feeding study. It is likely that the difference in the background intake of phosphorus, protein, and total energy intake between periods would not have occurred in a controlled feeding study. However, we used unaltered, commercially-available beverages and bars to produce the phosphorus contrast rather than administering supplements to more closely resemble a “real-life” situation. While our choice of calcium as the accompanying cation (rather than sodium or potassium) may have been superior for studying effects on albuminuria, differences in absorbed calcium between periods may have had independent effects on markers of bone mineral metabolism. Second, we used albuminuria as an outcome rather than a clinical end point such as doubling of serum creatinine or eGFR decline. However, findings from randomized trials provide support for proteinuria reduction as a valid surrogate outcome. 47 Third, our sample size was small, although we used a cross-over study design, which enhances statistical power as each person serves as his or her own control. Fourth, this study was not designed to examine changes in serum phosphorus, which was only measured in the morning after fasting. Multiple measurements, particularly in the afternoon, may be needed to detect an effect of higher phosphorus intake on serum phosphorus levels. 48
There were also a number of strengths of our study. Our predominantly urban African-American population is extremely relevant given their high burden of kidney disease and disproportionately low access to healthy food. 49 Still, findings may not be generalizable to other populations as mineral metabolism may be different in African- Americans compared to whites. 50 Second, we had high rates of adherence and follow-up, evidenced by the large contrast achieved in phosphorus excretion between study periods. Lastly, we rigorously collected multiple 24-hour urine collections and dietary recalls using high-quality methods.
In conclusion, higher phosphorus additive consumption did not significantly increase albuminuria. Further research is needed to determine whether reduction in phosphorus additive consumption improves cardiovascular and kidney disease outcomes.
Supplementary Material
Acknowledgments
Results of this study were presented in Phoenix, Arizona, at the American Heart Association Epidemiology and Prevention/Lifestyle and Cardiometabolic Health 2016 Scientific Sessions on March 2, 2016.
Support: Dr Chang received support from the American Heart Association National Clinical Research Program (13CRP16970085), the National Kidney Foundation Satellite Dialysis Clinical Investigator Grant, and Geisinger Health System for this study. Currently, Dr Chang receives support from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant K23 DK106515-01. Funders of this study did not have any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: ARC, ERM, MM, JC, LJA; data acquisition: JC, KW, MM, BH, CK, SO; data analysis/interpretation: ARC, CK, SPJ, ERM, LJA, CAA; supervision or mentorship: LJA, ERM, CAA. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. ARC takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
Peer Review: Evaluated by 3 external peer reviewers, a Statistical Editor, a CoEditor, and the Editor-in-Chief.
Trial registration: www.ClinicalTrials.gov; study number: NCT02020785.
Table S1: Sensitivity analyses of effect of higher phosphorus additive consumption on outcomes.
Note: The supplementary material accompanying this article (doi:____________) is available at www.ajkd.org
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). Sensitivity analyses of effect of higher phosphorus additive consumption on outcomes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calvo MS, Uribarri J. Contributions to total phosphorus intake: all sources considered. Semin Dial. 2013 Jan-Feb;26(1):54–61. doi: 10.1111/sdi.12042. [DOI] [PubMed] [Google Scholar]
- 2.Leon JB, Sullivan CM, Sehgal AR. The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. J Ren Nutr. 2013 Jul;23(4):265–270. e2. doi: 10.1053/j.jrn.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karp H, Ekholm P, Kemi V, Hirvonen T, Lamberg-Allardt C. Differences among total and in vitro digestible phosphorus content of meat and milk products. J Ren Nutr. 2012 May;22(3):344–349. doi: 10.1053/j.jrn.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Karp HJ, Vaihia KP, Karkkainen MU, Niemisto MJ, Lamberg-Allardt CJ. Acute effects of different phosphorus sources on calcium and bone metabolism in young women: a whole-foods approach. Calcif Tissue Int. 2007 Apr;80(4):251–258. doi: 10.1007/s00223-007-9011-7. [DOI] [PubMed] [Google Scholar]
- 5.Chang AR, Lazo M, Appel LJ, Gutierrez OM, Grams ME. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am J Clin Nutr. 2014 Feb;99(2):320–327. doi: 10.3945/ajcn.113.073148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez OM, Luzuriaga-McPherson A, Lin Y, Gilbert LC, Ha SW, Beck GR., Jr Impact of Phosphorus-Based Food Additives on Bone and Mineral Metabolism. J Clin Endocrinol Metab. 2015 Nov;100(11):4264–4271. doi: 10.1210/jc.2015-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvo MS, Uribarri J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am J Clin Nutr. 2013 Jul;98(1):6–15. doi: 10.3945/ajcn.112.053934. [DOI] [PubMed] [Google Scholar]
- 8.Calvo MS, Moshfegh AJ, Tucker KL. Assessing the health impact of phosphorus in the food supply: issues and considerations. Adv Nutr. 2014 Jan 1;5(1):104–113. doi: 10.3945/an.113.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food, Nutrition Board IoM. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. The National Academies Press; 1997. [PubMed] [Google Scholar]
- 10.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter, Suppl. 2013;3:1–150. [Google Scholar]
- 11.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor–23 concentrations in healthy men. J Clin Endocrinol Metab. 2006 Aug;91(8):3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 12.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006 Aug;21(8):1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 13.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011 Nov;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2012 Jul 17;60(3):200–207. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishi T, Shuto E, Ogawa M, et al. Excessive dietary phosphorus intake impairs endothelial function in young healthy men: a time- and dose-dependent study. J Med Invest. 2015;62(3–4):167–172. doi: 10.2152/jmi.62.167. [DOI] [PubMed] [Google Scholar]
- 16.Calvo MS, Kumar R, Heath H. Persistently elevated parathyroid hormone secretion and action in young women after four weeks of ingesting high phosphorus, low calcium diets. J Clin Endocrinol Metab. 1990 May;70(5):1334–1340. doi: 10.1210/jcem-70-5-1334. [DOI] [PubMed] [Google Scholar]
- 17.Ritskes-Hoitinga J, Lemmens AG, Danse LH, Beynen AC. Phosphorus-induced nephrocalcinosis and kidney function in female rats. J Nutr. 1989 Oct;119(10):1423–1431. doi: 10.1093/jn/119.10.1423. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki H, Uehara M, Suzuki K, et al. High phosphorus diet rapidly induces nephrocalcinosis and proximal tubular injury in rats. J Nutr Sci Vitaminol (Tokyo) 1997 Dec;43(6):627–641. doi: 10.3177/jnsv.43.627. [DOI] [PubMed] [Google Scholar]
- 19.Finch JL, Lee DH, Liapis H, et al. Phosphate restriction significantly reduces mortality in uremic rats with established vascular calcification. Kidney Int. 2013 Dec;84(6):1145–1153. doi: 10.1038/ki.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang A, Batch BC, McGuire HL, et al. Association of a reduction in central obesity and phosphorus intake with changes in urinary albumin excretion: the PREMIER study. Am J Kidney Dis. 2013 Nov;62(5):900–907. doi: 10.1053/j.ajkd.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011 Jun;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002 Apr;13(4):1034–1039. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- 24.Moser M, White K, Henry B, et al. Phosphorus Content of Popular Beverages. Am J Kidney Dis. 2015 Apr 8; doi: 10.1053/j.ajkd.2015.02.330. [DOI] [PubMed] [Google Scholar]
- 25.Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004 Apr;104(4):595–603. doi: 10.1016/j.jada.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005 Mar;90(3):1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 27.Haut LL, Alfrey AC, Guggenheim S, Buddington B, Schrier N. Renal toxicity of phosphate in rats. Kidney Int. 1980 Jun;17(6):722–731. doi: 10.1038/ki.1980.85. [DOI] [PubMed] [Google Scholar]
- 28.Alfrey AC. Effect of dietary phosphate restriction on renal function and deterioration. Am J Clin Nutr. 1988 Jan;47(1):153–156. doi: 10.1093/ajcn/47.1.153. [DOI] [PubMed] [Google Scholar]
- 29.Karlinsky ML, Haut L, Buddington B, Schrier NA, Alfrey AC. Preservation of renal function in experimental glomerulonephritis. Kidney Int. 1980 Mar;17(3):293–302. doi: 10.1038/ki.1980.35. [DOI] [PubMed] [Google Scholar]
- 30.Di Iorio BR, Bellizzi V, Bellasi A, et al. Phosphate attenuates the anti-proteinuric effect of very low-protein diet in CKD patients. Nephrol Dial Transplant. 2013 Mar;28(3):632–640. doi: 10.1093/ndt/gfs477. [DOI] [PubMed] [Google Scholar]
- 31.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994 Mar 31;330(13):877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 32.Grimm M, Muller A, Hein G, Funfstuck R, Jahreis G. High phosphorus intake only slightly affects serum minerals, urinary pyridinium crosslinks and renal function in young women. Eur J Clin Nutr. 2001 Mar;55(3):153–161. doi: 10.1038/sj.ejcn.1601131. [DOI] [PubMed] [Google Scholar]
- 33.Vervloet MG, van Ittersum FJ, Buttler RM, Heijboer AC, Blankenstein MA, ter Wee PM. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin J Am Soc Nephrol. 2011 Feb;6(2):383–389. doi: 10.2215/CJN.04730510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isakova T, Gutierrez OM, Smith K, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011 Feb;26(2):584–591. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kremsdorf RA, Hoofnagle AN, Kratz M, et al. Effects of a high-protein diet on regulation of phosphorus homeostasis. J Clin Endocrinol Metab. 2013 Mar;98(3):1207–1213. doi: 10.1210/jc.2012-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isakova T, Barchi-Chung A, Enfield G, et al. Effects of dietary phosphate restriction and phosphate binders on FGF23 levels in CKD. Clin J Am Soc Nephrol. 2013 Jun;8(6):1009–1018. doi: 10.2215/CJN.09250912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Meer R, De Vries HT. Differential binding of glycine- and taurine-conjugated bile acids to insoluble calcium phosphate. Biochem J. 1985 Jul 1;229(1):265–268. doi: 10.1042/bj2290265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block GA, Wheeler DC, Persky MS, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012 Aug;23(8):1407–1415. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005 Nov;289(5):F1088–95. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 40.Carrigan A, Klinger A, Choquette SS, et al. Contribution of food additives to sodium and phosphorus content of diets rich in processed foods. J Ren Nutr. 2014 Jan;24(1):13–9. 19e1. doi: 10.1053/j.jrn.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013 Dec;24(12):2096–2103. doi: 10.1681/ASN.2013030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slagman MC, Waanders F, Hemmelder MH, et al. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ. 2011 Jul 26;343:d4366. doi: 10.1136/bmj.d4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan C, Sayre SS, Leon JB, et al. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. JAMA. 2009 Feb 11;301(6):629–635. doi: 10.1001/jama.2009.96. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez OM, Isakova T, Enfield G, Wolf M. Impact of poverty on serum phosphate concentrations in the Third National Health and Nutrition Examination Survey. J Ren Nutr. 2011 Mar;21(2):140–148. doi: 10.1053/j.jrn.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suarez JJ, Isakova T, Anderson CA, Boulware LE, Wolf M, Scialla JJ. Food Access, Chronic Kidney Disease, and Hypertension in the U.S. Am J Prev Med. 2015 Dec;49(6):912–920. doi: 10.1016/j.amepre.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martins D, Tareen N, Zadshir A, et al. The association of poverty with the prevalence of albuminuria: data from the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2006 Jun;47(6):965–971. doi: 10.1053/j.ajkd.2006.02.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inker LA, Levey AS, Pandya K, et al. Early change in proteinuria as a surrogate end point for kidney disease progression: an individual patient meta-analysis. Am J Kidney Dis. 2014 Jul;64(1):74–85. doi: 10.1053/j.ajkd.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ix JH, Anderson CA, Smits G, Persky MS, Block GA. Effect of dietary phosphate intake on the circadian rhythm of serum phosphate concentrations in chronic kidney disease: a crossover study. Am J Clin Nutr. 2014 Nov;100(5):1392–1397. doi: 10.3945/ajcn.114.085498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buczynski A, Freishtat H, Buzogany S. [Accessed June 15, 2016];Mapping Baltimore City's Food Environment: 2015 Report. 2015 Available at: http://mdfoodsystemmap.org/wp-content/uploads/2015/06/Baltimore-Food-Environment-Report-2015-11.pdf.
- 50.Gutierrez OM, Isakova T, Smith K, Epstein M, Patel N, Wolf M. Racial differences in postprandial mineral ion handling in health and in chronic kidney disease. Nephrol Dial Transplant. 2010 Dec;25(12):3970–3977. doi: 10.1093/ndt/gfq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.