Abstract
Background
The development of tobacco use treatments that are effective for all smokers is critical to improving clinical and public health. The Multiphase Optimization Strategy (MOST) uses highly efficient factorial experiments to evaluate multiple intervention components for possible inclusion in an optimized tobacco use treatment. Factorial experiments permit analyses of the influence of patient characteristics on main and interaction effects of multiple, relatively discrete, intervention components. This study examined whether person-factor and smoking characteristics moderated the main or interactive effects of intervention components on 26-week self-reported abstinence rates.
Methods
This fractional factorial experiment evaluated six smoking cessation intervention components among primary care patients (N=637): Prequit Nicotine Patch vs. None, Prequit Nicotine Gum vs. None, Preparation Counseling vs. None, Intensive Cessation In-Person Counseling vs. Minimal, Intensive Cessation Telephone Counseling vs. Minimal, and 16 vs. 8 Weeks of Combination Nicotine Replacement Therapy (NRT; nicotine patch + nicotine gum).
Results
Both psychiatric history and smoking heaviness moderated intervention component effects. In comparison with participants with no self-reported history of a psychiatric disorder, those with a positive history showed better response to 16- vs. 8-weeks of combination NRT, but a poorer response to counseling interventions. Also, in contrast to light smokers, heavier smokers showed a poorer response to counseling interventions.
Conclusions
Heavy smokers and those with psychiatric histories demonstrated a differential response to intervention components. This research illustrates the use of factorial designs to examine the interactions between person characteristics and relatively discrete intervention components. Future research is needed to replicate these findings.
Keywords: Smoking cessation, treatment, moderators, factorial design, psychiatric comorbidity
1. INTRODUCTION
Tobacco smoking remains the leading preventable cause of mortality and morbidity in developed countries, underscoring the continued need for highly efficacious smoking treatments (U.S. Department of Health and Human Services, 2014). Even with the best smoking cessation treatments that comprise both counseling and pharmacotherapy, about two-thirds of smokers fail to achieve long-term abstinence (Fiore et al., 2008; West et al., 2015). Smoking rates remain especially high amongst certain groups of smokers, such as those with psychiatric comorbidity and those with lower educational attainment (Centers for Disease Control and Prevention, 2013; Jamal et al., 2014). Therefore, it is of considerable public health importance that such populations benefit from smoking cessation treatments. Further, it is important to determine whether person factors (e.g., gender, race) or smoking-related factors (e.g., tobacco dependence) might effectively guide treatment selection or allocation (Hughes, 2013; Loh et al., 2012).
Most prior attempts to evaluate the moderation of treatment response have been obscured by the use of randomized controlled trials (RCTs) that evaluated only groups of components (e.g., pharmacotherapy + various counseling elements) and did not manipulate discrete intervention components. The Multiphase Optimization Strategy (MOST) has been proposed as an efficient way to engineer more effective treatment packages by using screening experiments to identify especially effective intervention components which can be combined into a treatment package and ultimately evaluated in a traditional RCT (Collins, et al., 2016, 2005). Screening experiments frequently use factorial designs, which allow researchers to identify the main and interactive effects of the evaluated intervention components (Collins et al., 2016, 2005). Factorial designs can also reveal how person factors moderate the effects of individual intervention components, or combinations of components. In other words, factorial designs can provide insight into how individuals’ characteristics predict differential response to multiple, discrete intervention components or to combinations of components. Identifying differential response to interventions by different types of smokers could be used to personalize treatment and provide insight into factors that influence intervention effectiveness. Conversely, a lack of interactions between intervention components and person factors would support the robustness and stability of treatment effects.
A recent smoking cessation screening fractional factorial experiment (Piper et al., 2016) evaluated the main and interactive effects of six intervention components selected to address the challenges smokers face during different phases of smoking treatment (Baker et al., 2011). This screening study is part of a program of research using MOST (Piper et al., 2016) to engineer an optimized smoking cessation treatment. There were no significant main effects on long-term (26 week) point-prevalence abstinence, but there were three significant two-way interactions. As an important step in the MOST approach to treatment development, the goal of this research was to explore the stability of the effects of multiple, relatively discrete intervention components. Despite the absence of main effects, some of the intervention components might be meaningfully effective in some subgroups of participants and this could guide the development of treatment algorithms. Examining moderation effects might also shed light on the unexpected finding of significant interaction effects amongst intervention components. Such moderation effects could also have theoretical value; they could provide information about individual risk factors (e.g., high tobacco dependence) that are especially addressed by different intervention components. Therefore, this research sought to determine whether easily assessable person factors (e.g., gender, race, education, psychiatric history) and smoking-related variables (e.g., dependence, smoking rate, living with a smoker) moderated the individual and joint effects of six smoking cessation intervention components.
2. METHODS
2.1 Procedure
This is a secondary data analysis of a fractional factorial screening experiment that assessed the effects of six smoking cessation intervention components on long-term abstinence (see Piper et al., 2016 for additional details including the CONSORT diagram). A total of 637 participants were recruited during primary care clinic visits and screened for eligibility: ≥18 years old; ≥5 cigarettes/day for the previous 6 months; motivated to quit; not currently taking bupropion or varenicline; agreeing to use only study medication for the duration of the study; no medical contraindications to NRT; no self-reported history of psychosis or bipolar disorder; and, for women of childbearing potential, agreeing to use an approved method of birth control during treatment. Eligible participants provided written informed consent, completed initial assessments, and received their interventions at their primary care clinic. A research database created intervention and assessment schedules, based on randomly assigned treatment conditions, which guided delivery of the interventions by bachelor’s level case managers supervised by licensed clinical psychologists.
2.2 Experimental Design
This experiment used a balanced fractional factorial design with six factors: 1) Prequit Nicotine Patch vs. None; 2) Prequit Nicotine Gum vs. None; 3) Preparation Counseling vs. None; 4) Intensive Cessation In-Person Counseling vs. Minimal; 5) Intensive Cessation Phone Counseling vs. Minimal; and 6) 16 vs. 8 Weeks of Combination NRT. These factors were chosen to address specific challenges that emerge early in the quit attempt, based on theory and extant research (Baker et al., 2011), and to be easily translated into real-world healthcare settings (Piper et al., 2016). The Resolution VI fractional factorial design reduced the number of conditions from 64 to 32 and allowed for the estimation of main effects and two-way interactions only (Collins et al., 2016; Piper et al., 2016). Randomization was stratified by gender and clinic. Staff were blinded to randomization until eligibility was confirmed; participants were blinded until consent was provided.
2.3 Experimental Factors
2.3.1 Prequit Nicotine Patch
Half the participants were assigned to the active condition and received 14-mg patches for the 3 weeks prior to the target quit day (TQD) while the other half did not receive prequit patches.
2.3.2 Prequit Nicotine Gum
Participants in the active condition received 2-mg nicotine gum for the 3 weeks prior to the TQD (≥9 pieces of gum/day, 1 piece/1–2 hours); the other half did not. Participants who received both Prequit Patch and Gum were told to use at least 5 pieces/day of gum, unless such use produced adverse effects.
2.3.3 Preparation Counseling
Participants in the active condition received three 20-minute counseling sessions prior to the TQD, focused on coping skills, reduction, and making practice quit attempts, while the other half of participants did not. The sessions 3 weeks and 1 week before the TQD (Weeks-3 and -1) were in-person, and the Week-2 session was over the phone.
2.3.4 In-Person Counseling
Participants in the intensive condition received three 20-minute face-to-face counseling sessions: one week pre-TQD, on the TQD, and at Week 1. Sessions focused on skill building and intra-treatment social support. Participants assigned to the minimal level received one 3-minute in-person session at Week-1.
2.3.5 Phone Counseling
Participants in the intensive condition received three 15-minute phone sessions (TQD, Days 2 and 10), focused on coping skills, avoiding smoking cues, and intra-treatment social support. Participants assigned to the minimal condition received one 10-minute session on the TQD. Thus, all participants received some TQD phone counseling.
2.3.6 Extended Medication
All participants received combination NRT (nicotine patch + nicotine gum) starting on their TQD. Half were assigned to receive 8 weeks of patches and 8 weeks of nicotine gum. The other half received 16 weeks of patches and 16 weeks of gum. Participants were advised to use one piece of gum every 1–2 hours until 2 weeks before treatment termination (Fiore et al., 2008), and at least 5 pieces/day unless such use produced adverse effects.
2.4 Assessments
Participants completed baseline assessments of demographics, smoking history, and tobacco dependence (Fagerström Test of Nicotine Dependence; FTND; Heatherton et al., 1991). Participants also reported whether they had ever been diagnosed with or treated for each of the following psychiatric conditions: depression, anxiety, bipolar disorder, attention-deficit disorder, panic, post-traumatic stress disorder, or schizophrenia. Follow-up calls assessed self-reported 7-day point-prevalence abstinence at 26 weeks post-TQD. Those missing data at the 26-week follow-up were considered to be smoking.
2.5 Analytic Plan
After characterizing the study population, we used cross-tab Pearson chi-square analyses to compare 26-week abstinence rates between: 1) men vs. women, 2) whites vs. non-whites, 3) those with a high school education or less vs. at least some college, 4) those with no self-reported psychiatric history vs. self-reported psychiatric history, 5) those smoking within 5 minutes of waking vs. delaying smoking for more than 5 minutes after waking, 6) those smoking at least 20 cigarettes per day vs. smoking less than 20 cigarettes per day, and 7) those living with a smoker vs. not living with a smoker.
We used two general linear models to examine the moderation of the 6 main effects and the 3 statistically significant 2-way interactions on 26-week point-prevalence abstinence. The first model examined potential person-factor moderators (i.e., gender, race, education, and psychiatric history) entered simultaneously. The second model examined smoking-focused variables (i.e., time to first cigarette, cigarettes per day, and living with a smoker) entered simultaneously. The person-factor and smoking-focused models each included: 1) the 6 intervention component main effects, 2) the interactions between potential moderators for that model and the intervention component main effects, 3) the three significant 2-way intervention component interactions; and 4) the three-way interactions between the potential moderators for that model and each of the 2-way intervention component interactions.
Although we did not control for family-wise error, we graphed the significant interaction effects with 95% confidence intervals for interpretation. It should be noted that non-overlapping 95% confidence intervals represent a very conservative test (i.e., p<.006) whereas 95% confidence intervals that overlap by up to 25% are still statistically significant (Cumming and Finch, 2005). These graphs allowed us to examine the effects of different intervention components by group as well as the effects of a single intervention component or factor level across groups. Finally, this study was powered to detect 2-way interactions between randomly assigned intervention components, but it was not powered to detect 2-way interactions between an intervention component and an individual difference variable or a 3-way interaction between two intervention components and an individual difference variable. All analyses were conducted using SPSS (IBM Corporation, 2013).
3. RESULTS
The mean 26-week point-prevalence abstinence rate of the 637 participants was 27.9%. Group differences in 26-week point-prevalence abstinence rates as a function of person factors are reported in Table 1. Men (compared with women) had statistically significantly higher 26-week abstinence rates, as did those with no self-reported psychiatric history (compared to those with such a history). It should be noted that one of the largest differences in abstinence rates (a 12 percentage point difference) was between white and non-white smokers, but was not statistically significant possibly due to the small sample of non-white smokers (n=85).
Table 1.
Group differences in 7-day point-prevalence abstinence rates at 26 weeks after the target quit day
| n | 26 week abstinence rate (%) | χ2 | p-value | |
|---|---|---|---|---|
| Total Sample | 637 | 27.9 | ||
| Men | 288 (45%) | 32.3 | 4.65 | 0.03 |
| Women | 346 (55%) | 24.6 | ||
| White | 552 (87%) | 38.3 | 0.21 | 0.65 |
| Non-white | 85 (13%) | 25.9 | ||
| High school or less | 262 (41%) | 26.3 | 0.59 | 0.44 |
| At least some college | 371 (59%) | 29.1 | ||
| No self-reported psychiatric history | 372 (59%) | 32.3 | 8.11 | 0.004 |
| Self-reported psychiatric history† | 264 (41%) | 22.0 | ||
| Attention Deficit Disorder | 38 (6%) | 26.3 | ||
| Anxiety Disorder | 126 (20%) | 20.6 | ||
| Bipolar Disorder | 6 (1%) | 16.7 | ||
| Depression | 210 (33%) | 19.5 | ||
| Panic Disorder | 48 (8%) | 8.3 | ||
| PTSD | 31 (5%) | 22.6 | ||
| Schizophrenia | 2 (.3%) | 0.0 | ||
| Smoke within 5 minutes of waking | 213 (34%) | 24.4 | 2.06 | 0.51 |
| Smoke > 5 minutes after waking | 419 (66%) | 29.8 | ||
| Smoke a pack or more a day | 310 (49%) | 24.8 | 3.03 | 0.08 |
| Smoke < 1 pack a day | 322 (51%) | 31.1 | ||
| Does not live with a smoker | 503 (79%) | 28.0 | 0.01 | 0.92 |
| Lives with a smoker | 134 (21%) | 27.6 |
The rates of individual reported diagnoses are a percentage of the total sample. Participants could endorse more than one diagnosis. The abstinence rates for specific diagnoses were not statistically compared with the no psychiatric history group due to the small sample sizes. It should be noted that during the initial telephone screen all participants reported that they did not have a history of psychosis or bipolar disorder, but at the initial visit 6 participants reported a history of bipolar disorder diagnosis or treatment and 2 reported a history of schizophrenia.
In the person-factor moderator model, there was a main effect of psychiatric history: participants reporting psychiatric history were significantly less likely to be abstinent (B=-.61, p=.01). Psychiatric history was the only statistically significant moderator; it moderated the main effects of three interventions (Preparation Counseling, In-Person Counseling, and Extended Medication) and it moderated two of the 2-way treatment interactions (In-Person x Phone Counseling and In-Person Counseling x Prequit Patch; see Table 2). When we examined the effects of a history of depression and anxiety separately, we found similar results: those with a history of depression and those with a history of anxiety had lower abstinence rates than those without such histories. A history of depression significantly moderated the In-Person Counseling x Prequit Patch (B=.57, p=.02) and the In-Person x Phone Counseling (B=.65, p=.01) effects and a history of an anxiety disorder or panic moderated the In-Person Counseling (B=-.92, p=.02) and the In-Person x Phone Counseling (B=.63, p=.03) effects.
Table 2.
Significant moderation effects
| No Psychiatric History | Psychiatric History | Moderation Effect | |||
|---|---|---|---|---|---|
| % Abstinent (95% CI) | % Abstinent (95% CI) | B | p-value | ||
| Preparation Counseling | Preparation Counseling | 38.2 (31.0–45.8) | 20.1 (13.8–27.8) | -.446 | .035 |
| No Preparation Counseling | 26.8 (20.7–33.6) | 24.0 (16.8–32.5) | |||
| In-Person Counseling | Intensive | 34.7 (27.7–42.2) | 19.0 (12.8–26.6) | -.495 | .038 |
| Minimal | 30.1 (23.8–37.0) | 25.2 (17.9–33.7) | |||
| Medication Duration | 8 weeks | 37.0 (30.1–44.2) | 17.0 (11.2–24.3) | .623 | .004 |
| 16 weeks | 27.2 (20.9–34.3) | 27.6 (20.0–36.4) | |||
| Phone x In-Person Counseling | Minimal Phone & Minimal In-Person | 19.1 (11.5–28.8) | 25.4 (15.8–37.1) | .636 | .003 |
| Intensive Phone & Minimal In-Person | 39.9 (30.0–49.2) | 25.0 (14.4–38.4) | |||
| Minimal Phone & Intensive In-Person | 40.2 (29.9–51.3) | 15.7 (8.1–26.4) | |||
| Intensive Phone & Intensive In-Person | 29.2 (20.1–39.8) | 22.4 (13.1–34.2) | |||
| In-Person Counseling x Prequit Patch | Minimal In-Person & No Prequit Patch | 29.8 (20.8–40.1) | 29.5 (18.5–42.6) | .561 | .014 |
| Minimal In-Person & Prequit Patch | 30.4 (21.7–40.3) | 21.2 (12.1–33.0) | |||
| Intensive In-Person & No Prequit Patch | 34.1 (24.2–45.2) | 7.5 (2.5–16.6) | |||
| Intensive In-Person & Prequit Patch | 35.2 (25.4–45.9) | 30.0 (19.6–42.1) | |||
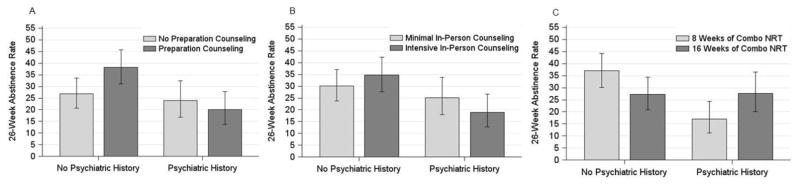
Preparation Counseling produced significantly higher 6-month abstinence rates amongst participants with no psychiatric history than amongst those with a psychiatric history (see Figure 1a). However, amongst participants with no psychiatric history, there was no significant benefit of such counseling, consistent with our conservative interpretation of their overlapping 95% confidence intervals. Intensive In-Person Counseling produced slightly higher abstinence rates than did Minimal In-Person Counseling amongst those with no history of psychiatric disorder (see Table 2 and Figure 1b). However, amongst those with psychiatric histories, Intensive In-Person Counseling produced lower abstinence rates than did Minimal In-Person Counseling (see Figure 1b). Inspection of abstinence rates across groups revealed that amongst participants receiving Intensive In-Person Counseling, those with no psychiatric history had significantly higher abstinence rates at 6-months than did those with such a history. Finally, psychiatric history interacted with Extended Medication (see Table 2 and Figure 1c). There was no significant difference in abstinence rates by medication duration within each psychiatric group. However, amongst participants who received combination NRT for 8 weeks, those with no history of psychiatric disorder reported abstinence rates 20 percentage points higher than did those with such histories. In comparison, psychiatric history was unrelated to abstinence amongst those getting medication for 16 weeks.
Figure 1. Treatment main effects on Week 26 abstinence rates moderated by psychiatric history. These graphs depict the mean Week 26 abstinence rates and 95% confidence intervals for each treatment group by the presence (n=264; 41%) or absence (n=372; 59%) of a psychiatric history.
Figure 1a. Week 26 abstinence rates for the psychiatric history x Preparation Counseling interaction
Figure 1b. Week 26 abstinence rates for the psychiatric history x In-Person Counseling interaction
Figure 1c. Week 26 abstinence rates for the psychiatric history x Medication Duration interaction
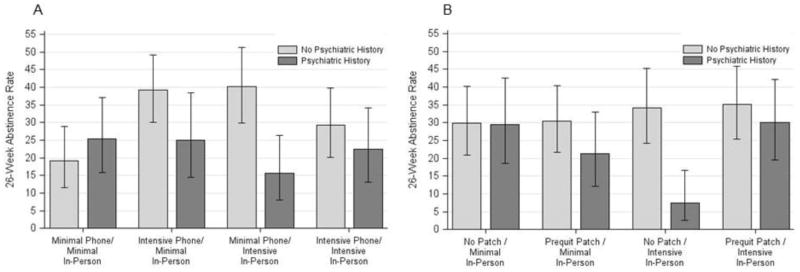
The In-Person x Phone Counseling interaction was also moderated by psychiatric history (see Table 2 and Figure 2a). Examination of the subgroups involved in this interaction shows significantly higher abstinence rates amongst participants without a history of psychiatric disorder if they received Minimal Phone and Intensive In-Person counseling or Intensive Phone and Minimal In-Person counseling, relative to those who received minimal levels of both types of cessation counseling. However, individuals receiving intensive levels of both types of cessation counseling did not differ from participants receiving minimal levels of both types of counseling. Amongst participants with histories of a psychiatric disorder, no combination of the two intervention components meaningfully enhanced abstinence rates (Figure 2a). In addition to differences in treatment response within each group, groups differed significantly in their response to Minimal Phone and Intensive In-Person counseling. Participants with a psychiatric history had significantly lower abstinence rates in response to that treatment combination than did those without a positive psychiatric history.
Figure 2. Treatment interaction effects on Week 26 abstinence rates moderated by psychiatric history. These graphs depict the mean Week 26 abstinence rates and 95% confidence intervals for each combination of treatments by the presence (n=264; 41%) or absence (n=372; 59%) of a psychiatric history.
Figure 2a. Week 26 abstinence rates for the psychiatric history x Phone Counseling x In-Person Counseling interaction
Figure 2b. Week 26 abstinence rates for the psychiatric history x Prequit Patch x In-Person Counseling interaction
Psychiatric history also moderated the In-Person x Prequit Patch interaction (see Table 2 and Figure 2b). The use of Prequit Patch did not affect response to Minimal or Intensive Counseling among participants with no history of psychiatric disorders. Participants with a psychiatric history responded significantly better to Intensive In-Person counseling (i.e., had higher abstinence rates) if they also received the Prequit Patch than if they did not. However, if such participants received Prequit Patch, Intensive In-Person Counseling did not significantly improve abstinence rates beyond those produced by Minimal Counseling.
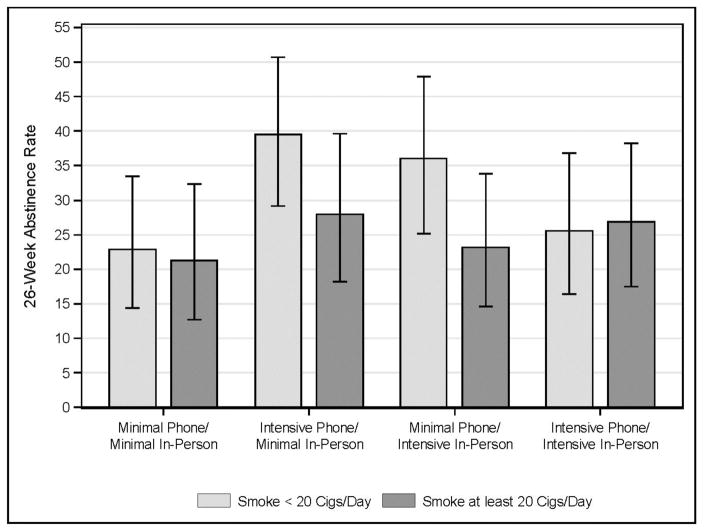
The smoking-focused moderators model revealed only one statistically significant effect: a cigarettes per day x In-Person Counseling x Phone Counseling interaction (B=.39, p=.047). Participants who smoked 20 cigarettes per day or more attained similar abstinence rates regardless of the in-person or phone counseling they received (see Figure 3). However, amongst lighter smokers, the best results were obtained with the use of either type of intensive counseling intervention, but not both. None of these simple effects was statistically significant as all had considerable overlap of confidence intervals (interpreting the confidence intervals in a conservative manner: Cumming and Finch, 2005).
Figure 3.
Week 26 abstinence rates for the interaction of heavy smoking (smoking >20 cigs/day) x Phone Counseling x In-Person Counseling. This graph depicts the mean Week 26 abstinence rates and 95% confidence intervals for each treatment combination by the amount of cigarettes smoked.
4. DISCUSSION
This research analyzed data from a fractional factorial screening experiment of smokers attending primary care clinics who were willing to make a quit attempt. The factorial nature of the study design permitted examination of whether participant characteristics moderated the effects of six discrete intervention components, either separately or in combination, on long-term cessation. The main and interaction effects of the six intervention components on 26-week abstinence rates were not moderated by gender, race, education, time to first cigarette, or living with a smoker. This suggests that the effects of these intervention components are somewhat robust with respect to these five participant characteristics. However, both psychiatric history and baseline number of cigarettes smoked per day moderated the effects of some intervention components.
A history of psychiatric disorder (reflected by self-reporting either diagnosis or treatment for depression, anxiety, bipolar disorder, attention-deficit disorder, panic, post-traumatic stress disorder, schizophrenia, or any combination of these disorders) predicted lower abstinence rates and interacted with both counseling and medication intervention components. It should be noted that only 3% of participants reported serious mental illness (i.e., schizophrenia or bipolar disorder). The vast majority of participants with a psychiatric history reported depression and/or anxiety disorders. Given the large disparity in smoking prevalence between those with and without psychiatric disorders (Grant, et al., 2004; Lasser et al., 2000), it is vital to understand how psychiatric history may influence response to smoking cessation interventions.
With regard to medication, the moderation analyses revealed a cross-over interaction; participants with histories of a psychiatric disorder responded somewhat better to 16-weeks of combination NRT than to 8 weeks, while participants without such histories responded somewhat better to 8 weeks of combination NRT (Figure 4). It is unclear why extended medication would increase abstinence rates only in individuals with a psychiatric history. It is possible that extended NRT is problematic because the use of prn medication (i.e., the nicotine gum) might elicit smoking motivation because acute doses of nicotine could produce priming effects (LeSage et al., 2004; Shaham et al., 2003) or because extended medication might sustain physical dependence. However, it is hard to see why these effects would not similarly, adversely affect participants with psychiatric histories. The benefits of extended medication displayed by patients with histories of psychopathology seem more comprehensible. For instance, psychiatric disorders such as depression, schizophrenia, and PTSD often are associated with high levels of negative affect and anhedonia (Kashdan et al., 2006; Treadway and Zald, 2013), which are also withdrawal symptoms (Cook et al., 2015), and, as such, can be ameliorated by nicotine (Chaudhri et al., 2006; Cook et al., 2015; Markou and Paterson, 2009). While such withdrawal symptoms tend to peak relatively early in the quit attempt, they can also be elicited by nicotine-related cues (e.g., Kenny and Markou, 2005). Further, smokers with psychiatric histories are more likely to use cigarettes for instrumental purposes, such as coping with negative affect, which may or may not be withdrawal related (Piper et al., 2010a). It may be that extended access to prn medication that can substitute for smoking as a coping response to negative affect may facilitate long-term cessation. This moderation effect may explain why studies have not consistently found that extended NRT is efficacious in broad groups of smokers (e.g., Carpenter et al., 2013; Fiore et al., 2008; Schnoll et al., 2015; Stead et al., 2012).
Psychiatric history also moderated response to several counseling intervention components (i.e., Preparation, In-Person, and Phone Counseling). While more intensive counseling, at least in some combinations, enhanced abstinence rates in participants with no psychiatric history, it failed to do so for those with psychiatric histories. For instance, for participants with no psychiatric history, either Intensive Phone or Intensive In-Person Counseling produced 6-month abstinence rates that were approximately double those produced by Minimal Phone and Minimal In-Person Counseling (Figure 2a). These findings are consistent with a prior study of extended cognitive behavioral therapy that found that participants with a history of depression had higher abstinence rates if they received less intensive phone counseling (Killen et al., 2008). Also, research has found that smokers with elevated negative affect receive little benefit from skill training counseling; however, such smokers do benefit from supportive counseling that comprises little content or training (Zelman et al., 1992). Both the Preparation and In-Person Counseling comprised considerable training and content related to recognition of danger situations, planning daily activities, and coping response acquisition and practice. It is possible that the demands of such counseling outweighed its potential benefits. It is also important to note that the relation between negative affect and counseling content has not been found consistently (Mermelstein et al., 2003). In fact, some types of counseling have been found to be effective for smokers with psychiatric histories such as mood management counseling for depressed smokers (Hall et al., 1994, 2006; van der Meer et al., 2013). However, it is clear that the counseling approaches studied here were not particularly effective in addressing the disparity between those with and without a psychiatric history. Further, in this study counseling ended at 2 weeks post-quit; perhaps extended counseling would benefit such smokers (cf. Hall et al., 2006). Of course, the need for longer counseling would not explain the detrimental effects of more intense early counseling in smokers with a psychiatric history. Finally, integrating smoking treatment with psychiatric care, versus delivering it in primary care, might improve its effectiveness for smokers who are currently being treated for psychiatric disorders (McFall et al., 2005).
In sum, these results suggest that almost half of smokers who present for smoking cessation treatment at their primary care clinic (i.e., those with a psychiatric history) may not be helped by counseling that emphasizes skill training and that occurs shortly before, or during, a quit attempt. Further research is needed to determine the robustness of this effect, and whether this finding is related to counseling content (e.g., support vs. skills training), intensity (amount of contact), timing (3 sessions during the first 2 weeks of a quit attempt vs. sessions spread out over a longer period of time), or other factors. Future research is also needed to explore whether the observed moderating relations are due to a broad personality trait such as neuroticism that is shared by many psychiatric disorders (Leventhal, et al., 2013; Leventhal and Zvolensky, 2015) or to diagnosis-specific factors.
Participants were recruited and treatment was provided in primary care settings (versus recruited via media for an efficacy study), enhancing the generalizability and translation of these finding. In addition, the magnitude of the relations between psychiatric history and treatment effects (see Figures 1 and 2) and the fact that psychiatric status appeared to similarly modulate the effects of the intensity of several different types of counseling suggests the robustness of the relations. However, these results need to be interpreted within the context of certain limitations. First, the factorial design is efficient, but there was limited power to detect interactions with some person and smoking factors that were highly unevenly distributed (e.g., there were only 85 non-white participants; 13% of the sample). Second, these were exploratory analyses, and we did not control for experiment-wise error. These exploratory findings suggest hypotheses but do not confirm them; thus, these findings must be considered tentative in the absence of replication. In this regard, it is important to recognize that only a small number of moderating effects were detected amongst numerous ones analyzed. Third, variables correlated with the nonrandomly assigned person factors (i.e., 3rd variables) or sampling error may have affected the observed moderating effects. Fourth, we were not able to examine how recently participants were diagnosed or treated for psychiatric disorders nor were we able to tease apart the effects of each diagnosis. Fifth, the effects of the counseling and medication interventions cannot be generalized with any confidence to other sorts of smoking interventions (e.g., non-NRT medications or counseling interventions of different contents or intensities). Sixth, this paper concentrated on person characteristics that moderated treatment effects. It did not explore characteristics such as gender, which was significantly related to 6-month abstinence (32.3% vs. 24.6% abstinent in men and women, respectively), but that did not interact with treatment. This gender difference in outcomes emerged late in follow-up (after 16 weeks), suggesting that women especially may need extended treatment (e.g., McKee et al., 2016; Piper et al., 2010b). Finally, in studying interactions, we evaluated interactions with respect to the multiplicative scale associated with logistic regression; different results might be obtained on an additive scale (VanderWeele and Knol, 2014).
In conclusion, factorial screening experiments permit testing of differential response to individual intervention components and combinations of components. This research demonstrates that psychiatric history significantly moderates the effect of both medication duration and counseling intervention components; smokers with a history of psychiatric disorder appear to benefit especially from extended combination NRT but not from intensive in-person counseling with an emphasis on skill building. These findings illustrate the importance of examining individual intervention components of smoking cessation treatment to determine whether the components are effective for all smokers. Results of such analyses may suggest strategies for personalizing smoking treatment.
Highlights.
Factorial experiments allow researchers to examine how person-factors influence response to individual intervention components.
Self-reported history of psychiatric diagnoses or treatment affected response to smoking cessation counseling treatments and to the duration of combination nicotine replacement therapy.
Participants who smoked more than a pack of cigarettes per day had a poorer response to smoking cessation counseling interventions.
Acknowledgments
Role of Funding Source: This research was supported by grants 9P50CA143188 and 1K05CA139871 from the National Cancer Institute to the University of Wisconsin Center for Tobacco Research and Intervention and by the Wisconsin Partnership Program. Dr. Collins is also supported by NIH grants P50DA10075, R01DK097364, and R01AA022931. This work was carried out in part while Dr. Schlam was a Primary Care Research Fellow supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine. Dr. Cook is also supported by Merit Review Award 101CX00056 from the US Department of Veterans Affairs. Dr. Loh is also supported by NSF grant DMS-1305725.
Footnotes
Contributors: All authors listed have seen, approved, and contributed to the manuscript. All authors conceived the research idea, MEP conducted the analyses, MEP and TBB wrote the manuscript, and all authors provided interpretation and editorial comments.
Conflict of Interest: No conflict declared.
Clinical Trial Registration: NCT01116986
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, Christiansen BA, Schlam TR, Cook JW, Fiore MC. New methods for tobacco dependence treatment research. Ann Behav Med. 2011;41:192–207. doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Jardin BF, Burris JL, Mathew AR, Schnoll RA, Rigotti NA, Cummings KM. Clinical strategies to enhance the efficacy of nicotine replacement therapy for smoking cessation: a review of the literature. Drugs. 2013;73:407. doi: 10.1007/s40265-013-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: current cigarette smoking among adults aged >/=18 years with mental illness - United States, 2009–2011. MMWR. 2013;62:81–87. [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Collins LM, Kugler KC, Gwadz MV. Optimization of multicomponent behavioral and biobehavioral interventions for the prevention and treatment of HIV/AIDS. AIDS Behav. 2016;20(Suppl 1):S197–214. doi: 10.1007/s10461-015-1145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, Nair VN, Strecher VJ. A strategy for optimizing and evaluating behavioral interventions. Ann Behav Med. 2005;30:65–73. doi: 10.1207/s15324796abm3001_8. [DOI] [PubMed] [Google Scholar]
- Cook JW, Piper ME, Leventhal AM, Schlam TR, Fiore MC, Baker TB. Anhedonia as a component of the tobacco withdrawal syndrome. J Abnorm Psychol. 2015;124:215–225. doi: 10.1037/abn0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming G, Finch S. Inference by eye: confidence intervals and how to read pictures of data. Am Psychol. 2005;60:170–180. doi: 10.1037/0003-066X.60.2.170. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, … Wewers ME. Treating tobacco use and dependence: 2008 update. 2008 Retrieved from http://bphc.hrsa.gov/buckets/treatingtobacco.pdf.
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Hall SM, Munoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62:141–146. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- Hall SM, Tsoh JY, Prochaska JJ, Eisendrath S, Rossi JS, Redding CA, Rosen AB, Meisner M, Humfleet G, Gorecki JA. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96:1808–1814. doi: 10.2105/AJPH.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. An updated algorithm for choosing among smoking cessation treatments. J Subst Abuse Treat. 2013;45:215–221. doi: 10.1016/j.jsat.2013.01.011. [DOI] [PubMed] [Google Scholar]
- IBM Corporation. IBM SPSS Statistics for Windows, Version 22.0. IBM Corporation; Armonk, NY: 2013. [Google Scholar]
- Jamal A, Agaku IT, O’Connor E, King BA, Kenemer JB, Neff L. Current cigarette smoking among adults - United States, 2005–2013. MMWR. 2014;63:1108–1112. [PMC free article] [PubMed] [Google Scholar]
- Kashdan TB, Barrios V, Forsyth JP, Steger MF. Experiential avoidance as a generalized psychological vulnerability: comparisons with coping and emotion regulation strategies. Behav Res Ther. 2006;44:1301–1320. doi: 10.1016/j.brat.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci. 2005;25:6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg AF, Arredondo C, Murphy G, Hayward C, Celio M, Cromp D, Fong D, Pandurangi M. Extended cognitive behavior therapy for cigarette smoking cessation. Addiction. 2008;103:1381–1390. doi: 10.1111/j.1360-0443.2008.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–513. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, Langdon KJ. Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug Alcohol Depend. 2013;133:324–329. doi: 10.1016/j.drugalcdep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: a transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychol Bull. 2015;141:176–212. doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh WY, Piper ME, Schlam TR, Fiore MC, Smith SS, Jorenby DE, Cook JW, Bolt DM, Baker TB. Should all smokers use combination smoking cessation pharmacotherapy? Using novel analytic methods to detect differential treatment effects over 8 weeks of pharmacotherapy. Nicotine Tob Res. 2012;14:131–141. doi: 10.1093/ntr/ntr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Paterson NE. Multiple motivational forces contribute to nicotine dependence: Nebraska Symposium on Motivation. In: Bevins R, Caggiula AR, editors. The Motivational Impact Of Nicotine And Its Role In Tobacco Use. Vol. 55. New York: Springer Science and Business Media, LLC; 2009. pp. 65–90. [DOI] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Thompson CE, Yoshimoto D, Malte C, Straits-Troster K, Kanter E, Zhou XJ, Dougherty CM, Steele B. Improving the rates of quitting smoking for veterans with posttraumatic stress disorder. Am J Psychiatry. 2005;162:1311–1319. doi: 10.1176/appi.ajp.162.7.1311. [DOI] [PubMed] [Google Scholar]
- McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH. Sex differences in varenicline efficacy for smoking cessation: a meta-analysis. Nicotine Tob Res. 2016;18:1002–1011. doi: 10.1093/ntr/ntv207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein R, Hedeker D, Wong SC. Extended telephone counseling for smoking cessation: Does content matter? J Consult Clin Psychol. 2003;71:565–574. doi: 10.1037/0022-006x.71.3.565. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, Loh WY. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010b;12:647–657. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, Mermelstein R, Schlam TR, Cook JW, Jorenby DE, Loh WY, Baker TB. Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction. 2016;111:129–141. doi: 10.1111/add.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, Brown JL, Leitzke CJ, Zehner ME, Fiore MC, Baker TB. Psychiatric disorders in smokers seeking treatment for tobacco dependence: relations with tobacco dependence and cessation. J Consult Clin Psychol. 2010a;78:13–23. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, Gariti P, Wileyto EP, Hitsman B. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504–511. doi: 10.1001/jamainternmed.2014.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Curr Dir Psychol Sci. 2013;22:244–249. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The health consequences of smoking: 50 years of progress. a report of the Surgeon General. 2014 Retrieved from http://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf.
- van der Meer RM, Willemsen MC, Smit F, Cuijpers P. Smoking cessation interventions for smokers with current or past depression. Cochrane Database Syst Rev. 2013:CD006102. doi: 10.1002/14651858.CD006102.pub2. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Method. 2014;3:33–72. [Google Scholar]
- West R, Raw M, McNeill A, Stead L, Aveyard P, Bitton J, Stapleton J, McRobbie H, Pokhrel S, Lester-George A, Borland R. Health-care interventions to promote and assist tobacco cessation: a review of efficacy, effectiveness and affordability for use in national guideline development. Addiction. 2015;110:1388–1403. doi: 10.1111/add.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelman DC, Brandon TH, Jorenby DE, Baker TB. Measures of affect and nicotine dependence predict differential response to smoking cessation treatments. J Consult Clin Psychol. 1992;60:943–952. doi: 10.1037//0022-006x.60.6.943. [DOI] [PubMed] [Google Scholar]