Abstract
Background:
Hypertension is closely related with obesity in pediatric population. Obesity indices were used for screening elevated blood pressure (BP) in children and adolescents. The present study was to perform a meta-analysis to assess the performance of obesity indices, body mass index (BMI), waist circumference (WC), and waist-to-height ratio (WHtR), for identifying elevated BP in children and adolescents.
Methods:
Data sources were PubMed, EMBASE, Web of Science, Cochrane, and SCOPUS up to May 2016. Studies providing measures of diagnostic performance of obesity indices and using age-, sex-, and height-specific BP 95% as reference standard (the definition of United State Fourth Report) were included. We extracted available data on true-positive, false-positive, true-negative, and false-negative to construct a 2 × 2 contingency table and computed the pooled summary statistics for the sensitivities and specificities to estimate the diagnostic performance.
Results:
Nine eligible studies that evaluated 25,424 children and adolescents aged 6 to 18 years were included in the meta-analysis. The pooled sensitivities were 42% (BMI), 42% (WC), and 43% (WHtR). The pooled specificities were 80% (BMI), 75% (WC), and 77% (WHtR). The areas under the curve (AUCs) of obesity indices were 0.7780 (BMI), 0.7181 (WC), and 0.6697 (WHtR), respectively. In this meta-analysis, the BP measurements were based on 3 visits in only 1 study. The prevalence of hypertension may be overestimated in these studies.
Conclusions:
The present meta-analysis showed that the performance of obesity indices for identifying elevated BP was poor. Our findings do not support the performance of WC and WHtR is superior to BMI to help identify children with elevated BP.
Keywords: children, hypertension, meta-analysis, obesity indices
1. Introduction
In the last decade, childhood hypertension has become an important health issue due to its rising prevalence and associated damage.[1] Childhood blood pressure (BP) is associated with BP in later life. Systematic analysis had showed the tracking of BP from childhood to adulthood.[2] Hypertension in children is associated with early vascular aging and left ventricular hypertrophy or dysfunction.[3–6]
The epidemic of childhood obesity plays an important role in the development of hypertension. Childhood overweight and obesity have increased dramatically since 1990.[7] Recent systematic analysis shows that the overall prevalence of overweight including obesity has reached approximately 23.8% for boys and 22.6% for girls in developed countries and 12.9% for boys and 13.4% for girls in developing countries.[8] Obese children and adolescents are often accompanied with increased cardiovascular risk factor. Obesity increases the occurrence of hypertension 3-fold while favoring the development of insulin resistance, hyperlipidemia, and salt sensitivity.[9,10] Obese children and adolescents also have unhealthy living habit, such as excess dietary Na intake and low physical activity.[11,12] Children with hypertension, especially with hypertension coexisting with obesity, have more serious left ventricular hypertrophy and dysfunction.[13]
In the absence of evidence-based guidelines for high BP screening in asymptomatic children and adolescents,[14] a reasonable strategy is to screen those who are at high risk. Obesity indices can be used to target a high-risk population for BP screening until current evidence gaps are filled.[15] Therefore, obesity indices were used for screening elevated BP in pediatric population. Body mass index (BMI), waist circumference (WC), and waist-to-height ratio (WHtR) are the most common indices used to determine elevated BP in children and adolescents. The aim of this systematic review was to meta-analyze the performance of obesity indices, BMI, WC, and WHtR, for identifying elevated BP in children and adolescents.
2. Methods literature review
2.1. Search strategy and eligibility criteria
We performed a systematic review of published articles in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines (http://www.prisma-statement.org/). The predefined inclusion criteria for study selection were: the study must have assessed the performance of BMI, WC, and WHtR to identify elevated BP or hypertension in children and adolescents aged 0 to 18 years; (ii) provided a 2 × 2 diagnostic table to allow for meta-analysis or information to calculate these values; and used an age-, sex-, and height-specific BP 95% as reference standard (the definition of US Fourth Report).[16] We excluded editorials, reviews, and abstracts from conference proceedings. The research was limited to studies in humans and the English language.
We conducted systematic search in the bibliographic databases PubMed, EMBASE, Web of Science, Cochrane, and SCOPUS from inception to May 4, 2016. First, 4 keyword categorical searches were conducted: hypertension or synonyms (e.g., elevated blood pressure); children or adolescents or synonyms (e.g., teenage); screening or equivalent (e.g., sensitivity and specificity); (iv) body mass index or BMI, waist or waist circumference or WC, waist-to-height ratio or WHtR or waist to stature ratio or WSR. Second, categories “i” to “iv” were combined using “and,” and duplicates were removed. In addition, the related literatures and reference lists of the identified articles were searched for relevant publications. This study was approved by the ethics committee of the First Hospital of Qinhuangdao.
2.2. Study selection and quality assessment
We eliminated irrelevant articles from our primary search on the basis of information in title and abstract. Once papers had been identified on the basis of information in the title and abstract, full papers were obtained for all relevant studies. Two reviewers (C-MM and RW) independently read the full papers obtained from the search for relevance. All studies that did not meet the inclusion criteria or that met the exclusion criteria were removed. Disagreements between the 2 reviewers regarding study inclusion were resolved by a face-to-face discussion upon the full-text assessment. Eligible studies were further reviewed.
The methodological quality of each study was assessed using a checklist based on the Quality Assessment for Studies of Diagnostic Accuracy (QUADAS) tool,[17] which enables reviewers to evaluate the quality of studies. Disagreement between the reviewers on individual items was resolved by a consensus meeting with a 3rd reviewer (F-ZY).
2.3. Data extraction
Data were extracted from primary studies by the 2 reviewers (C-MM and RW) independently. Data regarding the population, screening tools used to define elevated BP and diagnostic criteria of elevated BP were extracted. We also extracted available data on true-positive, false-positive, true-negative, and false-negative to construct a 2 × 2 contingency table.
2.4. Data synthesis and statistical analysis
From the extracted data, arranged in 2 × 2 contingency tables, we computed the pooled summary statistics for the sensitivities, specificities, likelihood ratios, and diagnostic odds ratios (DORs) to estimate the diagnostic performance. All statistics are reported as point values with 95% confidence intervals (CIs). The heterogeneity of all diagnostic test parameters was evaluated with the inconsistency index (I2). The I2 statistic is defined as the percentage of variability due to heterogeneity beyond that from chance; values greater than 50% represent the possibility of substantial heterogeneity.
The comparison of diagnostic accuracy between BMI, WC, and WHtR were performed by constructing the summary receiver operating characteristic curves with pertinent areas under the curve (AUCs). AUC presents an overall summary of test performance. Perfect tests have AUCs close to 1, whereas poor tests have AUCs close to 0.5. The summary receiver operating characteristic plots were constructed using the Moses–Shapiro–Littenberg model.
In addition, multivariate meta-regression analyses were conducted to compare the diagnostic performance between BMI, WC, and WHtR after the adjustment of other study-specific covariates. These variables were chosen a priori as potential causes of between-study heterogeneity and included: BP measurement method, the number of office visit, sample size, region, and quality assessment score.
Studies were also grouped based on BP measurement method: auscultatory method, oscillometric method or Finapres apparatus; and the number of office visit: 3 visits, other. Given geographic differences, studies were grouped based on region of the study population into: North America and South America, Europe, Asia, and Africa. Finally, studies were categorized into 2 subgroups based on QUADAS score (QUADAS score < 10 and QUADAS score ≥ 10).
The diagnostic threshold (cut-off) bias was also evaluated as a cause between study heterogeneity. Begg test and Egger test was conducted to examine the possible publication bias of the studies regarding the performance of obesity indices in detecting elevated BP. Analyses were performed using the Meta-Disc 1.4 statistical software (Unit of the Clinical Biostatistics team of the Ramony Cajal Hospital in Madrid, Spain) and STATA version 12.0 (STATA Corporation, College Station, TX).
3. Results
Figure 1 summarizes the selection process of studies. In total, 3607 references were obtained using PubMed, EMBASE, Web of Science, Cochrane, and SCOPUS. An additional 6 full-text articles were included after scanning the related literatures and reference lists of the studies selected for inclusion. Eventually, a total of 9 studies were included in the present review.[18–26] The quality of the included articles is summarized in Table 1.
Figure 1.
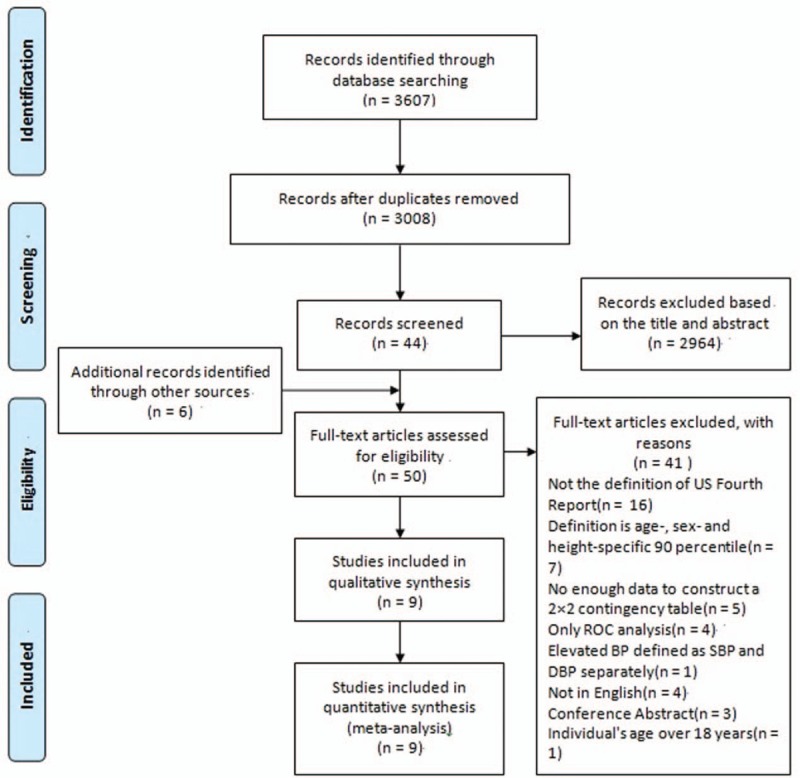
Flowchart of selection of studies and specific reasons for exclusion from the meta-analysis.
Table 1.
Characteristics of studies included in the meta-analysis.
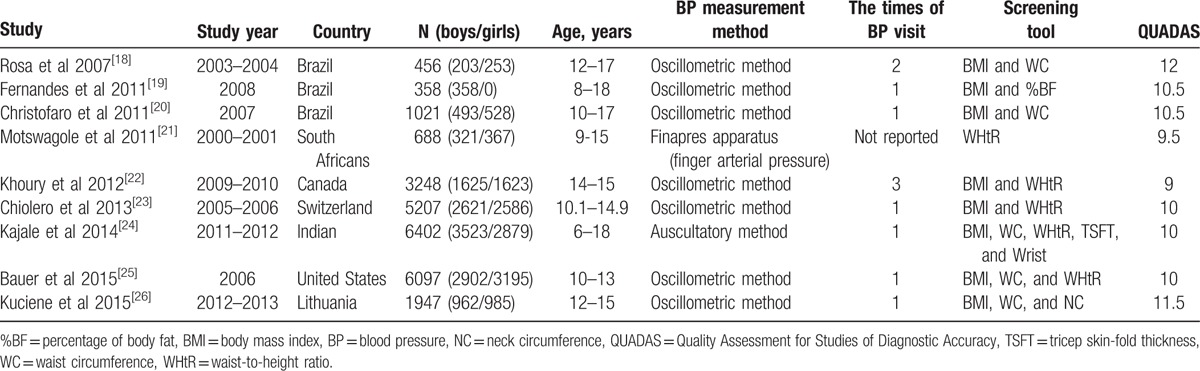
Table 1 shows details of these 9 papers. Studies were between 2007 and 2015, in 7 different countries, United States, Brazil, South Africans, Canada, Switzerland, Indian, and Lithuania. Seven eligible studies that evaluated 25,424 children and adolescents aged 6 to 18 years were included in the meta-analysis. The study population size ranged from 358 to 6402 participants. BMI was used in 8 studies, WC was used in 5 studies, and WHtR was used in 5 studies.
3.1. The performance of BMI
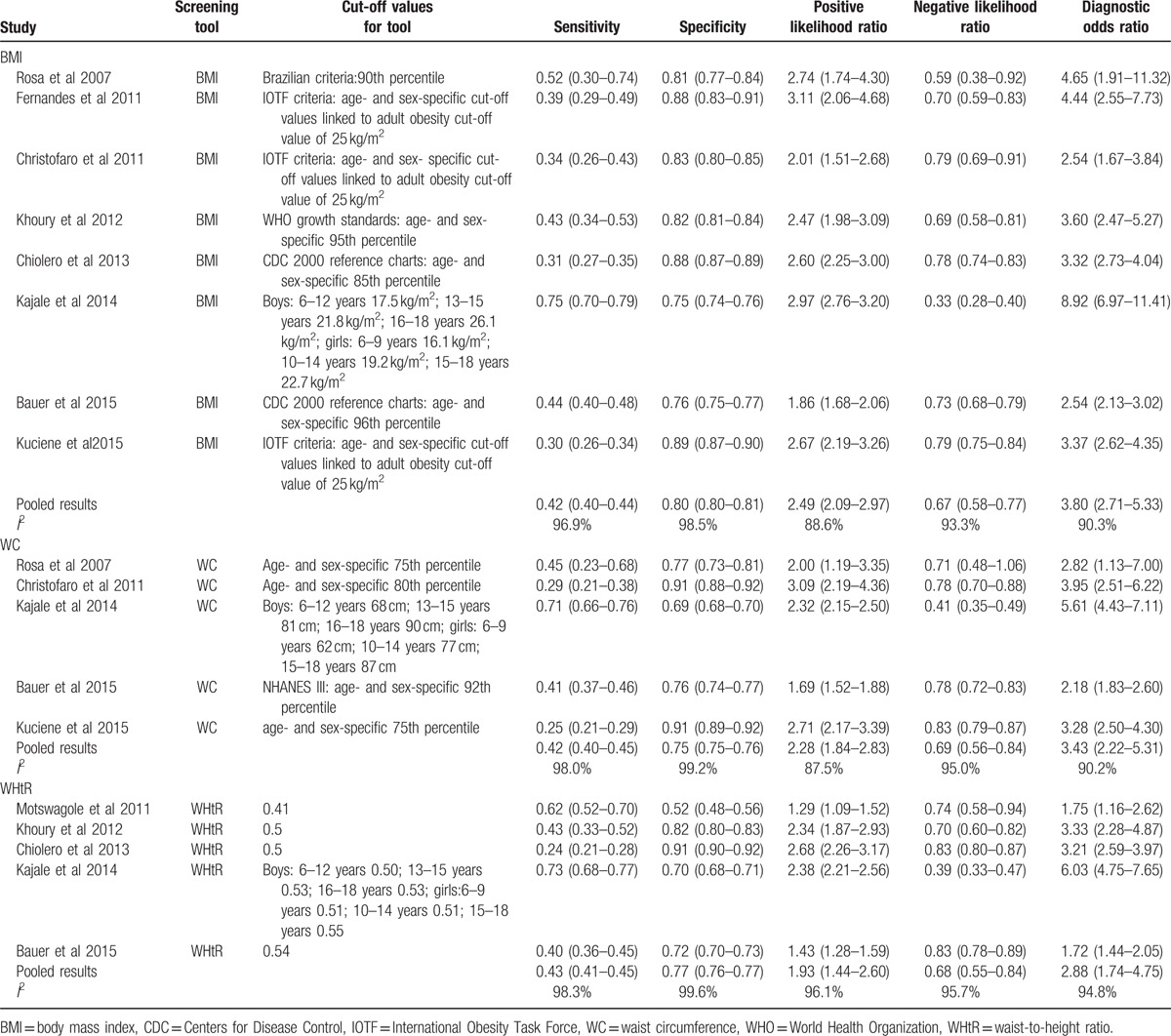
Meta-analysis showed pooled sensitivity to detect elevated BP of 0.42 (95%CI: 0.40–0.44) and pooled specificity of 0.80 (95%CI: 0.80–0.81). Positive likelihood ratio (LR+) was 2.49 (95%CI: 2.09–2.97), negative likelihood ratio (LR−) was 0.67 (95%CI: 0.58–0.77), and DOR was 3.80 (95%CI: 2.71–5.33) (Table 2). Heterogeneity was observed across studies (I2 = 88.6%–98.5%). Spearman correlation coefficient (Logit [sensitivity] vs Logit [1 − specificity]) was 0.952 (P = 0.000). They revealed evidence supporting the diagnostic threshold (cut-off) bias as a cause of heterogeneity. The Begg test and Egger test did not reveal significant publication bias (P = 0.266 and 0.634).
Table 2.
The sensitivities, specificities, likelihood ratios, and diagnostic odds ratios of obesity indices for identifying elevated blood pressure.
3.2. The performance of WC
Meta-analysis showed pooled sensitivity to detect the elevated BP of 0.42 (95%CI: 0.40–0.45) and pooled specificity of 0.75 (95%CI: 0.75–0.76). LR+ was 2.28 (95%CI: 1.84–2.83), LR− was 0.69 (95%CI: 0.56–0.84), and DOR was 3.43 (95%CI: 2.22–5.31) (Table 2). Heterogeneity was observed across studies (I2 = 87.5%–99.2%). Spearman correlation coefficient (Logit [sensitivity] vs Logit [1 − specificity]) was 0.900 (P = 0.037). They revealed evidence supporting the diagnostic threshold (cut-off) bias as a cause of heterogeneity. The Begg test and Egger test did not reveal significant publication bias (P = 1.000 and 0.641).
3.3. The performance of WHtR
Meta-analysis showed pooled sensitivity to detect the elevated BP of 0.43 (95%CI: 0.41–0.45) and pooled specificity of 0.77 (95%CI: 0.76–0.77). LR+ was 1.93 (95%CI: 1.44–2.60), LR− was 0.68 (95%CI: 0.55–0.84), and DOR was 2.88 (95%CI: 1.74–4.75) (Table 2). Heterogeneity was observed across studies (I2 = 94.8%–99.6%). Spearman correlation coefficient (Logit [sensitivity] vs Logit [1 − specificity]) was 0.800 (P = 0.104). The diagnostic threshold (cut-off) bias was not a cause of heterogeneity. The Begg test and Egger test did not reveal significant publication bias (P = 1.000 and 0.739).
3.4. Comparison between BMI, WC, and WHtR
The AUCs of obesity indices were 0.7780 (BMI), 0.7181 (WC), and 0.6697 (WHtR), respectively (Fig. 2). Multivariate meta-regression analysis showed that the diagnostic accuracy of BMI and WC was similar even after adjusting covariates (RDOR = 0.83, 95%CI: 0.65–1.05, P = 0.112) (Table 3). Multivariate meta-regression analysis showed that the diagnostic accuracy of BMI and WHtR was similar even after adjusting covariates (RDOR = 0.79, 95%CI: 0.59–1.05, P = 0.098) (Table 3).
Figure 2.
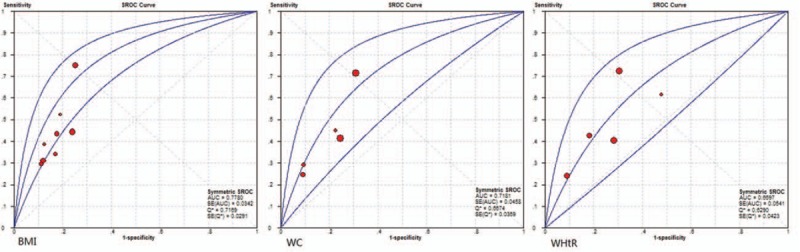
The summary receiver operating characteristic (SROC) curves for the studies examining obesity indices for the assessment of elevated blood pressure in children and adolescents.
Table 3.
Multivariate meta-regression analyses to compare the diagnostic performance between BMI, WC, and WHtR after the adjustment of other study-specific covariates.

4. Discussion
The present meta-analysis showed that the obesity indices have low pooled sensitivity and moderate pooled specificity in identifying elevated BP. Pooled results from the obesity indices showed that sensitivities were only 42% and 43%, suggesting over half of children with undiagnosis. Although the prevalence of hypertension in normal weight is low, the number of children living with hypertension in normal weight is not low. Obesity indices are not appropriate screening methods for elevated BP in children. However, this does not mean that obesity indices cannot be used in clinical practice.
Obesity is an important driver of the increased prevalence in childhood hypertension.[27] A lot of epidemiologic research confirmed the association between obesity and increased risk for hypertension.[27–31] Successful weight loss can improve BP status in obese children.[32,33] The evaluation of obesity is very important in childhood hypertension management. However, BP also should be measured in children with normal weight.
As is well known, body fat distribution is closely related to the occurrence and development of cardiovascular disease.[34] Visceral adipose tissue (VAT) accumulation was associated with greater free fatty acids flux[35] and insulin resistance and increased risk of hypertension.[36] WC and WHtR are good markers of abdominal obesity, and BMI is a marker of increases in overall adiposity. Magnetic resonance imaging and computed tomography are considered to be the most accurate approaches for the quantification of VAT. WC and WHtR were also strongly correlated with VAT assessed by magnetic resonance imaging and computed tomography.[37,38] However, our study indicates that WC and WHtR were no better than BMI to identify the risk of elevated BP in children.
Unlike adults, BMI is equivalently predictive and provides sufficient information to assess visceral adiposity in children and adolescents. WC does not add additional predictive value.[39] Harrington et al[40] found that 95th Centers for Disease Control and Prevention BMI percentile is a useful threshold for the prediction of elevated levels of VAT in children and adolescents. WC and BMI are equally correlated with VAT in a pediatric population. The amount of VAT in young adult men was associated with BMI changes specifically during adolescence.[41] So, WC and WHtR are not better screening tools than BMI for childhood hypertension.
The study has 2 limitations. First, repeated BP measurements are required to confirm the diagnosis of hypertension in children and adolescents. The prevalence of hypertension tends to decrease in subsequent visits.[28] In this meta-analysis, the BP measurements were based on 3 visits in only 1 study.[22] The prevalence of hypertension may be overestimated in these studies. Second, some new obesity indices, such as neck circumference[26] and mid-upper arm circumference,[42] had been used to screen elevated BP in children. These obesity indices were not in the meta-analysis because related research is few.
In conclusion, the present meta-analysis showed that the association with BP may not be strong enough for both indices to be used as efficient tools to identify elevated BP in children. Our findings do not support the performance of WC and WHtR is superior to BMI to help identify children with elevated BP.
Footnotes
Abbreviations: AUC = area under the curve, BMI = body mass index, BP = blood pressure, CI = confidence interval, DOR = diagnostic odds ratio, LR− = negative likelihood ratio, LR+ = positive likelihood ratio, QUADAS = Quality Assessment for Studies of Diagnostic Accuracy, VAT = visceral adipose tissue, WC = waist circumference, WHtR = waist-to-height ratio.
Authorship: FY contributed to the study's conception and design. All authors contributed to the study's performance.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Feber J, Ahmed M. Hypertension in children: new trends and challenges. Clinl Sci (Lond, Engl: 1979) 2010; 119:151–161. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation 2008; 117:3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litwin M, Feber J, Ruzicka M. Vascular aging: lessons from pediatric hypertension. Can J Cardiol 2016; 32:642–649. [DOI] [PubMed] [Google Scholar]
- 4.Sorof JM, Alexandrov AV, Garami Z, et al. Carotid ultrasonography for detection of vascular abnormalities in hypertensive children. Pediatr Nephrol 2003; 18:1020–1024. [DOI] [PubMed] [Google Scholar]
- 5.Lande MB, Carson NL, Roy J, et al. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension 2006; 48:40–44. [DOI] [PubMed] [Google Scholar]
- 6.Dobson CP, Eide M, Nylund CM. Hypertension prevalence, cardiac complications, and antihypertensive medication use in children. J Pediatr 2015; 167:92–97. [DOI] [PubMed] [Google Scholar]
- 7.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92:1257–1264. [DOI] [PubMed] [Google Scholar]
- 8.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension 2002; 40:441–447. [DOI] [PubMed] [Google Scholar]
- 10.Luma GB, Spiotta RT. Hypertension in children and adolescents. Am Fam Physician 2006; 73:1558–1568. [PubMed] [Google Scholar]
- 11.Lee SK, Kim MK. Relationship of sodium intake with obesity among Korean children and adolescents: Korea National Health and Nutrition Examination Survey. Br J Nutr 2016; 115:834–841. [DOI] [PubMed] [Google Scholar]
- 12.Gunter KB, Nader PA, John DH. Physical activity levels and obesity status of Oregon Rural Elementary School children. Prev Med Rep 2015; 2:478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baszun-Stepaniuk E, Urban M, Glowinska B. Left ventricular mass and systolic-diastolic function of the heart in children and adolescents with hypertension and hypertension accompanying obesity. Przegl Lek 2005; 62:206–209. [PubMed] [Google Scholar]
- 14.Thompson M, Dana T, Bougatsos C, et al. Screening for hypertension in children and adolescents to prevent cardiovascular disease. Pediatrics 2013; 131:490–525. [DOI] [PubMed] [Google Scholar]
- 15.Koebnick C, Black MH, Wu J, et al. High blood pressure in overweight and obese youth: implications for screening. J Clin Hypertens 2013; 15:793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004; 114 (2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 17.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003; 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosa ML, Mesquita ET, da Rocha ER, et al. Body mass index and waist circumference as markers of arterial hypertension in adolescents. Arq Bras Cardiol 2007; 88:573–578. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes RA, Christofaro DG, Buonani C, et al. Performance of body fat and body mass index cutoffs in elevated blood pressure screening among male children and adolescents. Hypertens Res 2011; 34:963–967. [DOI] [PubMed] [Google Scholar]
- 20.Christofaro DG, Ritti-Dias RM, Fernandes RA, et al. High blood pressure detection in adolescents by clustering overall and abdominal adiposity markers. Arq Bras Cardiol 2011; 96:465–470. [DOI] [PubMed] [Google Scholar]
- 21.Motswagole BS, Kruger HS, Faber M, et al. The sensitivity of waist-to-height ratio in identifying children with high blood pressure. Cardiovasc J Afr 2011; 22:208–211. [DOI] [PubMed] [Google Scholar]
- 22.Khoury M, Manlhiot C, Dobbin S, et al. Role of waist measures in characterizing the lipid and blood pressure assessment of adolescents classified by body mass index. Arch Pediatr Adolesc Med 2012; 166:719–724. [DOI] [PubMed] [Google Scholar]
- 23.Chiolero A, Paradis G, Maximova K, et al. No use for waist-for-height ratio in addition to body mass index to identify children with elevated blood pressure. Blood Press 2013; 22:17–20. [DOI] [PubMed] [Google Scholar]
- 24.Kajale NA, Khadilkar AV, Chiplonkar SA, et al. Body fat indices for identifying risk of hypertension in Indian children. Indian Pediatr 2014; 51:555–560. [DOI] [PubMed] [Google Scholar]
- 25.Bauer KW, Marcus MD, El ghormli L, et al. Cardio-metabolic risk screening among adolescents: understanding the utility of body mass index, waist circumference and waist to height ratio. Pediatr Obes 2015; 10:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuciene R, Dulskiene V, Medzioniene J. Association of neck circumference and high blood pressure in children and adolescents: a case-control study. BMC Pediatr 2015; 15:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostchega Y, Carroll M, Prineas RJ, et al. Trends of elevated blood pressure among children and adolescents: data from the National Health and Nutrition Examination Survey1988–2006. Am J Hypertens 2009; 22:59–67. [DOI] [PubMed] [Google Scholar]
- 28.Meng L, Liang Y, Liu J, et al. Prevalence and risk factors of hypertension based on repeated measurements in Chinese children and adolescents. Blood Press 2013; 22:59–64. [DOI] [PubMed] [Google Scholar]
- 29.Marrodan Serrano MD, Cabanas Armesilla MD, Carmenate Moreno MM, et al. Association between adiposity and blood pressure levels between the ages of 6 and 16 years. Analysis in a student population from Madrid, Spain. Rev Esp Cardiol 2013; 66:110–115. [DOI] [PubMed] [Google Scholar]
- 30.Abolfotouh MA, Sallam SA, Mohammed MS, et al. Prevalence of elevated blood pressure and association with obesity in Egyptian school adolescents. Int J Hypertens 2011; 2011:952537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aounallah-Skhiri H, El Ati J, Traissac P, et al. Blood pressure and associated factors in a North African adolescent population. A national cross-sectional study in Tunisia. BMC Public Health 2012; 12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalarchian MA, Levine MD, Arslanian SA, et al. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics 2009; 124:1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shalitin S, Ashkenazi-Hoffnung L, Yackobovitch-Gavan M, et al. Effects of a twelve-week randomized intervention of exercise and/or diet on weight loss and weight maintenance, and other metabolic parameters in obese preadolescent children. Horm Res 2009; 72:287–301. [DOI] [PubMed] [Google Scholar]
- 34.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012; 126:1301–1313. [DOI] [PubMed] [Google Scholar]
- 35.Adler-Wailes DC, Periwal V, Ali AH, et al. Sex-associated differences in free fatty acid flux of obese adolescents. J Clin Endocrinol Metab 2013; 98:1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JA, Park HS. Association of abdominal fat distribution and cardiometabolic risk factors among obese Korean adolescents. Diabetes Metab 2008; 34:126–130. [DOI] [PubMed] [Google Scholar]
- 37.Savgan-Gurol E, Bredella M, Russell M, et al. Waist to hip ratio and trunk to extremity fat (DXA) are better surrogates for IMCL and for visceral fat respectively than for subcutaneous fat in adolescent girls. Nutr Metab 2010; 7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Connor M, Ryan J, Foley S. Best single-slice location to measure visceral adipose tissue on paediatric CT scans and the relationship between anthropometric measurements, gender and VAT volume in children. Br J Radiol 2015; 88:20140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koren D, Marcus CL, Kim C, et al. Anthropometric predictors of visceral adiposity in normal-weight and obese adolescents. Pediatr Diabetes 2013; 14:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrington DM, Staiano AE, Broyles ST, et al. BMI percentiles for the identification of abdominal obesity and metabolic risk in children and adolescents: evidence in support of the CDC 95th percentile. Eur J Clin Nutr 2013; 67:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kindblom JM, Lorentzon M, Hellqvist A, et al. BMI changes during childhood and adolescence as predictors of amount of adult subcutaneous and visceral adipose tissue in men: the GOOD Study. Diabetes 2009; 58:867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma CM, Li Y, Gao GQ, et al. Mid-upper arm circumference as a screening measure for identifying children with hypertension. Blood Press Monit 2015; 20:189–193. [DOI] [PubMed] [Google Scholar]


