High-frequency neuromuscular electrical stimulation (HF NMES) induces muscular contractions that partially match physiological motor control. Here, we tested whether HF NMES applied to the upper limb influences interhemispheric inhibition. Our results show that interhemispheric inhibition was increased after HF NMES and that this increase was correlated to the electromyographic activity within the contralateral homologous muscle. This opens up original perspectives for the implementation of HF NMES in sport training and neurorehabilitation.
Keywords: corticospinal excitability, upper limb
Abstract
High-frequency neuromuscular electrical stimulation (HF NMES) induces muscular contractions through neural mechanisms that partially match physiological motor control. Indeed, a portion of the contraction arises from central mechanisms, whereby spinal motoneurons are recruited through the evoked sensory volley. However, the involvement of supraspinal centers of motor control during such stimulation remains poorly understood. Therefore, we tested whether a single HF NMES session applied to the upper limb influences interhemispheric inhibition (IHI) from left to right motor cortex (M1). Using noninvasive electrophysiology and transcranial magnetic stimulation, we evaluated the effects of a 10-min HF NMES session applied to a right wrist flexor on spinal and corticospinal excitability of both arms, as well as IHI, in healthy subjects. HF NMES induced a rapid decline in spinal excitability on the right stimulated side that closely matched the modulation of evoked force during the protocol. More importantly, IHI was significantly increased by HF NMES, and this increase was correlated to the electromyographic activity within the contralateral homologous muscle. Our study highlights a new neurophysiological mechanism, suggesting that HF NMES has an effect on the excitability of the transcallosal pathway probably to regulate the lateralization of the motor output. The data suggest that HF NMES can modify the hemispheric balance between both M1 areas. These findings provide important novel perspectives for the implementation of HF NMES in sport training and neurorehabilitation.
NEW & NOTEWORTHY High-frequency neuromuscular electrical stimulation (HF NMES) induces muscular contractions that partially match physiological motor control. Here, we tested whether HF NMES applied to the upper limb influences interhemispheric inhibition. Our results show that interhemispheric inhibition was increased after HF NMES and that this increase was correlated to the electromyographic activity within the contralateral homologous muscle. This opens up original perspectives for the implementation of HF NMES in sport training and neurorehabilitation.
although neuromuscular electrical stimulation (NMES) has been useful in neuromuscular research as well as sport and rehabilitation for decades (Maffiuletti and Hortobagyi 2011; Maffiuletti et al. 2013), it is only recently that methods of stimulation have been developed with the ability of inducing muscular contractions through neurophysiological mechanisms that mirror physiological motor control. Whereas early protocols triggered contractions through peripheral mechanisms of the neuromuscular system, recent methods of stimulation have implicated both peripheral and central pathways (Collins 2007). Indeed, conventional NMES with narrow stimulus pulses (<400 μs) delivered at low frequencies (15–40 Hz) and high current intensities (Hainaut and Duchateau 1992) generates contractions through the activation of motor axons and induces large antidromic transmissions (Bergquist et al. 2011a). This produces a nonphysiological motor unit recruitment leading to high metabolic demands (Theurel et al. 2007) and a high level of muscular fatigue that may limit the use of NMES in rehabilitation (Gregory and Bickel 2005). Alternatively, when adopting wider pulse widths (e.g., 1 ms) and higher frequencies (e.g., 80–100 Hz) of NMES, a portion of the contraction arises from central mechanisms, whereby spinal motoneurons are recruited through the evoked sensory volley (Collins et al. 2001, 2002). Long pulse durations (0.5–1 ms) favor the recruitment of sensory axons (Kiernan et al. 2004), and low current intensities used in high-frequency (HF) NMES minimize the antidromic collision allowing orthodromically transmitted signals to descend from spinal circuits (Collins 2007; Dean et al. 2007). Various studies have thus shown that HF NMES produces modulations in spinal excitability and asynchronous discharge of motor units (Bergquist et al. 2011b; Klakowicz et al. 2006; Wegrzyk et al. 2015), thus highlighting a central contribution for these kinds of electrically induced contractions.
However, the involvement of supraspinal neural networks during HF NMES is not well known. Several studies using transcranial magnetic stimulation (TMS) have suggested that HF NMES can change corticospinal excitability (Chipchase et al. 2011a; Mang et al. 2010, 2011; Myata and Usuda 2015). In the present study, we were interested in understanding if the hemispheric balance between the primary motor cortices (M1) contralateral and ipsilateral to a stimulated muscle can be modified through the application of HF NMES. Indeed, most electrophysiological studies have focused on the contralateral corticospinal system without paying attention to interhemispheric interactions, despite the fact that the efficient control of movement and interlimb coordination both rely upon interhemispheric neural networks, capable of lateralizing the motor output (Swinnen 2002). Balanced interhemispheric interactions between both M1 areas are crucial to the generation of proper voluntary movements (Carson 2005; Ferbert et al. 1992). For instance, during unilateral hand actions, the active M1, contralateral to the moving hand, exerts an inhibitory influence on the ipsilateral M1, likely to prevent mirror activity (Ferbert et al. 1992; Giovanelli et al. 2009; Mochizuki et al. 2004; Morishita et al. 2012). Interhemispheric inhibition (IHI) thus refers to the neurophysiological mechanism by which one M1 inhibits the opposite one, and arises from pyramidal neurons in one M1 area projecting, via the corpus callosum, on GABAergic inhibitory interneurons in the other M1 area, which in turn modulates the excitability of pyramidal neurons in the same hemisphere (Ferbert et al. 1992).
In this study we investigated whether a single HF NMES session applied to the right upper arm influences the hemispheric balance between the two M1 areas. We evaluated spinal and corticospinal excitabilities of both arms using TMS, as well as the IHI between the two M1 in healthy subjects who underwent a NMES training of a right wrist flexor. We hypothesized that, in addition to modulating spinal excitability, HF NMES would influence supraspinal centers of motor control and change IHI. Interestingly, it is known that IHI is modulated through diverse modes of activation of the neuromuscular system. For instance, short sessions of unilateral voluntary contractions have specific and transitory effects on hemispheric balance (Avanzino et al. 2014a; Perez et al. 2007), as well as proprioceptive stimulation through muscle vibration (Avanzino et al. 2014b; Swayne et al. 2006). Indeed, Swayne et al. (2006) showed that IHI is increased during muscle vibration, and Avanzino et al. (2014) demonstrated that muscle vibration prevents the IHI modulation that is normally observed after hours of arm immobilization. However, there is still no experimental evidence that a protocol using HF NMES can induce a lasting effect on IHI.
Beyond its theoretical relevance, this topic is also of great functional significance, since it may help us better understand performance gains induced through electrical stimulation methods, e.g., the cross-education phenomenon (Hortobàgyi et al. 1999), and may help designing rehabilitation techniques for patients with abnormal interhemispheric balance (Murase 2004), e.g., by targeting abnormal interhemispheric communication from the intact to the lesioned hemisphere in stroke patients (Hummel and Cohen 2006).
METHODS
Participants
Ten right-handed healthy males from 19 to 45 yr old (average of 30.5 ± 4.2 yr) volunteered to participate in the study. All were volunteers and did not present neurological or psychiatric disorders and had no family history of epilepsy. Participants gave their informed consent to the experimental procedures, which were approved by the Regional Ethics Committee. The study was performed in accordance with the Declaration of Helsinki.
General Experimental Design
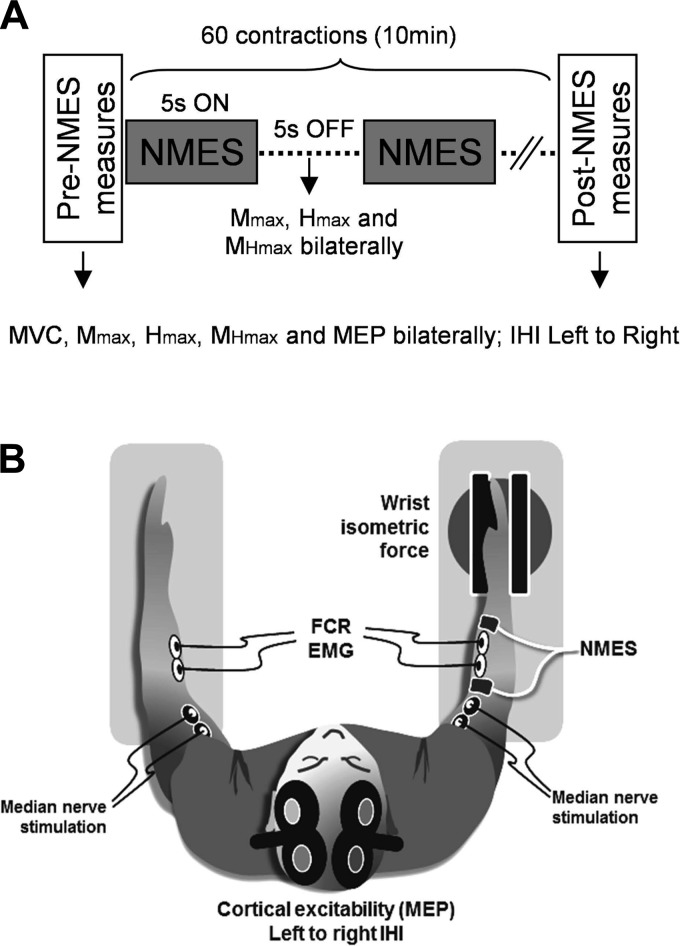
The experimental protocol and setup are shown in Fig. 1. Participants underwent a single session of HF NMES applied to the right flexor carpi radialis (FCR). Mechanical and neurophysiological parameters from the right and left FCR were measured before, during, and immediately after the intervention (see details below). The experiment lasted ∼1.5 h, with participants receiving adequate break periods when necessary.
Fig. 1.
Experimental set up. A: schematic representation of the timeline of events during the experimental sessions. Neuromuscular electrical stimulation (NMES) was applied to the right flexor carpi radialis (FCR) with a 5 s on/5 s off duty cycle for 60 contractions. Black arrows before, during, and after the NMES session indicate the recording times of the different variables. B: illustration of the subject's position and equipment during the NMES session (see methods for details).
Neurophysiological Measurements
Force recordings.
Subjects were seated comfortably in an armchair with both arms flexed at the elbow by ∼90° and the wrists in a neutral position (palms facing one another). The right forearm was attached to a dynamometer (Biodex, Shirley, NY), which measured the force (torque signal) exerted by the subjects during isometric wrist flexion (Fig. 1B). The motor axis was aligned with the styloid process of the ulna. Particular care was taken in monitoring subjects' posture and head position to maintain constant corticovestibular influences on spinal excitability during the experiment (Schieppati 1987). The trunk was stabilized using a shoulder harness, and head rotations and tilt were avoided by using a neck brace fastened to the seat. The force signal was digitized online (sampling frequency 5 kHz) and stored for analysis in TIDA software (Heka Elektonik, Lambrecht/Pfalz, Germany). After a warm-up of three submaximal contractions of the wrist flexors, subjects had to perform two maximal voluntary isometric contractions (MVC), the average of which constituted their pre-NMES values. They also had to repeat one MVC immediately after the NMES session, resulting in a post-NMES value. Both right and left MVC were assessed before and after NMES. For the left MVC the Biodex armchair was rotated so as to attach the left forearm to the dynamometer. The force signal was also used to track the NMES-induced force at the right wrist level during the protocol (see Data Collection and Analysis).
Electromyographic recordings.
After the skin was shaved and cleaned, two silver chloride surface electrodes (8 mm diameter) were positioned side by side on both the right and left FCR. The two electrodes were centered over the muscle belly at 1/3 of the distance from the medial epicondyle to the radial styloid. A common reference electrode was placed over the medial epicondyle of the left arm. EMG signals were amplified with a bandwidth frequency ranging from 15 to 5 kHz (gain = 1,000), then digitized online (sampling frequency: 5 kHz), and stored for offline analysis using the Tida software (Heka Elektonik).
H and M waves.
H reflex responses were quantified to explore spinal contributions during the evoked contractions, whereas M wave provided information on peripheral muscle mechanisms. Median nerve was stimulated via a rectangular pulse (1 ms width) delivered by a Digitimer stimulator (model DS7; Hertfordshire, UK). Two silver chloride surface electrodes were positioned in line with the nerve in the cubital fossa with the cathode ∼2.5 cm proximal to the anode. The best stimulation site to obtain a FCR H reflex was first located by a small hand-held cathode ball electrode. Once determined, the stimulation electrodes were fixed with straps. To determine the stimulation intensity to evoke maximal H reflex (Hmax) and maximal M wave (Mmax), the intensity was progressively increased (0.5-mA steps) from H reflex threshold to Mmax. The same procedure was applied for both arms. Stimulation intensities ranged from 1.2 to 17.0 mA and from 1.0 to 18.0 mA for left and right Hmax, respectively, and ranged from 2.3 to 22.0 mA and from 2.4 and 20.0 mA for the left and right Mmax, respectively.
Transcranial magnetic stimulation.
TMS was delivered to the optimal scalp position for activation of the FCR muscles overlying left (and right) hand M1. Motor evoked potentials (MEP) were elicited by magnetic stimuli delivered from a Magstim 200 stimulator (Magstim) through a figure-eight coil (loop diameter, 8 cm) with a monophasic current waveform. The coil was held tangential to the scalp with the handle pointing backward and 45° away from the midline (see Fig. 1B) to activate the corticospinal system preferentially trans-synaptically via horizontal corticocortical connections (Di Lazzaro et al. 2004). Optimal stimulation site for FCR activation was identified by moving the coil in 0.5-cm steps around the presumed motor hand area and marked on a bathing cap worn by subjects. The resting motor threshold (RMT) was defined as the lowest intensity of TMS output required to evoke FCR MEP >50 μV in peak-to-peak amplitude in at least three of five consecutive trials (Rossini et al. 1994). Measures of motor cortical excitability included MEP amplitudes of the right and left FCR and interhemispheric inhibition (IHI) from left to right M1. One coil per hemisphere was used and were secured and oriented using lockable articulated arms to ensure that the same area of M1 was stimulated through the experiment.
For the right and left MEP, M1 was stimulated at 120% of RMT. To study IHI we used a randomized conditioning-test design (Ferbert et al. 1992). A suprathreshold-conditioning stimulus (CS) was given to the left M1 10 ms before a test stimulus (TS) delivered to the right M1. In all testing, the TS was maintained to produce a 0.3- to 0.4-mV MEP amplitude, whereas the CS was set at 120% of RMT (Perez and Cohen 2008). A 10-ms interstimulus interval and the conditioning and test intensities were chosen because they have been reported to be effective for studying IHI (Ni et al. 2009). Stimuli were randomly delivered in sets of 20 trials: 10 conditioned (TScond) and 10 unconditioned (TS); one set was performed before NMES and one set after NMES. IHI was expressed as a ratio between the mean peak-to-peak MEP amplitude in TScond vs. TS (see Data Collection and Analysis, hereafter).
Neuromuscular Electrical Stimulation
The NMES session consisted of 60 evoked contractions with on/off periods of 5 s each (Fig. 1A). This duty cycle was chosen because it can be easily implemented in rehabilitation (Dirks et al. 2015). Short-duration contractions were preferred to prevent central force development during evoked contractions (Collins et al. 2001; Dean et al. 2007) and limit muscular fatigue (Neyroud et al. 2014). The evoked contractions induced an isometric wrist flexion at an initial force level of 15% of the MVC. Stimulation intensity was set before the NMES session to evoke a force level corresponding to 15% MVC for a 1-s train (by matching a line on a computer screen). The intensity was kept constant throughout the protocol. A low-force target was chosen from earlier studies to minimize antidromic block (Bergquist et al. 2011; Collins et al. 2002).
Electrical stimuli were delivered to the right FCR using a high-voltage (maximal voltage 400 V) constant-current stimulator (model DS7AH). Two large electrodes (10 × 5 cm; Compex, Ecublens, Switzerland) were placed over the FCR. Current characteristics were 100 Hz frequency/1 ms pulse. The left arm was freely resting on an armrest, and subjects were carefully instructed to keep it completely relaxed during the protocol (left FCR EMG was constantly checked by an experimenter).
Careful attention was paid to electrode placement, since both recording (EMG signal) and stimulation (NMES train) electrodes were positioned over the same muscle in the right stimulated arm (Fig. 1B). Stimulation electrodes were placed over the right FCR. The centers of the proximal anode and the distal cathode were placed ∼5 and ∼12 cm, respectively, distal to the crease of the cubital fossa, with an interelectrode distance of 7 cm. We considered the placement of the stimulation electrode as optimal when clear wrist flexions where observed during the setting of NMES intensity. EMG recording electrodes were thus placed as accurately as possible over the flexor carpi radialis muscle given the location of the stimulating electrodes. Anatomical differences between subjects warranted small variations in stimulating and recording electrode placement. This setup was adapted from Baldwin et al. (2006), since it allows muscle stimulation and the recording of neurophysiological signals from the same muscle.
Data Collection and Analysis
Pre- and post-NMES measures.
MVC force was considered as the highest force attained during maximal muscular contraction. Pre- to post-NMES variations of MVC torque were considered as an index of global muscle fatigue (Burnley et al. 2012). Hmax and Mmax amplitudes of both right and left FCR were recorded and averaged from two stimuli pre- and post-NMES. M waves at Hmax (MatHmax) were quantified from the same traces pre- and post-NMES. Variations in MatHmax amplitude would be used to identify any modulation in nerve stimulation intensity (Grosprêtre and Martin 2012). Ten MEPs pre- and post-NMES were recorded from both right and left FCR and averaged offline. For each neurophysiological measure we considered the peak-to-peak amplitude of the EMG response. Trials in which MEPs were larger than two SDs, and those where muscle activity exceeded 100 μV during the 100 ms before the TMS pulse were considered as outliers (see Methods in Gueugneau et al. 2015). On average 0.9 (SE: 1.2) out of 10 MEP were excluded. To reduce intersubject variability and allow reliable pre to post comparisons, MEP, Hmax, and MatHmax were expressed as a percentage of Mmax (Lackmy and Marchand-Pauvert 2010; Palmieri et al. 2004). IHI pre- and post-NMES was expressed as a percentage of TS and quantified as follows: IHI%ofTS = (TScond/TS) × 100. Thus, high and low values indicate weak and strong IHI, respectively. To better present the data graphically, IHI was also quantified using the following formula: IHI = [(TScond − TS)/TS] × 100, which directly indicates a percentage of decrease from TS.
The recording order of pre-NMES measures was randomized, whereas postmeasures were fixed due to methodological constraints. Particularly, the TMS coil's position had to be carefully controlled before the NMES session ended to avoid any delay between the end of NMES and post measures. Briefly, after the pre measures few TMS pulses were given before the NMES session to ensure reliable coil positions. Just following the NMES session, IHI, MEP (conditioned and nonconditioned MEP were applied randomly), Hmax (and MatHmax), Mmax, and MVC were measured in this order so that TMS variables could be recorded without interruption after NMES. Post measures lasted ∼4 min.
Measures during NMES.
Isometric force at the wrist level for each evoked contraction was assessed by quantifying the force-time integral (FTI), defined as the integrated area under each torque-time curve during the 5 s evoked contraction (in N·m·s). FTI for each evoked contraction was averaged across subjects and expressed both as a percentage of the baseline level (1st contraction FTI) and as raw values. Hmax, MatHmax, and Mmax were measured during the off periods from both the right and left FCR. For the sake of participants' comfort, these parameters were recorded alternatively every two contractions. Participants indeed received only one nerve stimulation per side for each off period, e.g., Hmax (and MatHmax) of the right FCR and Mmax of the left FCR after the first NMES train, vice versa after the second NMES train, and so on (see Fig. 1A). The starting order (right or left) of the neuromuscular parameters was randomly determined between participants. The timing of nerve stimulation was randomized (between 1.5 and 4 s during the off period) so that participants could not anticipate the pulses. Each variable was expressed as a percentage of their corresponding baseline levels (pre-NMES value) and averaged across subjects.
EMG mirroring was assessed during the protocol (see Giovannelli et al. 2009; Hübers et al. 2008). It was quantified as the ratio between the on and off periods (5 s) of the root mean square (RMS) of the left FCR EMG activity:
where RMSON is the RMS value during the evoked contractions, and RMSOFF is the RMS value during the resting period before the contraction. A value of 0% of EMG mirroring indicates an absence of mirror activity, and a value of 100% indicates that EMG mirroring is two times the background EMG. For each subject EMG mirroring was computed for both the entire NMES session (mean of the 60 contractions) and for the first five and last five contractions so as to identify EMG mirroring modulations during the protocol.
Statistics
Once the data have been tested for normal distribution using a Kolmogorov-Smirnov test, variations for variables concerning both hands as MVC, Hmax, MatHmax, Mmax, and MEP were quantified using two-way repeated-measures ANOVA with time [pre-, post-NMES] and side [left, right FCR] as within factors. Pre- to post-NMES variations for IHI were quantified using two-tailed paired t-tests. Two-tailed paired t-tests were also applied to detect variations in EMG mirroring (first 5 vs. last 5 contractions).
For the variables during the NMES, one-way repeated-measures ANOVA with time as main factor were used to detect any modulation for FTI, Hmax, MatHmax, and Mmax for both hands. Post hoc Scheffé tests were used when necessary.
Variables showing a monotonic decrease were fitted to a power function of the form:
where Ec is the number of evoked contractions, a is a scaling factor depending of the initial value of the variable, and b is the decreasing rate. Data were adjusted to the model through a least-squares method. A level of P < 0.05 was used to identify statistical significance. The statistical analyses were performed using Statistica software for Windows (Statsoft, version 6.1; Statistica, Tulsa, OK).
RESULTS
Pre- to Post-NMES Modulations
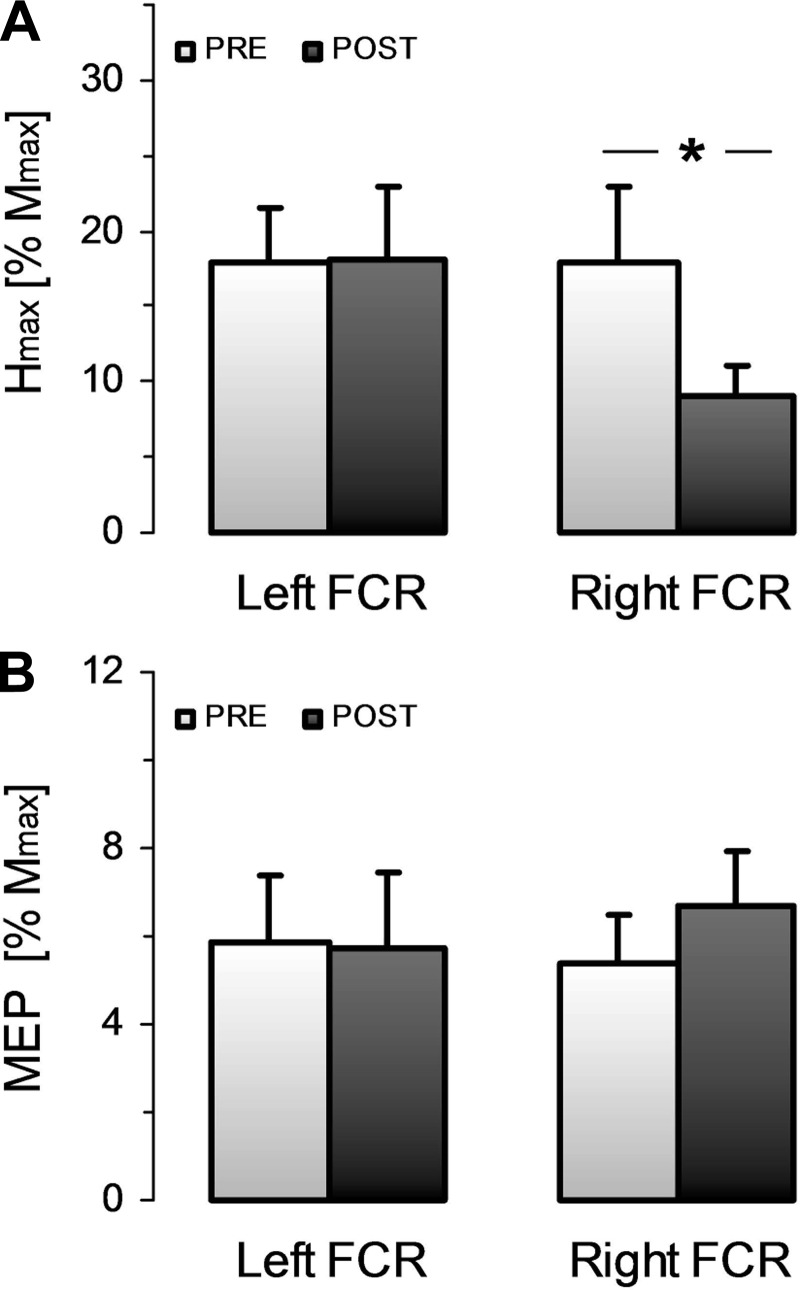
Average values for all measures pre- and post-NMES are given in Table 1. Of all the measures, only Hmax (in mV) in the right FCR showed a significant difference between pre- and post-NMES. ANOVA gave significant time [F(1,1) = 9.7, P = 0.012] and interaction [F(1,1) = 12.11, P = 0.006] effects, whereas the side effect did not reach significance [F(1,1) = 0.5, P = 0.91]. Post hoc comparisons indicated that the right Hmax dropped significantly by ∼50% (P = 0.007), whereas the left Hmax was not altered after NMES (P = 0.99). Importantly, MVC torque did not change after NMES for either hand. ANOVA yielded no main effect of time [F(1,1) = 0.005, P = 0.95] and side [F(1,1) = 4.5, P = 0.07], and no interaction effect [F(1,1) = 0.13, P = 0.72]. This underlines the absence of muscular fatigue. Likely related to this, both MatHmax and Mmax of left and right FCR did not show any significant change, since ANOVA (time × side) conducted for MatHmax and Mmax yielded no main or interaction effect (P > 0.09 in all cases).
Table 1.
Behavioral and neurophysiological data
| Left FCR |
Right FCR |
|||||
|---|---|---|---|---|---|---|
| Pre | Post | P value | Pre | Post | P value | |
| MVC, N·m | 20.47 ± 1.28 | 20.59 ± 1.29 | 0.80 | 23.19 ± 0.66 | 23.10 ± 0.73 | 0.82 |
| Mmax, mV | 3.17 ± 0.33 | 3.03 ± 0.33 | 0.96 | 3.80 ± 0.37 | 3.02 ± 0.40 | 0.31 |
| Hmax, mV | 0.50 ± 0.10 | 0.51 ± 0.11 | 0.99 | 0.72 ± 0.17 | 0.30 ± 0.08 | 0.01* |
| MatHmax, mV | 1.06 ± 0.24 | 0.98 ± 0.19 | 0.76 | 0.62 ± 0.15 | 0.47 ± 0.06 | 0.78 |
| MatHmax, %Mmax | 32.09 ± 5.59 | 34.26 ± 6.71 | 0.99 | 17.59 ± 4.00 | 18.27 ± 3.38 | 0.98 |
| MEP, mV | 0.16 ± 0.02 | 0.16 ± 0.03 | 0.61 | 0.17 ± 0.02 | 0.16 ± 0.02 | 0.67 |
| TS, mV | 0.28 ± 0.04 | 0.28 ± 0.06 | 0.98 | |||
| TSconditionned, mV | 0.22 ± 0.04 | 0.17 ± 0.05 | 0.07 | |||
| IHI, %TS | 75.38 ± 4.13 | 61.39 ± 4.10 | 0.02* | |||
Values are means ± SE for each variable before and after the application of the neuromuscular electrical stimulation (NMES) protocol for both right and left flexor carpi radialis (FCR). MVC, maximal voluntary isometric contractions; Mmax, maximal M wave; Hmax, maximal H wave; MatHmax, M waves at Hmax; MEP, motor evoked potentials; TS, test stimulus; TSconditioned, conditioned test stimulus; IHI, interhemispheric inhibition. P values from Scheffé pairwise comparisons are reported for both right and left FCR.
Statistical differences between pre- and post-NMES values for P < 0.05.
Figure 2 shows the normalized values for Hmax and MEP in both hands. Figure 2A clearly shows that there was a post-NMES decrease of Hmax in the right FCR, but not in the left FCR. ANOVA indeed gave a significant time-by-side interaction effect [F(1,1) = 12.46, P = 0.006], whereas no main effect reached significance [F(1,1) = 0.48, P = 0.46 and F(1,1) = 4.17, P = 0.07 for time and side effect, respectively]. Post hoc comparisons revealed significant pre to post differences for the right (P = 0.01) but not for the left (P = 0.99) FCR. Moreover, there was no significant change between pre- and post-NMES MEP amplitudes for either left or right FCR, since ANOVA gave no main or interaction effects [F(1,1) = 0.42, P = 0.84; F(1,1) = 0.51, P = 0.49; and F(1,1) = 3.17, P = 0.10 for time, side, and interaction effect, respectively, see Fig. 2B].
Fig. 2.
Pre- to post-NMES modulation of spinal and corticospinal excitability. A: mean ± SE of the normalized maximal H reflex (Hmax) for the right and left FCR before and after NMES. B: mean ± SE of the normalized motor evoked potentials (MEP) for the right and left FCR before and after NMES. *Statistical difference between pre- and post-NMES values for P < 0.05.
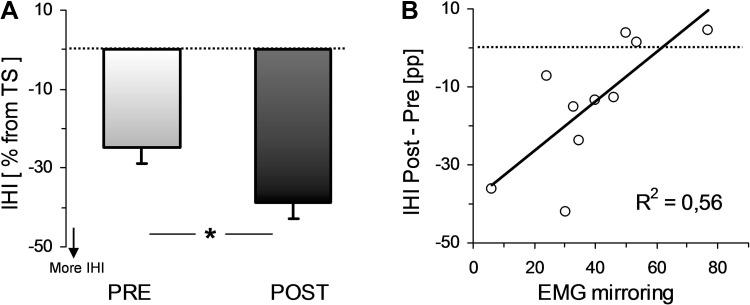
Figure 3 shows the IHI data. Figure 3A indicates that IHI was significantly increased after the NMES session, since the inhibition was −24.62 ± 4.13% and −38.61 ± 4.10%, pre- and post-NMES, respectively [t(9) = 2.76, P = 0.02]. Note that IHI expressed as a percentage of TS gave the very same result (see IHI in Table 1). Our IHI protocol was highly consistent, since the pre-NMES amplitude of the TScond was systemically lower than the TS [0.22 ± 0.04 vs. 0.28 ± 0.04 mV for TScond and TS, respectively; t(9) = 4.21, P < 0.001]. Moreover, there was no pre- to post-NMES significant difference in the TS (in mV, see Table 1).
Fig. 3.
Pre- to post-NMES modulation of interhemispheric inhibition (IHI) and its relation with the EMG mirror activity. A: mean ± SE of IHI before and after NMES. IHI is here directly expressed as the relation between unconditioned (TS) and conditioned (TScond) test stimulus in percentage (see methods). B: pre to post changes in IHI are plotted vs. average EMG mirroring for all subjects and are fitted to their best linear model. *Statistical differences between pre- and post-NMES values for P < 0.05.
Finally, pre- to post-NMES IHI modulation was correlated with the level of EMG mirroring during the NMES session (R2 = 0.56, P < 0.05; see Fig. 3B). Precisely, subjects who had the highest increase in IHI (as expressed by post-NMES IHI minus pre-NMES IHI in percentage points) were those who had the lowest average EMG mirroring during the protocol. Note that the IHI variation as expressed through a pre-to-post ratio also correlated well with EMG mirroring (R2 = 0.64, P < 0.05). Importantly, no modulation was detected in EMG mirroring throughout the protocol, since it did not change significantly [t(9) = 1.49, P = 0.16] from the first five (44.78 ± 5.63%) to the last five (39 ± 6.61%) evoked contractions.
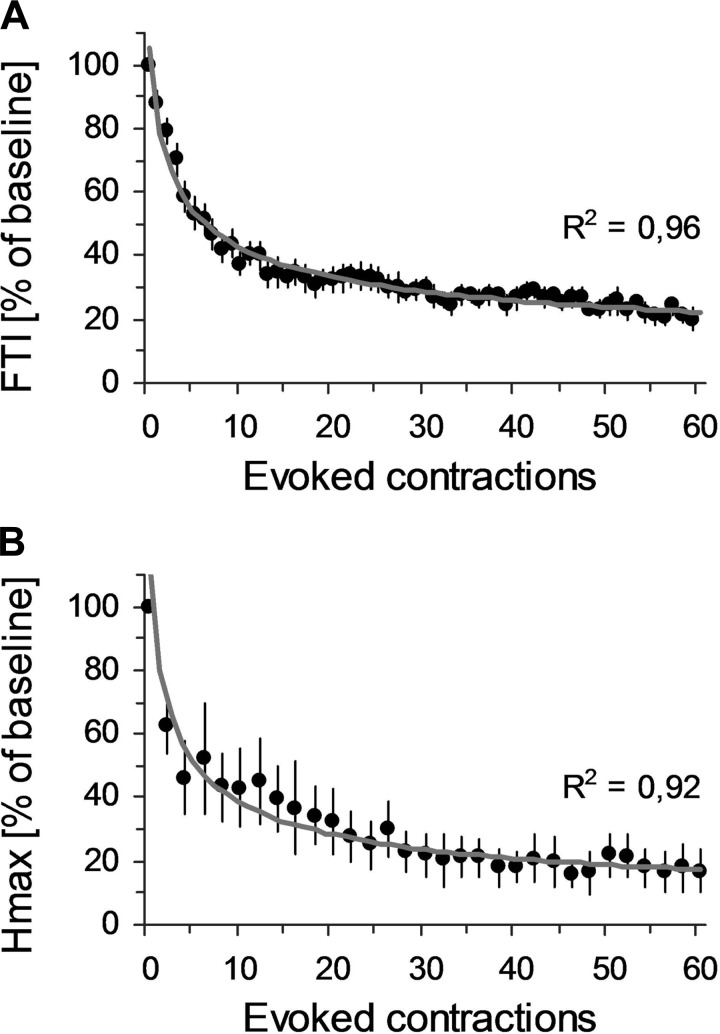
Contraction-Dependent Modulations during NMES
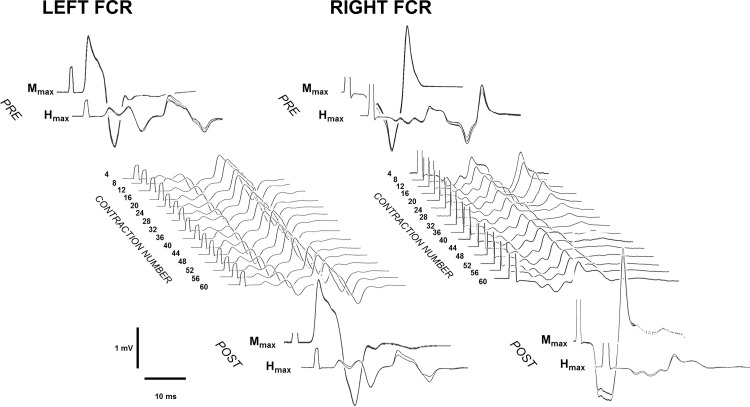
The mean FTI during the first evoked contraction was 13.7 ± 1.15 N·m·s and then decreased rapidly during the subsequent contractions. ANOVA gave a significant time effect [F(1,59) = 22.78, P < 0.001], and FTI was significantly depressed after the second contraction (P < 0.001). This rapidly declined to reach ∼40% of the initial value at the 10th contraction; next, FTI declined slower until reaching ∼20% of the initial value at the last contraction (see Fig. 4A). Right Hmax also showed a rapid decrease during NMES (Fig. 4B). Figure 5 depicts qualitatively this modulation. ANOVA showed a significant time effect [F(1,30) = 22.78, P < 0.001] for the right Hmax amplitude. The pattern of modulation was qualitatively similar to the FTI, with a rapid reduction early on (Hmax dropped by ∼40% and ∼60% after the 2nd and the 8th contractions, respectively; P < 0.001 for both comparisons) and then a slower decrease until the end of the protocol (∼20% of the baseline after the last contraction). Left Hmax, MatHmax, and Mmax bilaterally did not show any specific change throughout the protocol (P > 0.05 for each analysis).
Fig. 4.
Contraction-dependent modulations of force-time integral (FTI) and right Hmax during NMES. A: means ± SE of FTI at each evoked contraction are expressed as %baseline, i.e., FTI during the 1st evoked contraction. B: means ± SE of Hmax for every two evoked contractions are expressed as %baseline, i.e., pre-NMES values. Data of both variables are fitted to their best-fitted power function of the form y = a × x−b with their respective R2.
Fig. 5.
Typical Hmax and maximal M wave (Mmax) signals during the NMES protocol. Pre (top)- and post (bottom)-NMES Mmax and Hmax of left and right FCR are depicted from a representative subject. In the middle, typical Hmax signals from the same subject recorded during the protocol are shown for both left and right FCR. It can be seen that Hmax amplitude of the right FCR is clearly modulated, whereas Hmax amplitude of the left FCR remains stable.
FTI and right Hmax variations fitted well to a decreasing power function (see the fitting curves and their corresponding R2 for average data in Fig. 4). For the whole group, mean R2 were high for both FTI and right Hmax and did not differ significantly between the two variables [0.70 ± 0.03 vs. 0.61 ± 0.07 for FTI and right Hmax, respectively; t(9) = 1.79, P = 0.10]. Interestingly, the decreasing rates of the power functions were not significantly different between FTI and right Hmax [0.39 ± 0.02 vs. 0.49 ± 0.07 for FTI and right Hmax, respectively; t(9) = 1.35, P = 0.20].
DISCUSSION
This study investigated if HF NMES alters the hemispheric balance between the two motor cortices (M1 areas) evaluated by measuring IHI. To do so, we examined the effects of 10 min of HF NMES applied to the right wrist flexor on spinal and corticospinal excitability of both the contralateral and ipsilateral sides, and on the IHI from left to right M1. HF NMES induced: 1) a strong and rapid decline of both evoked force and ipsilateral spinal excitability, 2) no significant change in corticospinal excitability, and 3) a modulation of IHI. Although various effects of HF NMES on spinal and corticospinal excitability have been previously reported (Chipchase et al. 2011b; Collins 2007), the modulation of IHI is a novel finding. Our data suggest that a short period of HF NMES changes the excitability of the transcallosal pathway and may support its use in disorders where abnormal IHI is a potential target.
Behaviorally, evoked force from the stimulated arm showed a rapid and monotonic decrease with the continuing NMES trains (Fig. 3A). Interestingly, similar results have been recorded for lower limb muscles during short NMES sessions (Matkowski et al. 2015; Neyroud et al. 2014; Papaiordanidou 2014). This may reflect a certain degree of neuromuscular fatigue; however, it does not account for central or muscular fatigue, since MVC and M wave were not modified by the protocol. Evoked torque and MVC are differently affected by NMES (Matkowski et al. 2015; Neyroud et al. 2014). Indeed, although a reduction in MVC reflects central impairments such as lower muscle activation (Zory et al. 2005), the decrease in NMES-evoked torque is more likely the result of a progressive loss in the number of recruited motor units caused by a change in motor axon excitability (Matkowski et al. 2015; Papaiordanidou 2014). We have shown that ipsilateral H reflex was considerably decreased (∼50%) after the protocol, and, interestingly, the pattern of modulation across the course of NMES trains matched to a high degree the modulation of evoked force. HF NMES has been shown to be suitable for motor unit recruitment through reflex pathways by enhancing afferent volleys and is likely to induce adaptations within the central nervous system (Collins et al. 2007). Thus, the ipsilateral H reflex modulation is in line with studies that have documented modifications in spinal excitability through HF NMES (Collins et al. 2001, 2002; Lagerquist et al. 2009) and further confirm that this type of stimulation leads to central adaptations within the reflex pathway. Modulations of both NMES-evoked torque and H reflex throughout the protocol may also be explained by processes related to the efficacy of neurotransmission at the level of Ia terminals. Precisely, mechanisms presynaptic to the motoneuronal membrane (Collins et al. 2007) and/or postactivation depression may be potential mechanisms accounting for the reported decrease of H reflex and reflex-induced force (Hutborn et al. 1996). A depletion of neurotransmitter release from Ia terminals could indeed partially mediate the observed decline during the stimulation protocol (Crone and Nielsen 1989; Grey el al. 2008). Taken together, the H reflex and evoked force data provide solid evidence for the involvement of spinal circuitry in motor unit recruitment during HF NMES.
Our main observation was that the IHI changed following HF NMES. IHI refers to the neurophysiological mechanism by which the contralateral M1 inhibits the opposite one via transcalosal mechanisms (Ferbert et al. 1992; Giovanelli et al. 2009; Morishita et al. 2012). Transcallosal control from left to right M1 arises from pyramidal neurons of cortical layer III in left M1 projecting, via corpus callosum, on GABAergic inhibitory interneurons on right M1, which in turn modulate the excitability of corticospinal pyramidal neurons of cortical layer V in right M1 (Ferbert et al. 1992). In this study, we have shown that 10 min of right hand HF NMES increased IHI from left to right M1. Because neither MEP nor H reflex changed in the left FCR, we may conclude that IHI modulation probably does not depend on corticospinal excitability emerging from the right M1 but rather on an increased excitability of transcallosal cells from the left hemisphere. Interestingly, similar IHI modulations have been observed following unilateral motor training and mirror visual feedback training (Avanzino et al. 2014a), or after muscular proprioceptive stimulation (Avanzino et al. 2014b; Swayne et al. 2006). Because 1) HF NMES does not involve voluntary motor commands (in contrast to volitional training, which contains both efferent and afferent signals) and 2) the muscle spindles activated during electrical stimulation have direct access to contralateral sensory and motor cortical areas (Heath et al. 1976; Hore et al. 1976), our data strongly suggest that the afferent signals provided by the stimulation (i.e., somatosensory inputs provided by HF NMES) have a direct influence on the excitability of transcallosal neurons projecting from left to right M1, and may thus affect interhemispheric plasticity.
Furthermore, the IHI modulation correlated with EMG mirroring in the left wrist flexor. More precisely, the subjects who kept a low background EMG level throughout the protocol within the left hand were those who had the greatest increase in IHI. Data from the literature indicate that interhemispheric communication between both M1 plays a role in unilateral hand movement control. Indeed, during unimanual finger movements, the contralateral M1 inhibits the ipsilateral one to a greater extent compared with a rest condition (Duque et al. 2007). From a functional perspective, the increase in IHI from the active to the opposite M1 during unilateral movements seems to be instrumental in suppressing mirror activity (Giovannelli et al. 2009; Hübers et al. 2008). The presence of a slight (unintentional) EMG mirror activity in the left resting hand during our HF NMES session is in line with both neuroimaging and neurophysiological studies. Indeed, ipsilateral neural activations within motor and somatosensory areas are generally observed during various forms of unilateral functional electrical stimulation (see Veldman et al. 2014 for a review); and, more importantly, low EMG signals are recorded in the contralateral homologous muscles during unilateral electro-induced contractions, even under strict instructions of task laterality (Hortobàgyi et al. 1999). It thus emerged from our study that the functional link between IHI and the control of contralateral EMG output is at play during HF NMES, further suggesting a key participation of transcalosal mechanisms in the regulation of mirror activity of the upper limb during electro-induced contractions. We can speculate that, in our paradigm, the priority for the motor system was to maintain the task laterality and that the increase in transcallosal inhibition from the left to right hemisphere reflects an increase in synaptic strength between the neural populations involved that may allow for a suppression of mirror activity.
A final finding to be discussed is the lack of change in right MEP amplitude (stimulated hand). Thus, despite a strong effect at the spinal level, the afferent volley induced during our protocol did not significantly change the cortical excitability in the contralateral M1. However, many studies have shown that NMES can modulate motor cortical excitability from a targeted muscle (Chipchase et al. 2011a; Mang et al. 2010, 2011; Miyata and Usuda 2015; Ridding et al. 2001). Unfortunately, the heterogeneity of experimental designs (stimulation intensity, frequency, and duration of stimuli) makes the interpretation of the data difficult and can lead to conflicting results (Chipchase et al. 2011b). Nonetheless, two studies from Mang et al. (2010, 2011) employing similar electrical stimuli as in our experiment but with longer durations (40 vs. 10 min) have shown an enhanced cortical excitability after HF NMES, suggesting that a longer HF NMES stimulation might have induced cortical plasticity in our case. Future studies are nevertheless necessary to confirm this hypothesis.
In a clinical context, NMES is a common method in rehabilitation programs since it could maintain contractile activity in immobilized muscles (Maffiuletti et al. 2013). Patients suffering from neurological injuries can also benefit from NMES. In particular, wide-pulse high-frequency NMES, by priming the central nervous system thought afferent feedback, could be considered in patients with impaired motor control (Thomson et al. 2011). For instance, motor performance of the paretic limb in post-stroke patients has been shown to improve after such stimulation (Clair-Auger et al. 2012). Our study shows that neural adaptations can be induced without large muscular solicitation, since interhemispheric adaptations were found without muscle fatigue (i.e., MVC decrease). Interestingly, our results may support the use of HF NMES in disorders where abnormal IHI is a potential target. These disorders include stroke, where the balance between the lesioned and the intact hemisphere plays a crucial role in motor recovery (Hummel & Cohen, 2006), and other neurological diseases, such as focal hand dystonia, Parkinson's disease, and multiple sclerosis, where abnormal interhemispheric communication is supported by clinical data (Bonzano et al. 2008; Cox et al. 2012; Sitburana et al. 2009). Also, HF NMES can evoke higher forces for lower intensities than conventional NMES protocols (Collins et al. 2007). This is of major interest to plebiscite its use in rehabilitation since it reduces the discomfort felt by patients with conventional protocols. This drawback is also associated with a rapid onset of muscular fatigue resulting from a nonphysiological recruitment that could limit the use of NMES (Gregory and Bickel 2005).
Potential limitations should be considered in our study. First, as mentioned in the Introduction, one-hand physical training could transitorily change hemispheric balance (Avanzino et al. 2014a; Perez et al. 2007). However, IHI targeting the resting hand is either decreased to facilitate the intermanual transfer of learning (Perez et al. 2007) or increased to maintain the laterality of the task (Avanzino et al. 2014a). Postexercise IHI modulation is thus closely related to the nature of the motor task. Here, because we aimed at determining whether one HF NMES session could alter IHI, we focused on a unique experimental condition where transcalosal excitability was evaluated after repeated electro-induced contractions. Thus, whereas our data strongly suggest that HF NMES provide somatosensory inputs capable of modulating transcallosal excitability, future studies could take advantage of both our current results and the literature to further assess neurophysiological differences between specific training modalities (i.e., varying in term of task demand and/or contraction mode). Second, a possible hemispheric asymmetry of IHI was not tested here, since only IHI from left to right M1 was evaluated. This may constitute a limitation of the study. Indeed, because hemispheric balance has been shown to be strongly use dependent (Avanzino et al. 2011), a protocol assessing IHI in both directions could refine the interhemispheric mechanisms subserving HF NMES. Thus, whereas we clearly show that HF NMES modulates transcalosal communication from the contralateral “active” to the ipsilateral “resting” M1, IHI in the opposite direction should be investigated in follow-up studies so as to figure out potential cortical modifications induced by an abnormal asymmetric stimulation of the two limbs (Avanzino et al. 2011, 2014b).
Perspectives and Significance
To conclude, our study gives original insights concerning the neurophysiological mechanisms involved in HF NMES. First, our data confirm that muscle contractions involve central pathways of motor control, since strong modulations of spinal excitability were observed during HF NMES and were located to (and here restricted to) the stimulated side. Second, and more importantly, we highlighted for the first time that HF NMES has a significant influence on the hemispheric balance between both motor cortexes, since our short HF NMES session induced an increase in IHI. Interestingly, this effect seems to be directly involved in the control of the motor output of the contralateral hemiside.
GRANTS
This research work was supported by the Région de Bourgogne (contract no. 9201AAO050S02953) and the Fonds Européen de Développement Régional.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.G. and R.L. conception and design of research; N.G. and S.G. performed experiments; N.G., S.G., and R.L. analyzed data; N.G., S.G., P.J.S., and R.L. interpreted results of experiments; N.G. and S.G. prepared figures; N.G., S.G., P.J.S., and R.L. drafted manuscript; N.G., S.G., P.J.S., and R.L. edited and revised manuscript; N.G., S.G., P.J.S., and R.L. approved final version of manuscript.
REFERENCES
- Avanzino L, Bassolino M, Pozzo & Bove MT. Use dependent hemispheric balance. J Neurosc 31(9): 3423–3428, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzino L, Raffo A, Pelosin E, Ogliastro C, Marchese R, Ruggeri P, Abbruzzese G. Training based on mirror visual feedback influences transcallosal communication. Eur J Neurosci 40: 2581–2588, 2014a. [DOI] [PubMed] [Google Scholar]
- Avanzino L, Pelosin E, Abbruzzese G, Bassolino M, Pozzo & Bove M T. Shaping motor cortex plasticity through proprioception. Cereb Cortex 24: 2807–2814, 2014b. [DOI] [PubMed] [Google Scholar]
- Baldwin ERL, Klakowicz PM, David Collins DF F. Wide-pulse-width, high-frequency neuromuscular stimulation: implications for functional electrical stimulation. J Appl Physiol 101: 228–240, 2006. [DOI] [PubMed] [Google Scholar]
- Bergquist AJ, Clair JM, Lagerquist O, Mang CS, Okuma Y, Collins DF. Neuromuscular electrical stimulation: implications of the electrically evoked sensory volley. Eur J Appl Physiol 111: 2409–2426, 2011. [DOI] [PubMed] [Google Scholar]
- Bergquist AJ, Clair JM, Collins DF. Motor unit recruitment when neuromuscular electrical stimulation is applied over a nerve trunk compared with a muscle belly: triceps surae. J Appl Physiol 110: 627–637, 2011. [DOI] [PubMed] [Google Scholar]
- Burnley M, Vanhatalo A, Jones AM. Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J Appl Physiol 113: 215–223, 2012. [DOI] [PubMed] [Google Scholar]
- Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Rev 49: 641–662, 2005. [DOI] [PubMed] [Google Scholar]
- Chipchase LS, Schabrun SM, Hodges PW. Corticospinal excitability is dependent on the parameters of peripheral electric stimulation: a preliminary study. Arch Phys Med Rehabil 92: 1423–1430, 2011a. [DOI] [PubMed] [Google Scholar]
- Chipchase LS, Schabrun SM, Hodges PW. Peripheral electrical stimulation to induce cortical plasticity: A systematic review of stimulus parameters. Clin Neurophysiol 122: 456–463, 2011b. [DOI] [PubMed] [Google Scholar]
- Clair-Auger JM, Collins DF, Dewald JP. The effects of wide pulse neuromuscular electrical stimulation on elbow flexion torque in individuals with chronic hemiparetic stroke. Clin Neurophysiol 123: 2247–2255, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci 21: 4059–4065, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behaviour in human motoneurones. J Physiol 538: 289–301, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF. Central contributions to contractions evoked by tetanic neuromuscular electrical stimulation. Exerc Sport Sci Rev 35: 102–109, 2007. [DOI] [PubMed] [Google Scholar]
- Cox BC, Cincotta & Espay AJ M. Mirror movements in movement disorders: a review. Tremor Other Hyperkinet Mov 2: PMCID, MC3569961, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus h-reflex in man. Exp Brain Res 78: 28–32, 1989. [DOI] [PubMed] [Google Scholar]
- Dean JC, Yates LM, Collins DF. Turning on the central contribution to contractions evoked by neuromuscular electrical stimulation. J Appl Physiol 103: 170–176, 2007. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115: 255–266, 2004. [DOI] [PubMed] [Google Scholar]
- Dirks ML, Hansen D, Van Assche A, Dendale P, Van Loon LJC. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin Sci 128: 357–365, 2015. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movementrelated interhemispheric inhibition. J Cognitive Neurosci 19: 204–213, 2007. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol 453: 525–546, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli F, Borgheresi A, Balestrieri F, Zaccara G, Viggiano MP, Cincotta M, Ziemann U. Modulation of interhemispheric inhibition by volitional motor activity: an ipsilateral silent period study. J Physiol 587: 5393–5410, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther 85: 358-64, 2005. [PubMed] [Google Scholar]
- Grey MJ, Klinge K, Crone C, Lorentzen J, Biering-Sørensen F, Ravnborg M, Nielsen JB. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp Brain Res 185: 189–197, 2008. [DOI] [PubMed] [Google Scholar]
- Grosprêtre S, Martin A. H reflex and spinal excitability: methodological considerations. J Neurophysiol 107: 1649–1654, 2012. [DOI] [PubMed] [Google Scholar]
- Gueugneau N, Mc Cabe SI, Villalta JI, Grafton ST, Della-Maggiore V. Direct mapping rather than motor prediction subserves modulation of corticospinal excitability during observation of actions in real time. J Neurophysiol 113: 3700–3707, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut K, Duchateau J. Neuromuscular electrical stimulation and voluntary exercise. Sports Med 14: 100–113, 1992. [DOI] [PubMed] [Google Scholar]
- Heath CJ, Hore J, Phillips CG. Inputs from low threshold muscle and cutaneous afferents of hand and forearm to areas 3a and 3b of baboon's cerebral cortex. J Physiol 257: 199–227, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hore J, Preston JB, Cheney PD. Responses of cortical neurons (areas 3a and 4) to ramp stretch of hindlimb muscles in the baboon. J Neurophysiol 39: 484–500, 1976. [DOI] [PubMed] [Google Scholar]
- Hortobàgyi T, Scott K, Lambert J, Hamilton G, Tracy J. Crosseducation of muscle strength is greater with stimulated than voluntary contractions. Mot Control 3: 205–219, 1999. [DOI] [PubMed] [Google Scholar]
- Hübers A, Orekhov Y, Ziemann U. Interhemispheric motor inhibition: its role in controlling electromyographic mirror activity. Eur J Neurosci 28: 364–371, 2008. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res 108: 450–462, 1996. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 5: 708–712, 2006. [DOI] [PubMed] [Google Scholar]
- Klakowicz PM, Baldwin ER, Collins DF. Contribution of M-waves and H-reflexes to contractions evoked by tetanic nerve stimulation in humans. J Neurophysiol 96: 1293–1302, 2006. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Lin CS, Burke D. Differences in activity-dependent hyperpolarization in human sensory and motor axons. J Physiol 558: 341–349, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackmy A, Marchand-Pauvert V. The estimation of short intra-cortical inhibition depends on the proportion of spinal motoneurones activated by corticospinal inputs. Clin Neurophysiol 121: 612–621, 2010. [DOI] [PubMed] [Google Scholar]
- Lagerquist O, Walsh LD, Blouin JS, Collins DF, Gandevia SC. Effect of a peripheral nerve block on torque produced by repetitive electrical stimulation. J Appl Physiol 107: 161–167, 2009. [DOI] [PubMed] [Google Scholar]
- Maffiuletti & Hortobagyi T NA. Neural adaptations to electrical stimulation strength training. Eur J Appl Physiol 111: 2439–2449, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffiuletti NA, Roig M, Karatzanos E, Nanas S. Neuromuscular electrical stimulation for preventing skeletal-muscle weakness and wasting in critically ill patients: a systematic review. BMC Med 11: 137, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Lagerquist O, Collins DF. Changes in corticospinal excitability evoked by common peroneal nerve stimulation depend on stimulation frequency. Exp Brain Res 203: 11–20, 2010. [DOI] [PubMed] [Google Scholar]
- Mang CS, Clair JM, Collins DF. Neuromuscular electrical stimulation has a global effect on corticospinal excitability for leg muscles and a focused effect for hand muscles. Exp Brain Res 209: 355–363, 2011. [DOI] [PubMed] [Google Scholar]
- Matkowski B, Lepers R, Martin A. Torque decrease during submaximal evoked contractions of the quadriceps muscle is linked not only to muscle fatigue. J Appl Physiol 118: 1136–1144, 2015. [DOI] [PubMed] [Google Scholar]
- Miyata K, Usada S. Changes in corticospinal excitability with short-duration high-frequency electrical muscle stimulation: a transcranial magnetic stimulation study. J Phys Ther Sci 27: 2117–2120, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol 561: 331–338, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita T, Uehara K, Funase K. Changes in interhemispheric inhibition from active to resting primary motor cortex during a fine-motor manipulation task. J Neurophysiol 107: 3086–3094, 2012. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55: 400–409, 2004. [DOI] [PubMed] [Google Scholar]
- Neyroud D, Dodd D, Gondin J, Maffiuletti NA, Kayser B, Place J. Wide-pulse-high-frequency neuromuscular stimulation compared with conventional stimulation of triceps surae induces greater muscle fatigue. J Appl Physiol 116: 1281–1289, 2014. [DOI] [PubMed] [Google Scholar]
- Palmieri RM, Ingersoll CD, Hoffman MA. The Hoffmann Reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train 39: 268–277, 2004. [PMC free article] [PubMed] [Google Scholar]
- Papaiordanidou M, Stevenot JD, Mustacchi V, Vanoncini M, Martin A. Electrically induced torque decrease reflects more than muscle fatigue. Muscle Nerve 50: 604–607, 2014. [DOI] [PubMed] [Google Scholar]
- Perez MA, Wise SP, Willingham DT, Cohen LG. Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosc 27: 1045–1053, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28: 5631–5640, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, McKay DR, Thompson PD, Miles TS. Changes in corticomotor representations induced by prolonged peripheral nerve stimulation in humans. Neurophysiol Clin 112: 1461–1469, 2001. [DOI] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res 181: 615–626, 2007. [DOI] [PubMed] [Google Scholar]
- Schabrun SM, Chipchase LS. Priming the brain to learn: The future of therapy? Manual Therapy 17: 184–186, 2012. [DOI] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol 28: 345–376, 1987. [DOI] [PubMed] [Google Scholar]
- Sitburana O, Wu LJ, Sheffield JK, Davidson & Jankovic J A. Motor overflow and mirror dystonia. Parkinsonism Relat D 15: 758–761, 2009. [DOI] [PubMed] [Google Scholar]
- Swayne O, Rothwell J, Rosenkranz K. Transcallosal sensorimotor integration: effects of sensory input on cortical projections to the contralateral hand. Clin Neurophysiol 117: 855–863, 2006. [DOI] [PubMed] [Google Scholar]
- Swinnen SP. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci 3: 348–359, 2002. [DOI] [PubMed] [Google Scholar]
- Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res 186: 59–66, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurel J, Lepers R, Pardon L, Maffiuletti NA. Differences in cardiorespiratory and neuromuscular responses between voluntary and stimulated contractions of the quadriceps femoris muscle. Respir Physiol Neurobiol 157: 341–347, 2007. [DOI] [PubMed] [Google Scholar]
- Wegrzyk J, Fouré A, Vilmen C, Ghattas B, Maffiuletti NA, Mattei JP, Place N, Bendahan D, Gondin J. Extra Forces induced by wide-pulse, high-frequency electrical stimulation: Occurrence, magnitude, variability and underlying mechanisms. Clin Neurophysiol 126: 1400–1412, 2015. [DOI] [PubMed] [Google Scholar]
- Zory R, Boerio D, Jubeau M, Maffiuletti NA. Central and peripheral fatigue of the knee extensor muscles induced by electromyostimulation. Int J Sports Med 26: 847–853, 2005. [DOI] [PubMed] [Google Scholar]