Abstract
Background:
Pancreatic cancer (PC) remains difficult to treat, despite the recent advances in various anticancer therapies. Immuno-inflammatory response is considered to be a major risk factor for the development of PC in addition to a combination of genetic background and environmental factors. Although patients with PC exhibit evidence of systemic immune dysfunction, the PC microenvironment is replete with immune cells.
Methods:
We searched PubMed for all relevant English language articles published up to March 2016. They included clinical trials, experimental studies, observational studies, and reviews. Trials enrolled at Clinical trial.gov were also searched.
Results:
PC induces an immunosuppressive microenvironment, and intratumoral activation of immunity in PC is attenuated by inhibitory signals that limit immune effector function. Multiple types of immune responses can promote an immunosuppressive microenvironment; key regulators of the host tumor immune response are dendritic cells, natural killer cells, macrophages, myeloid derived suppressor cells, and T cells. The function of these immune cells in PC is also influenced by chemotherapeutic agents and the components in tumor microenvironment such as pancreatic stellate cells. Immunotherapy of PC employs monoclonal antibodies/effector cells generated in vitro or vaccination to stimulate antitumor response. Immune therapy in PC has failed to improve overall survival; however, combination therapies comprising immune checkpoint inhibitors and vaccines have been attempted to increase the response.
Conclusion:
A number of studies have begun to elucidate the roles of immune cell subtypes and their capacity to function or dysfunction in the tumor microenvironment of PC. It will not be long before immune therapy for PC becomes a clinical reality.
Keywords: B cell, dendritic cell, macrophage, myeloid cell, natural killer cell, pancreatic cancer, T cell
1. Introduction
Pancreatic ductal adenocarcinoma, commonly known as pancreatic cancer (PC), is among the deadliest of human malignancies. Despite the recent advances in surgery, chemotherapy, radiotherapy, and recently developed targeted therapies, PC continues to have less than a 10% 5-year survival rate.[1] Immunotherapy has demonstrated efficacy in the treatment of several types of solid tumors; there has been great in interest regarding the role of immune cells in PC and applying various immunotherapeutic approaches to PC.[2,3]
Although PC is distinguished by prominent desmoplasia (fibrosis), its microenvironment is also replete with immune cells.[4] Despite the presence of many immune cells in PC, immune dysfunction is observed in patients with PC where the tumor microenvironment is immunosuppressive, thus inhibiting the activation or function of immune effectors.[5,6] These immune defects develop in the earliest precancerous lesions.[7] Recent studies have reported that the immune cells in PC interact in the tumor microenvironment such as pancreatic stellate cells (PSCs),[8,9] and anticancer drugs have immune-modulatory effects in PC.[10–12] Therefore, the relationship of immune cells with neighboring stroma and chemotherapeutic reagents is critical to consider for future therapeutic development.
The present review provides updated discussion on cellular immunity in PC including interaction with PSC and chemotherapeutic reagents and its clinical therapeutic application. For this review, we searcheed PubMed for all relevant English language articles published up to March 2016. They included clinical trials, experimental studies, observational studies, and reviews. Trials enrolled at Clinical trial.gov were also searched. Ethical approval was not necessary because this study is a review without involving patients.
2. Immunopathogenesis of PC
Although a combination of genetic background and environmental factors is needed for development of PC, chronic inflammation is also considered to be a major risk factor. The general hypothesis for the pathogenesis of PC is that subclinical acute injuries accumulate and become chronic, leading to genetic instability and, ultimately, deleterious mutations. The cancer immunoediting theory posits that resultant malignancies are recognized by the immune system and are either eliminated, reach equilibrium, or achieve escape.[13] Elimination occurs when immuno-inflammatory cells destroy early-staged genetically unstable or altered premalignant cells. In the event that elimination is not entirely successful, the host immune system and the genetically altered cells that survive the elimination process enter into a dynamic equlibrium. When new variants with mutation accumulated continuously and exceed the limit, immunologic elimination response becomes insufficient, and tumor cell variants acquires resistance. Furthermore, the immuno-inflammatory cells exhibit altered function with subsequent production of immunosuppressive signals, as well as inflammatory cytokines that promote tumor growth and invasion.[14,15] Finally, the tumor microenvironment has a highly immunosuppressive composition that contributes further to immune evasion.
3. Immunosuppression in PC
PC has many T cells, thus it is classified as a T-cell rich tumor, like microsatellite instability colorectal cancer. Although both innate and adaptive immune responses are active against the tumors, PC by itself induces local and systemic immune dysfunction or immunosuppression to prevent eradication of PC by effector immune cells.[16,17] PC interferes with antigen cross-presentation to effector T cells by downregulating the expression of major histocompatibility complex (MHC) class I molecules or antigen insertion into the MHC class I groove (Fig. 1A).[18] Nonfunctional Fas receptors of PC cells render them resistant to Fas-mediated apoptosis, and the expression of functional Fas ligand on them induces apoptosis in cancer-infiltrating effector T cells and natural killer (NK) cells.[19] PC secretes soluble immunosuppressive factors such as interleukin (IL)-10 and transforming growth factor-beta (TGF-β) that encourage the influx of suppressive immune cells and augment suppressor cell function.[20,21] Indoleamine 2,3-dioxygenase (IDO) in PC catalyzes the breakdown of tryptophan to kynurenine, and suppresses antitumor T cell responses by starving T lymphocytes of tryptophan, thereby inducing tolerance to tumor-derived antigens (Fig. 1B).[22] In addition, the expression of IDO in metastatic PC cells of lymph node was associated with increase of regulatory T cells (Tregs).[23] PC expresses immune system checkpoint ligands such as programmed cell death ligand 1 (PD-L1), which is another mechanism through which effector cells are suppressed (Fig. 1C).[24] The immunosuppressive cells, including tumor-associated macrophages, myeloid-derived suppressor cells (MDSC), and Tregs, appear in the early precursor lesions of PC and persist through invasive cancer in a mouse model.[25]
Figure 1.
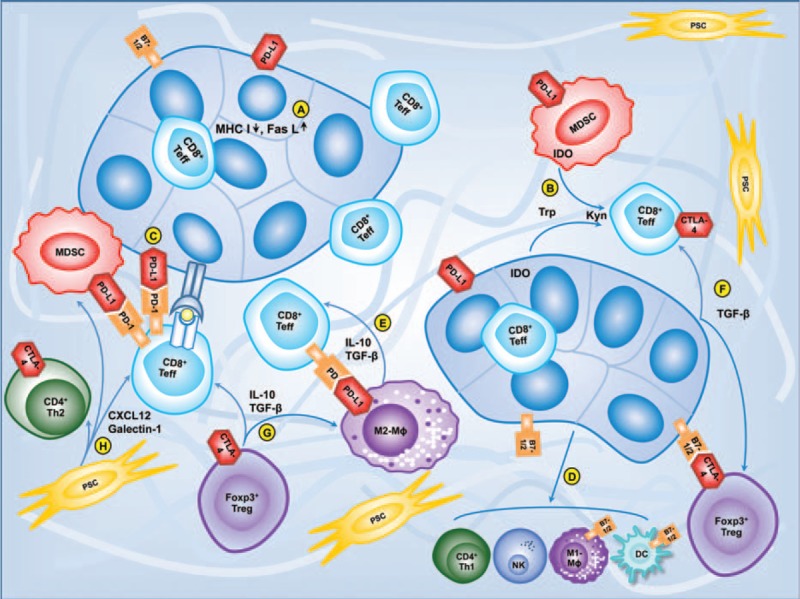
Immunosuppressive interactions among immune cells, pancreatic stellate cells, and pancreatic cancer cells. A, Pancreatic cancer cells downregulate the expression of MHC class I molecules and highly express Fas ligand. B, Pancreatic cancer cells and myeloid-derived suppressor cells suppress CD4+ and CD8+ T cells by activation of indoleamine 2,3-dioxygenase (IDO) which catalyzes the breakdown tryptophan (trp) to kynurenine (kyn). C, Pancreatic cancer cells express PD-L1 producing inhibitory signal by binding PD-1 on T cells. D, Pancreas cancer cells suppress dendritic cells and natural killer cells and skew differentiation of macrophages and Th cell. E, Tumor-associated macrophages produce inhibitory signal through TGF-β, IL-10, and PD-L1. F, Pancreatic cancer cells inhibit CD8+ T cells and recruit Treg cells by TGF-β. G, Treg cells suppress CD4+ and CD8+ T cells, macrophages, natural killer cells, and dendritic cells with secretion of IL-10 and TGF-β. H, Pancreatic stellate cells/fibroblasts reduce CD8+ T cell migration and promote Th2 cytokine secretion through CXCL12 and galectin-1. CXCL12 = chemokine ligand 12, IL = interleukin, MHC = major histocompatibility complex, PD-1 = programmed cell death-1, PD-L1 = programmed cell death ligand 1, TGF-β = transforming growth factor-beta, Th cells = helper T cells.
4. Innate immune cells in PC
4.1. Dendritic cells
Dendritic cells (DCs) are professional antigen-presenting cells that are among the main regulators of the antitumor immune response (Table 1 ).[4,7–9,26–55] DCs facilitate antigen presentation to CD4+ and CD8+ T cells through capture, internalization, processing, and presentation of tumor antigens via MHC class I and II molecules.[56] PC inhibits the capacity of DC through compromised recruitment, maturation, and survival (Fig. 1D).[57,58] When DCs and tumor interact, levels of cytokines and chemokines responsible for DCs suppression, such as IL-10, TGF-β, and granulocyte-macrophage colony-stimulating factor (GM-CSF), are increased, but those for DCs activation are inhibited mainly through activation of signal transducer and activator of transcription 3 (STAT3).[59] STAT3 ablation can lead to maturation of DCs and restore DC functions in tumor-bearing mice.[60]
Table 1.
Immune cells in the microenvironment of pancreas cancer.

Table 1 (Continued).
Immune cells in the microenvironment of pancreas cancer.
DCs are rare in the tumor microenvironment; the cells are located at the edges of the tumor, excluded from the tumor mass.[61] Reduction in the level of DCs in circulating blood and impairment in the stimulatory function of circulating DCs has been reported in patients with PC.[27,62] However, the presence of circulating DCs is related to prolonged survival in both resectable and unresectable PC.[27,62,63] In addition, higher preoperative circulating DCs count significantly reduced the risk of septic complications after pancreatectomy.[64] Radical resection, systemic chemotherapy, chemoradiotherapy, or immune-chemotherapy in patients with PC can increase the numbers of circulating DCs or restore their stimulatory function.[26–28] These findings suggest that immune therapy targeted at increasing DCs and improving DC function might be beneficial.
DC-based immunotherapy in PC has been investigated since the 1990s, and has been considered at various times to be a promising therapy for patients with advanced PC.[65] Administration of DCs pulsed with alpha-galactosylceramide, carcinoembryonic antigen (CEA) mRNA, tumor lysate, or apoptotic tumor cells in PC activates antigen-specific cellular components of antitumor response, leading to expansion of interferon gamma (IFN-γ) producing natural killer T cells, activation of cytotoxic T lymphocyte (CTL), suppression of tumor growth, and prolonged survival of patients.[66–68] Also, antigenic peptide-pulsed DCs have been recently reported to be superior to vaccines based on peptide and adjuvant.[69] Several studies have shown that DC vaccine and chemotherapeutic agents have a synergistic effect on PC, inducing tumor antigen-specific CTL. In cell and animal studies, gemcitabine-treated PC cell medium stimulated maturation of DCs inducing antitumor activity of CTL,[11] and concomitant gemcitabine and DC vaccine therapy increased the survival and facilitated recruitment of CD8+ T cells and CTL-mediated tumor cell lysis in a murine PC model.[70,71] In human studies, DCs were recovered and increased after cisplatin and gemcitabine chemotherapy,[29] and DC vaccine combined with chemotherapy was possibly regarded as safe and synergistically effective for inducing tumor antigen-specific CTLs.[30,72] A recent multicenter clinical study suggested the possible survival benefits of peptide-pulsed DC vaccines combined with standard chemotherapy in patients with PC.[31]
4.2. Natural killer cells
NK cells play a role both in innate and adaptive immunity against tumor by secreting cytokines such as IL-3, tumor necrosis factor-alpha, GM-CSF, and IFN-γ.[73] The numbers of circulating NK cells are reduced in patients with advanced PC[29], and pretreatment levels of peripheral NK cells positively correlate with survival in patients with PC.[32] These findings indicate that NK cells exert some control over tumor progression. Based on histologic evaluation, Ene-Obong et al[8] recently demonstrated reduction in numbers of NK cells and CD8+ T cells in the juxta-tumoral area as compared with the pan-stromal area of human PC, potentially due to sequestration and preferential migration to activated pancreatic satellite cells. Moreover, human PC highly expresses the Fas ligand that leads to apoptosis of tumor infiltrating lymphocytes, including NK cells.[74]
In addition to the afore-mentioned numerical suppression, significant impairment in cytotoxicity of NK cells was demonstrated in PC.[29,75] Funa et al[75] showed that both basal NK activity and in vitro responses of NK cells toward IFN-α were reduced in patients with PC. The activity of NK cells is controlled by signals that are produced from activating receptors or inhibitory receptors. The activating receptors such as natural killer group 2 member D (NKG2D), NKp30, and NKp46 are down-regulated in patients with PC, and the reduced levels of them were related to tumor progression in early stage.[76] Of these, NKG2D is a well-studied activating receptor expressed on NK cells, T cells, and natural killer T cells. MHC class I chain-related molecule A (MICA) is a ligand of NKG2D. It is not expressed on normal tissues but is frequently found on epithelial tumors.[77] The cells expressing MICA are susceptible toward attack by NK cells and antigen-specific T cells. Duan et al[78] reported that the level of preoperative soluble MICA and NKG2D expression are prognostic factors in patients who undergo resection of PC. NK activity of hepatic non-parenchymal cells has been demonstrated to be depressed in rats with obstructive jaundice, which may enhance growth of liver metastases in PC with obstructive jaundice.[79] Recently, CD56+CD16− NK cells have been reported as unique immune cells developed from a regressing metastatic lesion following the treatment with anticytotoxic T-lymphocyte associated protein-4 (CTLA-4) in patients with PC.[80] Although CD56+CD16− NK cells are small subsets of overall NK cells, they could lyse PC cell lines targets and secreted IFN-γ when cultured in the presence of high dose of IL-2. This finding supports the possibility that NK cell activation by immune check point inhibition can be a help in the treatment of PC. The efficacy of NK cell immunotherapy is being tested in a clinical trial in combination with irreversible electroporation for advanced pancreatic cancer (NCT02718859).
4.3. Macrophages
Macrophages in or near the tumor are particularly designated as tumor-associated macrophages. Macrophages are increased in the tumor, and the distribution of tumor-associated macrophages is related to prognosis in many human cancers.[81] In human PC, macrophages are prominent compared with normal pancreas.[82] Additionally, macrophages infiltrate in low-grade, preinvasive pancreatic tumor lesions and persist to invasive cancer in a mouse model of PC.[25] Tumor cells influence macrophages reciprocally in the tumor microenvironment. The PC cells induce differentiation and education of macrophages, and consequently tumor-educated macrophages enhance the progression of PC.[83,84] As a result, macrophages function in the tumor by facilitating tumor growth, angiogenesis, stromal remodeling, and metastasis.[83]
Macrophages are roughly divided into 2 subtypes: M1 and M2. M1 macrophages are classically activated to have proinflammatory properties, while M2 macrophages are alternately activated to have anti-inflammatory properties. For discriminating markers, CD68 is a pan-macrophage marker, HLA-DR and CD11c identify M1 macrophages, and CD163 and CD204 are expressed by M2 macrophages.[85] In relation to tumors, M1 macrophages have anti-tumor properties, while M2 macrophages are associated with tumor-promoting properties.[84] Cytokines like IL-4, IL-10, and IL-13 from the tumor and T cells induce differentiation of macrophages to the M2 phenotype in the tumor; thus M2 macrophages rather than M1 macrophages are predominant in tumors.[84] It has been demonstrated in vitro that naive macrophages can be converted to M2 phenotype by PC culture supernatants.[86] The protumor properties of M2 polarized macrophage include suppression of adaptive immune response and promotion of matrix remodeling and angiogenesis.[87,88] Furthermore, M2 macrophages induce epithelial–mesenchymal transition that is critically related to metastasis. Liu et al[89] reported that epithelial–mesenchymal transition was induced by activation of toll-like receptor 4 on M2 macrophages by stimulating an increase in IL-10 production by PC cells. The phenotype of macrophages in the tissues has been reported to be associated with the prognosis of PC. Ino et al[4] reported %M1/M2 in patients with PC as an independent positive prognostic factor. Furthermore, M2 macrophges are related to poor prognosis and presence of M2 macrophages in the tumor periphery has been associated with large tumor size, accelerated lymphatic metastasis, local recurrence, and reduced survival.[33,34] In recent studies, infiltration of M2 macrophages at extrapancreatic nerve plexuses was shown to be an independent negative prognostic factor,[35] and node-infiltrating M2 macrophages promoted regional lymph node metastasis through production of vascular endothelial growth factor C.[90] Although the M1-M2 dichotomous phenotyping is useful and generally accepted, many macrophages demonstrate overlapped M1 and M2 phenotype. Recently, a new multidimensional model of macrophage activation has been suggested to decode this complexity.[91]
Macrophages are recruited into tumors and interact with the immune system by a number of mechanisms involving various cytokines and chemokines. Vascular endothelial growth factor receptor 2 (VEGFR2) expressed on macrophages is associated with the recruitment of macrophages, and selective inhibition of VEGFR2 inhibited infiltration of macrophages into orthotopic murine pancreatic tumors.[92] Macrophages also contribute toward the creation of an immune suppressive tumor microenvironment through secretion or expression of cytokines and chemokines such as TGF-β, IL-10, CCL17, CCL18, CCL22, and PD-L1 (Fig. 1E).[93] Interaction of macrophage inflammatory protein-3α with its chemokine receptor 6 expressed in PC cells had been reported to promote the invasion of PC cell through up-regulation of matrix metalloproteinase-9.[94,95]
Immune therapy involving strategies to modulate or ablate the macrophages may have therapeutic potential. Colony-stimulating factor 1 receptor (CFS1R) is expressed by macrophages and monocytes. Recently, blockade of CFS1R has been reported to enhance antigen presentation and antitumor T-cell immune responses in PC mouse model.[96] Decoy receptor 3 (DcR3) is another therapeutic candidate. DcR3 is a member of the TNF receptor superfamily and is overexpressed in PC.[97] DcR3 up-regulates genes related to macrophages and down-regulates expression of MHC-II and HLA-DR on macrophages.[98] In addition, the expression level of DcR3 is inversely correlated with survival of patients with PC. Therefore, DcR3 is a potential target for immunotherapy to modulate macrophages to enhance anti-tumor immune responses against PC.
4.4. Myeloid-derived suppressor cells
MDSCs are a mixture of immature myeloid cells including immature stages of macrophages, granulocytes, and DCs. MDSCs comprise 2 types of cells, polymorphonuclear granulocytic MDSCs and mononuclear monocytic MDSCs.
The identification of MDSCs remains a difficult task due to their heterogeneity and lack of a single defining surface marker. MDSCs express CD11b/Gr-1 in mice and lack the markers of mature myeloid cells.[99] In humans, IL-4Rα, CD11b, CD33, and low levels of CD 15 are expressed in both MDSCs, whereas CD14 and low level of CD 15 are expressed in monocytic MDSCs.[100,101] In the presence of tumor, differentiation of immature myeloid cells is skewed toward the expansion of MDSCs. For the development and recruitment of MDSCs, tumor-derived GM-CSF has been suggested to be an important regulator in a genetically engineered mouse model (GEMM) of PC.[102] In the tumor, MDSCs equally suppress CD4+ and CD8+ T cells, and expand immunosuppressive Tregs. Moreover, MDSCs impede innate immunity by conversion of macrophages to M2 phenotype and suppression of NK cells and NK T cell.[103,104]
MDSCs suppress T cells through multiple mechanisms such as depletion of L-arginine, use of reactive oxygen species (ROS) or free radical peroxynitrate, and down-regulation of L-selectin.[105–107] Since both monocytic and granulocytic MDSCs use arginase to catabolize L-arginine, MDSCs expressing high levels of arginase deplete L-arginine in tumor environment, thus hindering protein synthesis by T cells by limiting L-arginine availability.[107] MDSCs can also sequester cysteine that is required for T cell activation.[108] Interaction of MDSCs with antigen-specific T cells results in increase in ROS production, and MDSCs suppress CD8+ cell response using ROS in tumor.[109] MDSCs are the predominant source of free radical peroxynitrate that can mediate tumor resistance to CTL by inhibiting binding of processed tumor peptides to MHC molecules.[106] In addition, MDSCs inhibit antitumor immunity empolying another mechanism that impairs T cell homing to lymph node via down-regulation of L-selectin in CD4+ and CD8+ T cells.[107]
MDSCs levels are increased in both the circulation and the microenvironment of PC. Clark et al[25] reported progressive increase of MDSCs from PanIN lesions to PC in GEMM, and also showed correlation between intratumoral MDSCs and the lack of tumor-infiltrating CD8+ cells. These findings indicate that immune suppression by MDSCs is related to the progression from premalignant lesions to PC, and MDSCs follow the histologic progression of PC. Similar to the tumor microenvironment, the MDSCs in the circulation are elevated and correlate with the stage in patients with PC. Gabitass et al[15] reported that the circulating MDSCs are significantly elevated in patients with PC as compared with healthy controls, and their levels correlate with circulating Treg levels. Moreover, Diaz-Montero et al[110] demonstrated correlation between circulating MDSCs and clinical stage of various cancers including PC. A recent study showed that patients with stable PC had lower circulating MDSCs before initiation of chemotherapy than those with progressive PC.[37] Therefore, the circulating MDSCs could be a predictive marker for establishing response to chemotherapy.
Inhibition of MDSC in PC is a potential method of cancer therapy. Selective depletion of granulocytic MDSCs in autochthonous GEMM of PC enhances apoptosis of tumor cells with increase in level of CD8+ T cells.[111] Zoledronic acid, a aminobisphosphonate and osteoclast inhibitor for osteoporotic bone disease or bone metastasis, has been studied for suppression of MDSCs. Zoledronic acid could inhibit MDSC accumulation and improved the host antitumor response in mice.[38] Unfortunately, a related human study did not demonstrate differences in overall or progression-free survival during pretreatment and posttreatment with zoledronic acid.[39] Various methods to inhibit MDSCs in patients with PC need to be explored.
5. Adaptive immune cells in PC
5.1. T cells
CD3+ T lymphocytes are the main immune infiltrates in PC, and are predominantly found in the stroma of both human and mouse PC.[112] The major components of CD3+ T lymphocytes are CD4+ helper T (Th) cell, CD8+ cytotoxic/effector T cell, and CD4+CD25+Forkhead box P3 (Foxp3)+ Treg. Tregs exhibit suppressive anti-tumor immunity; many studies regarding Tregs in PC have been performed.
5.2. CD4+ helper T cells
CD4+ T cells activate innate immune cells, such as macrophages, and modulate the function of B cells and CD8+ T cells through cytokine secretion and direct cell–cell signaling. A decrease in the circulating level of CD4+ T cells in patients with PC as compared with healthy control has been reported,[29,113] and the number of CD4+ T cells in the tissue was reportedly lower in PC than cases of chronic pancreatitis.[114] Studies employing immunohistochemistry showed higher level of tumor-infiltrating CD4+ T cells favored better survival in PC.[4,115] PC alters CD4+ T cell function by inhibition of CD4+ T cells proliferation and migration.[116]
Classically, CD4+ Th cells differentiate into 2 subsets of cells, Th1 and Th2. Th1 cells induce cell-mediated immune responses by secreting IL-2 and IFN-γ, while Th2 cells assist humoral immune responses by secreting IL-4, IL-5, IL-9, IL-10, and IL-13.[40] With respect to tumors, Th1 cells are involved in tumor-killing responses, but Th2 cells may promote tumor tolerance. Th differentiation is skewed in PC, predominately as Th2 rather than Th1 by the influence of immunosuppressive cytokines. Tassi et al[117] showed that Th cells population skewed toward Th2 cells based on immunohistochemical analysis of PC. Also, serum cytokine levels of Th cells in patients with PC were shifted toward a Th2 cytokine profile.[15,20] Interestingly, this immune deviation toward Th2 in PC is tumor specific, and antiviral CD4+ T cell immunity in patients with PC showed a Th1 type rather than Th2 type.[117] Th2 skewing in PC is influenced by various factors such as cytokines and stromal cells. IL-10 and TGF-β, which are aberrantly produced by PC, had been reported to contribute toward existence of Th2 phenotype.[20] In addition, the fibroblasts in the PC stroma are reported to be associated with a Th2 shift. Using immunohistochemistry for GATA-3, De Monte et al[41] showed that intratumoral Th2 cells infiltration is correlated with thymic stromal lymphopoietin from PC-associated fibroblasts. Moreover, the authors demonstrated that elevated GATA-3/T-bet cell ratios within the tumor-infiltrating cells are a negative survival marker in PC; however, it remains unclear if GATA-3 and T-bet, which are transcription factors, can be used to define Th2 and Th1 cells, respectively, in the absence of functional data.
CD4+ Th cells have functional plasticity in converting from Th2 to Th1, and vice versa. Accordingly, reversal of Th2 differentiation to Th1 is a potential therapeutic target. CEA-specific Th2 cells from PC patients could be reverted to Th1 type by combination of IL-12 and IL-27.[118] In another study, administration of immune-enhancing diets enriched with omega-3 fatty acids, arginine, and RNA before pancreaticoduodenectomy modulated Th differentiation into Th1 rather than Th2, and significantly reduced postoperative infectious complications.[40]
5.3. CD8+ cytotoxic/effector T cells
CD8+ T cells are primarily cytotoxic effector cells with the ability to lyse target cells. CD8+ T cells diminish in circulation and tumor tissues in PC patients. Several studies have revealed decrease in circulating level of CD8+ T cells in patients with PC as compared with the healthy controls.[28,29,119] The infiltration of CD8+ T cells may be inhibited by PC and tumor progression. However, we previously found that CD8+ effector T cells were the most prevalent T cell population in the majority of human PCs.[42] In another study, compared with chronic pancreatitis, the number of CD8+ T cells was found to be lower in PC.[114] Hiraoka et al[7] showed that CD8+ T cells markedly infiltrated in low-grade premalignant pancreatic lesion, but were reduced during the progression of PanINs and IPMNs. CD8+ T cells may be reduced near epithelial carcinoma cells, and 1 of the suggested reasons for this is that the pancreatic stellate cells affect the migration of CD8+ T cells, thus preventing their access to PC cells,[8] CD8+ T cells are associated with PC progression and prognosis of patients with PC. Tumor-infiltrating CD8+ cells together with CD4+ cells served as a favorable prognostic factor,[115] and high densities of CD8+ T cells in the juxta-tumoral area showed better survival in patients with PC.[8]
PC inhibits or prevents CD8+ T cells-mediated tumor cytotoxicity. With expression of TGF-β, PC inhibits CD8+ T cells from expressing genes encoding cytolytic proteins, such as perforin and granzyme (Fig. 1F).[120,121] Furthermore, PC cells frequently lose the expression of MHC class I, which prevents CD8+ T cells from exerting a cytotoxic effect on PC.[18] Importantly, PC cells express PD-L1 that binds to programmed cell death-1 (PD-1) expressed on the surface of activated T cells, and the binding impairs T cell function leading to T cell anergy or death.[122] Consequently, PD-1 activation blunts the host immune response toward PC, and subsequently promotes tumor progression. Similar to PD-1, CTLA-4 is an inhibitory receptor on T cells. Inhibition of CTLA-4 results in analogous outcomes as demonstrated by inhibition of PD-1.[123] PD-1 or CTLA-4 inhibition is a promising immune therapeutic method and has been actively tested in clinical trials with respect to various cancers. PD-1 inhibition leads to increases in effector CD8+ T cells with their production of tumor-specific IFN-γ in PC.[124] PD-1 blockade as combined with GM-CSF secreting PC vaccine (GVAX) improved the survival in mice compared with PD-1 blockade or GVAX monotherapy.[124] Rosiglitazone, a drug employed for the treatment of type II diabetes, has been introduced as it possesses immune modulating effects. Bunt et al[43] showed that rosiglitazone combined with gemcitabine increased peripheral CD8+ T cells and intratumoral CD4+ and CD8+ T cells in PC mice model. Since TGF-β imparts potent immunosupressive signals to CD8+ T cells and its expression is related to tumor progression, it is hypothesized that TGF-β gene silencing may restore antitumor immunity. Ellermeier et al[125] reported that TGF-β gene silencing with activation of retinoic acid-inducible gene I overcame tumor-induced CD8+ T cell suppression leading to prolonged survival in a PC mouse model.
5.4. Regulatory T cells
Immune tolerance and suppression by T cells has been known since 1970.[126] IL-2 receptor α-chain (CD25) was identified for the CD4+CD25+ cells down-regulating immnune response in 1995,[127] and Foxp3 was introduced as a key transcription factor for development and function of Treg in 2003.[128,129] Phenotypically, Tregs are defined as CD4+CD25+Foxp3+ cells.
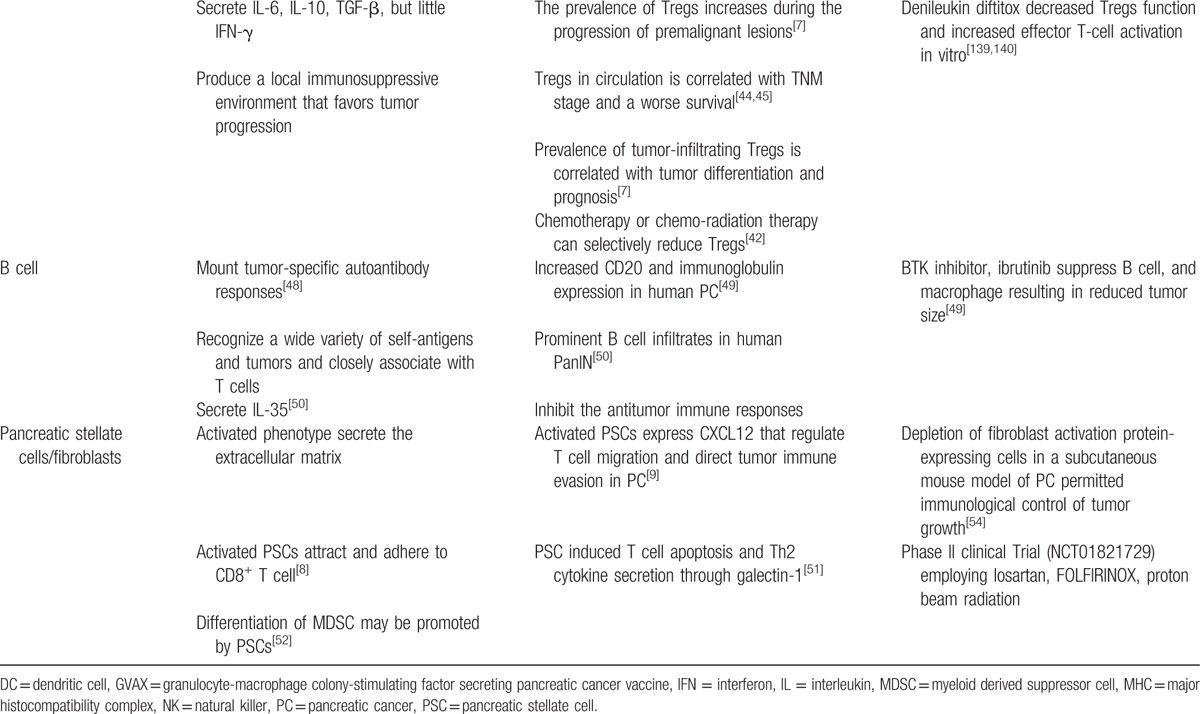
Tregs contribute toward immune suppressive activity through the expression of CTLA-4 and secretion of IL-10 and TGF-β (Fig. 1G). In physiological state, Tregs prevent autoimmune response; whereas in the tumor, Tregs suppress antitumor immune response favoring tumor growth. This involves suppression of tumor-specific CD4+ and CD8+ T cells, macrophages, NK cells, and DCs in tumor microenvironment.[5,14,130,131] Tregs appear in premalignant lesion of PC, and a gradual increase in Tregs through the progression of PanIN and IPMN to invasive ductal carcinoma has been demonstrated.[7,132] In PC tissue, Tregs are increased as compared with the stroma of non-neoplastic inflammatory pancreas.[7] Interestingly, Tregs more infiltrate adjacent to PC tissue.[133] These pathologic findings suggest that Tregs play a role in modulating the immune response to PC. Recently, α-enolase-specific Tregs have been studied. α-Enolase that is an enzyme expressed on the surface of PC cells is able to induce an immune response in PC patients and promote cell migration and cancer metastasis. The study demonstrated that the levels of α-enolase-specific Tregs increase in PC and functionally attenuate the recruited specific effector Th17 and Th1 cells.[134]
The prognosis of patients with PC is associated with both tumor-infiltrating and circulating Treg. Hiraoka et al[7] demonstrated that the prevalence of intratumoral Tregs is a negative prognostic factor and was related to tumor differentiation in PC. With respect to circulating Tregs, they are elevated in patients with PC as compared with healthy control.[15,44] The prevalence of circulating Tregs was reported to be correlated with the tumor stage and survival.[44,45] Amedei et al[134] showed that 86% of patients with a low α-enolase-specific Treg/Teffector ratio survived more than 10 months compared with those with a high ratio.
Migration of Tregs into PC is controlled by interactions between tumor chemokines and their receptor on Tregs or tumor-induced addressins on the endothelial cells and their ligands on Tregs. Tan et al[135] showed that pancreas cancer produced increased levels of ligands for chemokine receptor type 5 (CCR5) and Tregs expressed CCR5. They also demonstrated that when ligands for CCR5/CCR5 interaction are diminished or blocked, Tregs migrated to a lower extent to the tumor, and even the tumors became smaller in size. Addressins such as vascular cell adhesion molecule-1, mucosal addressin cell adhesion molecule-1, E-selectin, and activated leukocyte cell adhesion molecule (CD166) are highly expressed on tumor-derived endothelial cells. They interact with Tregs and allow selective Tregs trans-migration from peripheral blood to PC.[16] In addition, TGF-β from PC is associated with recruitment of inducible Tregs in the tumor. Tregs secrete TGF-β to suppress other immune cells, while PC secretes TGF-β to induce Tregs.[136] In a mouse model, TGF-β converted CD4+CD25− naïve T cells into Foxp3+ Tregs.[21]
Since Tregs are abundant and may suppress antitumor immune responses in PC, Tregs are an appealing target for immune therapy. CTLA-4 on Tregs produces inhibitory signal and interacts with its ligands CD80 (B7–1) and CD86 (B7–2) on antigen-presenting cells or target tissues. Therefore, anti-CTLA-4 therapy serves to reduce inhibitory signal and/or induce apoptosis of Tregs. Monoclonal antibodies to CTLA-4 have been developed to block this interaction. A previous phase II trial with ipilimumab (anti-CTLA-4) revealed no responses in patients with advanced PC.[46] However, a patient experienced significant delayed response with regression of the primary and hepatic metastasis lesions. CD25 is another target for inhibition of Tregs. Depletion of Tregs with anti-CD25 monoclonal antibodies alone or in combination with a whole tumor cell vaccine promotes smaller pancreatic tumor and longer survival in mouse model.[137] Denileukin diftitox, a fusion protein of IL-2 and active domain of diphtheria toxin, binds with IL-2 receptor and then is internalized into CD25+ cells, with subsequent Tregs death.[138] FDA approved denileukin diftitox for CD25+ cutaneous T-cell lymphoma and leukemia, and its clinical trials combined with anticancer cancer vaccine have been performed in metastatic cancers including PC.[139,140] Recently, antiglucocorticoid-induced TNF receptor monoclonal antibody has been introduced to suppress Tregs. Aida et al[47] showed this monoclonal antibody-induced suppression of Tregs infiltration in PC with down regulation of CCR5 and led to enhancement antitumor immunity of IFN-α gene therapy.
5.5. B cells
The role of B cells in modulating the immune response to PC has not been widely investigated. The majority of patients with cancers mount tumor-specific autoantibody responses,[141] and B cells infiltrate the human tumors.[48,142] Tumor-infiltrating lymphocyte B cells (TIL-Bs) recognize a wide variety of self-antigens and tumors, and closely associate with T cells and other immune cells. TIL-Bs generally show antigen-driven humoral immune response expressing immunoglobulin G.[143,144]
The role of TIL-Bs in relation to the tumors is conversial. There are conflicting reports showing TIL-Bs have tumor-protective or tumor-promoting function.[145,146] TIL-Bs have been reported to be prevalent in human PC tissues. Gunderson et al[147] demonstrated human PC show increased expression of CD20 and immunoglobulin compared with normal pancreas. Pylayeva-Gupta et al[49] detected distinct B cell infiltration in human PanIN as well as in oncogenic Kras-driven pancreatic neoplasms in a LSL-KrasG12D; p48Cre mouse model. They implanted pancreatic ductal epithelial cells expressing oncogenic Kras (KrasG12D-PDEC) into wild-type pancreata. KrasG12D-PDEC induced B cells accumulation in the regions near the newly established neoplastic lesions. This suggested the role of the transformed epithelium in B cell recruitment.
Recent studies have provided compelling evidence that TIL-Bs are involved in the initiation and progression of PC through a subset of B cells that inhibit the antitumor immune responses. KrasG12D-PDECs implanted into mice lacking B cells (μMT mice) had reduced tumor growth compared with tumors grown in wild-type mice. Adoptive transfer of regulatory B cells (CD1dhiCD5+ B-cell subsets) resored tumor growth.[49]KrasG12D mice with pancreas-specific deletion of hypoxia-inducible factor 1α showed significant increases in intrapancreatic B cells.[50]. They featured prominent influx of a CD19+CD43+IgMhiCD5− subset of B1b cells with increased PanIN progression. Treatment of hypoxia-inducible factor 1α-deficient mice with B cell-depleting αCD20 monoclonal antibodies prevented the progression of PanIN and development of invasive carcinomas.[50]
B cell recruitment by chemo-attractant in PC has been investigated. CXCL13 secreted by the fibro-inflammatory stroma in human and mouse PanIN lesions was shown to be responsible for the influx of B cells into the tumor.[49] In addition, treatment with a CXCL13-blocking monoclonal antibody resulted in decreased infiltration of B cells in mice orthotopically injected with KRASG12D-expressing PDECs, and the growth of orthotopic tumors was reduced.[49] Serum levels of B cell-activating factor in patients with PC that is associated with survival and maturation of B cells were significantly higher than in healthy subjects (P = 0.012), and those in patients with stage IV PC were higher than in patients with Ib-III (P = 0.018).[148] When PC cell lines were cultured with human recombinant B cell-activating factor, it induced enhancing PC cell motility and invasion.
The protumorigenic effect of B cells is mediated by IL-35 expression stimulating proliferation of PC cells.[49] Pylayeva-Gupta et al[49] demonstrated IL-35 produced by CD1dhiCD5+ B cells stimulate PDEC proliferation. Bruton tyrosine kinase (BTK) regulates B-cell and macrophage-mediated T-cell suppression in PC development. Both human and murine PCs were shown to exhibit high BTK activation in tumor-resident B cells and macrophages. Th2 polarization of macrophages developped following coculture with PC-derived B cells and was stopped by ibrutinib, BTK inhibitor. This suggests that B cells induce the protumorigenic macrophage phenotype.[147]
6. Immune modulation by pancreatic stellate cells/fibroblast
PSCs are myofibroblast-like cells and a major component of PC stroma. Pancreatic injuries activate quiescent PSCs, which transform into activated PSCs that secrete extracellular matrix materials, such as type I collagen. Recently, the association of PSC and immune cells has been studied. Ene-Obong et al[8] showed that activated PSCs that secrete chemokine ligand 12 (CXCL12) reduce the migration of CD8+ T cells into the juxtatumoral stroma of PC, and knockdown of CXCL12 by all-tans retinoic acid reverses these effects (Fig. 1H). Similarly, Feig et al[9] demonstrated that fibroblast activation protein positive carcinoma-associated fibroblasts produce CXCL12, which coat the cancer cells and prevent T cell infiltration. Although anti-PD-L1 did not promote T cell function in the mice, combination of anti-PD-L1 and inhibition of CXCL12 resulted in antitumor activity. Galectin-1 secreted by PSCs also promotes immune suppressive effects in the PC microenvironment. Tang et al[51] showed that galectin-1 promoted T cell apoptosis and Th2 cytokine secretion. It was suggested that myofibroblast in PC plays a role in recruitment of Tregs. Ozdemir et al reported that myofibroblast-depleted mouse pancreatic tumors show increased Tregs and correlated with reduced survival rate.[149] In addition to T cells, differentiation of MDSC may be promoted by PSCs. Recently, it has been reported that PSCs promoted differentiation of peripheral blood mononuclear cells into an MDSC phenotype that suppressed T cell proliferation.[52] Although the reported and ongoing studies regarding PSC and immune cell are still in the early stage, targeting this association has potential as a platform for immunotherapy of PC.
7. Effect of chemotherapy on immune cells
A number of studies have reported immune-modulatory effects of chemotherapeutic reagents such as gemcitabine, 5-fluorouracil (5-FU), and docetaxel. Gemcitabine has been associated with Tregs, DCs, and MDSC.[10–12,150] Gemcitabine reduced Tregs accumulation in an orthotopic Panc02 murine model with increase in survival rate.[10] Gemcitabine-containing PC cell medium reportedly stimulated DC maturation, and induced T cell proliferation resulting in CTL antitumor immune response.[11] Gemcitabine can directly suppress MDSCs in mice bearing mammary carcinoma, leading to increases in T cells and IFN-γ secretion.[150] Gemcitabine-based chemotherapy reduces the proportion and number of circulating Tregs in patients with PC.[12] Docetaxel reportedly enhanced IFN-γ secretion from CD8+ T cells without inhibition of Tregs functions.[151] Immune regulatory effects of 5-FU have also been demonstrated.[152,153] The immuno-chemotherapy employing 5-FU and IFN-α induced infiltration of NK cells in mouse model of PC.[152] These 5-FU and IFN-α treatment showed enhanced cytotoxicity on PC through increased expression of NKG2D ligands and MHC class I in Panc02 cells. Vincent et al[153] reported that 5-FU induced MDSC apoptosis and had a stronger efficacy than gemcitabine.
For evaluation of the effect of chemotherapeutic agents on human PC, tumor tissue from patients who underwent neoadjuvant chemotherapy provides a useful model. Our previous study showed that multimodal neoadjuvant treatment resulted in smaller numbers of myeloid cells and Tregs in human PC, than are seen in untreated tumors (P = 0.04 and 0.002, respectively).[42] Additionally, the ratios of CD4+ and CD8+ cells to Tregs were higher in patients with neoadjuvant therapy (P = 0.01 and 0.01, respectively), although CD8+ cells were decreased (P = 0.04).
8. Clinical immune-based therapeutic implementation
Conventional therapies for the PC have only marginally improved the survival rate; therefore, novel therapies are required. Immune therapy has many advantages, including the potential to generate lifelong immune responses while maintaining an acceptable safety profile. Recent excellent outcomes of immune therapy with antibodies modulating immune checkpoint pathway in melanoma, renal, and lung cancer have raised expectations for its application as a therapy for PC.[3,154,155] However, immune therapy for PC is still a challenging issue even though PC has a large number of immune cells in the tumor mircoenvironment. To date, immune therapy of PC includes passive immunotherapeutic approach using monoclonal antibodies or effector cells generated in vitro and active immunotherapeutic approach using vaccination to stimulate antitumor response. Monoclonal antibodies employed in passive immunotherapeutic approaches block ligand-receptor signaling for growth, thus leading to tumor cell death. They target tumor-associated antigens, such as mucin 1, Wilms tumor gene 1, human telomerase reverse transcriptase, mutated K-RAS, CEA, survivin, p53, HER-2/neu, vascular endothelial growth factor or epidermal growth factor receptor, and α-enolase.[156,157] Vaccination therapy for active immunotherapeutic approaches involves administering tumor-associated antigens to activate tumor-specific T cells. The available kinds of vaccines are whole cancer cell-based vaccines, antigen/peptide specific vaccines, and DC-based vaccines.[158] In addition, as previously described, immune checkpoint inhibition to activate effector T cells is one of the most actively studied themes. The most well-described therapeutically targeted immune checkpoint pathways that negatively regulate T cell function are those of PD-1 and CTLA-4. PD-L1 inhibitors (durvalumab, atezolizumab), PD-1 inhibitors (nivolumab, pembrolizumab, pidilizumab), and CTLA-4 inhibitors (ipilimumab, tremelimumab) have all been employed in clinical trials.
Although many clinical trials have been performed, phase 3 trials using monoclonal antibody or vaccination combined chemotherapeutic agent have failed to improve overall survival in cases of advanced PC.[158,159] To increase the response, combination therapies comprising immune checkpoint inhibitors and vaccines have been attempted. In a phase 1b trial, ipilimumab and GVAX were administered to patients with metastatic PC; the treatment increased overall survival as compared with patients administered with only ipilimumab.[53] In phase 2 trial, GVAX and cyclophosphamide followed by Listeria monocytogenes-expressing vaccine CRS-207 extended overall survival in PC patients[160]. Recently, new therapeutic attempts such as depletion of fibroblast activation protein-expressing cells in vitro and agonist CD40 antibody in vivo demonstrated promising results.[54,55]
Adoptive transfer of T cells is an approach in which patient's T cells are expanded and activated ex vivo then reinfused to the patient. Genetically modified T cells engineered to express a chimeric antigen receptor (CAR) is one of the methods of adoptive T cell transfer and has produced promising clinical results in CD19+ hematologic malignancies.[161,162] However, the efficacy of CAR T cells transfer still remains to be determined in solid tumors including PC.
Target antigen for adoptive T cell should be expressed at very low levels on normal tissue to reduce the on-target off-tumor effect. Mesothelin, MUC1, prostate stem cell antigen, and fibroblast activation protein have been introduced for target antigen of T cell regarding PC.[54,163,164] Beatty et al[163] presented adoptive transfer of mRNA CAR T cells that target mesothelin in a patient with metastatic pancreatic cancer. They engineered T cells to express CAR transiently using in vitro transcribed mRNA encoding a CAR to limit on-target off-tumor toxitcity against normal tissues. This CAR T cell therapy revealed an antitumor effect in the peritoneal lesion and ascitic fluid within a month without overt evidence for off-tumor toxicities. Kawaoka et al[165] performed adoptive immunotherapy employing MUC1-specific cytotoxic T lymphocytes in 28 patients with PC. They reported 19.4% 3-year survival rate in resectable PC patients and only 1 liver metastasis without side effects. Abate-Daga et al[164] showed transfer CAR T cell targetting prostate stem cell antigen induced significant antitumor activity in human PC xenograft model. Fibroblast activation protein-expressing stromal cells are immune-suppressive component of the tumor microenvironment in the tumors including PC.[54] Wang et al demonstrated adoptive transfer of fibroblast activation protein-CAR mouse T cells inhibits the growth of mutiple type of tumors with augmentation of the endogenous CD8+ T cell antitumor reosponses.
9. Conclusions and future prospective
PC has an assortment of immune-inflammatory tumor-infiltrating cells that establish immune suppressive tumor microenvironment. Due to the presence of an immune suppressive environment, PC has been described as an immune privilege tumor. However, the suppressive immune cells have emerged as excellent immune therapeutic targets, and immune therapy has shown remarkable outcomes in patients with melanoma and lung cancer. The immune cells in PC interact in the tumor microenvironment, therefore the relationship of immune cells with neighboring stromal and carcinoma cells is critical to consider for future therapeutic development. Numerous studies have begun to elucidate the roles of immune cell subtypes and their capacity to function or dysfunction in the tumor microenvironment of PC. Maintaining this focus will ensue that it will not be long before immune therapy for PC becomes a clinical reality.
Footnotes
Abbreviations: 5-FU = 5-fluorouracil, BTK = Bruton tyrosine kinase, CAR = chimeric antigen receptor, CCR5 = chemokine receptor type 5, CEA = crcinoembryonic antigen, CFS1R = colony-stimulating factor 1 receptor, CTL = cytotoxic T lymphocyte, CTLA-4 = cytotoxic T-lymphocyte associated protein-4, DC = dendritic cell, DcR3 = decoy receptor 3, Foxp3 = forkhead box P3, GEMM = genetically engineered mouse model, GM-CSF = granulocyte-macrophage colony-stimulating factor, GVAX = granulocyte-macrophage colony-stimulating factor secreting pancreatic cancer vaccine, IDO = indoleamine 2,3-dioxygenase, IFN = interferon, IL = interleukin, IPMN = intraductal papillary-mucinous neoplasm, MDSC = myeloid-derived suppressor cells, MHC = major histocompatibility complex, MICA = major histocompatibility complex class I chain-related molecule A, NK cells = natural killer cells, NKG2D = natural killer group 2 member D, PanIN = pancreatic intraepithelial lesion, PC = pancreatic cancer, PD-1 = programmed cell death-1, PDEC = pancreatic ductal epithelial cells, PD-L1 = programmed cell death ligand 1, PSC = pancreatic stellate cell, ROS = reactive oxygen species, STAT3 = signal transducer and activator of transcription 3, TGF = transforming growth factor, Th cells = helper T cells, TIL-Bs = tumor infiltrating lymphocyte B cells, TNF = tumor necrosis factor, Treg = regulatory T cell, VEGFR2 = vascular endothelial growth factor receptor 2.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg 2011;253:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 2013;108:914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A 2005;102:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res 2005;65:11743–51. [DOI] [PubMed] [Google Scholar]
- [7].Hiraoka N, Onozato K, Kosuge T, et al. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006;12:5423–34. [DOI] [PubMed] [Google Scholar]
- [8].Ene-Obong A, Clear AJ, Watt J, et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 2013;145:1121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shevchenko I, Karakhanova S, Soltek S, et al. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer 2013;133:98–107. [DOI] [PubMed] [Google Scholar]
- [11].Pei Q, Pan J, Zhu H, et al. Gemcitabine-treated pancreatic cancer cell medium induces the specific CTL antitumor activity by stimulating the maturation of dendritic cells. Int Immunopharmacol 2014;19:10–6. [DOI] [PubMed] [Google Scholar]
- [12].Homma Y, Taniguchi K, Nakazawa M, et al. Changes in the immune cell population and cell proliferation in peripheral blood after gemcitabine-based chemotherapy for pancreatic cancer. Clin Transl Oncol 2014;16:330–5. [DOI] [PubMed] [Google Scholar]
- [13].Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991–8. [DOI] [PubMed] [Google Scholar]
- [14].Ghiringhelli F, Menard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 2005;202:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gabitass RF, Annels NE, Stocken DD, et al. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011;60:1419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nummer D, Suri-Payer E, Schmitz-Winnenthal H, et al. Role of tumor endothelium in CD4+ CD25+ regulatory T cell infiltration of human pancreatic carcinoma. J Natl Cancer Inst 2007;99:1188–99. [DOI] [PubMed] [Google Scholar]
- [17].Panni RZ, Sanford DE, Belt BA, et al. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother 2014;63:513–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ryschich E, Notzel T, Hinz U, et al. Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res 2005;11:498–504. [PubMed] [Google Scholar]
- [19].von Bernstorff W, Spanjaard RA, Chan AK, et al. Pancreatic cancer cells can evade immune surveillance via nonfunctional Fas (APO-1/CD95) receptors and aberrant expression of functional Fas ligand. Surgery 1999;125:73–84. [DOI] [PubMed] [Google Scholar]
- [20].Bellone G, Turletti A, Artusio E, et al. Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol 1999;155:537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moo-Young TA, Larson JW, Belt BA, et al. Tumor-derived TGF-beta mediates conversion of CD4+Foxp3+ regulatory T cells in a murine model of pancreas cancer. J Immunother 2009;32:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003;9:1269–74. [DOI] [PubMed] [Google Scholar]
- [23].Witkiewicz A, Williams TK, Cozzitorto J, et al. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg 2008;206:849–54. [DOI] [PubMed] [Google Scholar]
- [24].Basso D, Fogar P, Falconi M, et al. Pancreatic tumors and immature immunosuppressive myeloid cells in blood and spleen: role of inhibitory co-stimulatory molecules PDL1 and CTLA4. An in vivo and in vitro study. PLoS One 2013;8:e54824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007;67:9518–27. [DOI] [PubMed] [Google Scholar]
- [26].Yanagimoto H, Takai S, Satoi S, et al. Impaired function of circulating dendritic cells in patients with pancreatic cancer. Clin Immunol 2005;114:52–60. [DOI] [PubMed] [Google Scholar]
- [27].Hirooka S, Yanagimoto H, Satoi S, et al. The role of circulating dendritic cells in patients with unresectable pancreatic cancer. Anticancer Res 2011;31:3827–34. [PubMed] [Google Scholar]
- [28].Bellone G, Novarino A, Vizio B, et al. Impact of surgery and chemotherapy on cellular immunity in pancreatic carcinoma patients in view of an integration of standard cancer treatment with immunotherapy. Int J Oncol 2009;34:1701–15. [DOI] [PubMed] [Google Scholar]
- [29].Bang S, Kim HS, Choo YS, et al. Differences in immune cells engaged in cell-mediated immunity after chemotherapy for far advanced pancreatic cancer. Pancreas 2006;32:29–36. [DOI] [PubMed] [Google Scholar]
- [30].Kimura Y, Tsukada J, Tomoda T, et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas 2012;41:195–205. [DOI] [PubMed] [Google Scholar]
- [31].Kobayashi M, Shimodaira S, Nagai K, et al. Prognostic factors related to add-on dendritic cell vaccines on patients with inoperable pancreatic cancer receiving chemotherapy: a multicenter analysis. Cancer Immunol Immunother 2014;63:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Davis M, Conlon K, Bohac GC, et al. Effect of pemetrexed on innate immune killer cells and adaptive immune T cells in subjects with adenocarcinoma of the pancreas. J Immunother 2012;35:629–40. [DOI] [PubMed] [Google Scholar]
- [33].Kurahara H, Shinchi H, Mataki Y, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res 2011;167:e211–9. [DOI] [PubMed] [Google Scholar]
- [34].Yoshikawa K, Mitsunaga S, Kinoshita T, et al. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Sci 2012;103:2012–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sugimoto M, Mitsunaga S, Yoshikawa K, et al. Prognostic impact of M2 macrophages at neural invasion in patients with invasive ductal carcinoma of the pancreas. Eur J Cancer 2014;50:1900–8. [DOI] [PubMed] [Google Scholar]
- [36].Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 2016;17:651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Markowitz J, Brooks TR, Duggan MC, et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol Immunother 2015;64:149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Porembka MR, Mitchem JB, Belt BA, et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother 2012;61:1373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sanford DE, Porembka MR, Panni RZ, et al. A study of zoledronic acid as neo-adjuvant, perioperative therapy in patients with resectable pancreatic ductal adenocarcinoma. J Cancer Ther 2013;4:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Suzuki D, Furukawa K, Kimura F, et al. Effects of perioperative immunonutrition on cell-mediated immunity, T helper type 1 (Th1)/Th2 differentiation, and Th17 response after pancreaticoduodenectomy. Surgery 2010;148:573–81. [DOI] [PubMed] [Google Scholar]
- [41].De Monte L, Reni M, Tassi E, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 2011;208:469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shibuya KC, Goel VK, Xiong W, et al. Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment. PLoS One 2014;9:e96565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bunt SK, Mohr AM, Bailey JM, et al. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother 2013;62:225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yamamoto T, Yanagimoto H, Satoi S, et al. Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas 2012;41:409–15. [DOI] [PubMed] [Google Scholar]
- [45].Ikemoto T, Yamaguchi T, Morine Y, et al. Clinical roles of increased populations of Foxp3+CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas 2006;33:386–90. [DOI] [PubMed] [Google Scholar]
- [46].Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010;33:828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Aida K, Miyakawa R, Suzuki K, et al. Suppression of Tregs by anti-glucocorticoid induced TNF receptor antibody enhances the antitumor immunity of interferon-alpha gene therapy for pancreatic cancer. Cancer Sci 2014;105:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Milne K, Kobel M, Kalloger SE, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 2009;4:e6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pylayeva-Gupta Y, Das S, Handler JS, et al. IL35-Producing B cells promote the development of pancreatic neoplasia. Cancer Discov 2016;6:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lee KE, Spata M, Bayne LJ, et al. Hif1a deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia. Cancer Discov 2016;6:256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tang D, Yuan Z, Xue X, et al. High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer. Int J Cancer 2012;130:2337–48. [DOI] [PubMed] [Google Scholar]
- [52].Mace TA, Ameen Z, Collins A, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res 2013;73:3007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013;36:382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010;330:827–30. [DOI] [PubMed] [Google Scholar]
- [55].Beatty GL, Torigian DA, Chiorean EG, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res 2013;19:6286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol 2007;7:543–55. [DOI] [PubMed] [Google Scholar]
- [57].Tjomsland V, Spangeus A, Sandstrom P, et al. Semi mature blood dendritic cells exist in patients with ductal pancreatic adenocarcinoma owing to inflammatory factors released from the tumor. PLoS One 2010;5:e13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bellone G, Carbone A, Smirne C, et al. Cooperative induction of a tolerogenic dendritic cell phenotype by cytokines secreted by pancreatic carcinoma cells. J Immunol 2006;177:3448–60. [DOI] [PubMed] [Google Scholar]
- [59].Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 2004;10:48–54. [DOI] [PubMed] [Google Scholar]
- [60].Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med 2005;11:1314–21. [DOI] [PubMed] [Google Scholar]
- [61].Dallal RM, Christakos P, Lee K, et al. Paucity of dendritic cells in pancreatic cancer. Surgery 2002;131:135–8. [DOI] [PubMed] [Google Scholar]
- [62].Tjomsland V, Sandstrom P, Spangeus A, et al. Pancreatic adenocarcinoma exerts systemic effects on the peripheral blood myeloid and plasmacytoid dendritic cells: an indicator of disease severity? BMC Cancer 2010;10:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yamamoto T, Yanagimoto H, Satoi S, et al. Circulating myeloid dendritic cells as prognostic factors in patients with pancreatic cancer who have undergone surgical resection. J Surg Res 2012;173:299–308. [DOI] [PubMed] [Google Scholar]
- [64].Takahashi K, Satoi S, Yanagimoto H, et al. Circulating dendritic cells and development of septic complications after pancreatectomy for pancreatic cancer. Arch Surg 2007;142:1151–7. [DOI] [PubMed] [Google Scholar]
- [65].Peiper M, Goedegebuure PS, Eberlein TJ. Generation of peptide-specific cytotoxic T lymphocytes using allogeneic dendritic cells capable of lysing human pancreatic cancer cells. Surgery 1997;122:235–41. [DOI] [PubMed] [Google Scholar]
- [66].Schnurr M, Scholz C, Rothenfusser S, et al. Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and gammadelta T cells. Cancer Res 2002;62:2347–52. [PubMed] [Google Scholar]
- [67].Akiyama Y, Maruyama K, Nara N, et al. Antitumor effects induced by dendritic cell-based immunotherapy against established pancreatic cancer in hamsters. Cancer Lett 2002;184:37–47. [DOI] [PubMed] [Google Scholar]
- [68].Morse MA, Nair SK, Boczkowski D, et al. The feasibility and safety of immunotherapy with dendritic cells loaded with CEA mRNA following neoadjuvant chemoradiotherapy and resection of pancreatic cancer. Int J Gastrointest Cancer 2002;32:1–6. [DOI] [PubMed] [Google Scholar]
- [69].Dissanayake D, Murakami K, Tran MD, et al. Peptide-pulsed dendritic cells have superior ability to induce immune-mediated tissue destruction compared to peptide with adjuvant. PLoS One 2014;9:e92380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bauer C, Bauernfeind F, Sterzik A, et al. Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut 2007;56:1275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bauer C, Sterzik A, Bauernfeind F, et al. Concomitant gemcitabine therapy negatively affects DC vaccine-induced CD8 (+) T-cell and B-cell responses but improves clinical efficacy in a murine pancreatic carcinoma model. Cancer Immunol Immunother 2014;63:321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hirooka Y, Itoh A, Kawashima H, et al. A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas 2009;38:e69–74. [DOI] [PubMed] [Google Scholar]
- [73].Perussia B. Lymphokine-activated killer cells, natural killer cells and cytokines. Curr Opin Immunol 1991;3:49–55. [DOI] [PubMed] [Google Scholar]
- [74].Ohta T, Elnemr A, Kitagawa H, et al. Fas ligand expression in human pancreatic cancer. Oncol Rep 2004;12:749–54. [PubMed] [Google Scholar]
- [75].Funa K, Nilsson B, Jacobsson G, et al. Decreased natural killer cell activity and interferon production by leucocytes in patients with adenocarcinoma of the pancreas. Br J Cancer 1984;50:231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Peng YP, Zhu Y, Zhang JJ, et al. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J Transl Med 2013;11:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Groh V, Rhinehart R, Secrist H, et al. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A 1999;96:6879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Duan X, Deng L, Chen X, et al. Clinical significance of the immunostimulatory MHC class I chain-related molecule A and NKG2D receptor on NK cells in pancreatic cancer. Med Oncol 2011;28:466–74. [DOI] [PubMed] [Google Scholar]
- [79].Hirazawa K, Hazama S, Oka M. Depressed cytotoxic activity of hepatic nonparenchymal cells in rats with obstructive jaundice. Surgery 1999;126:900–7. [PubMed] [Google Scholar]
- [80].Frankel TL, Burns W, Riley J, et al. Identification and characterization of a tumor infiltrating CD56(+)/CD16 (−) NK cell subset with specificity for pancreatic and prostate cancer cell lines. Cancer Immunol Immunother 2010;59:1757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002;196:254–65. [DOI] [PubMed] [Google Scholar]
- [82].Esposito I, Menicagli M, Funel N, et al. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol 2004;57:630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Menen RS, Hassanein MK, Momiyama M, et al. Tumor-educated macrophages promote tumor growth and peritoneal metastasis in an orthotopic nude mouse model of human pancreatic cancer. In Vivo 2012;26:565–9. [PubMed] [Google Scholar]
- [84].Karnevi E, Andersson R, Rosendahl AH. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol Cell Biol 2014;92:543–52. [DOI] [PubMed] [Google Scholar]
- [85].Barros MH, Hauck F, Dreyer JH, et al. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One 2013;8:e80908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Partecke LI, Gunther C, Hagemann S, et al. Induction of M2-macrophages by tumour cells and tumour growth promotion by M2-macrophages: a quid pro quo in pancreatic cancer. Pancreatology 2013;13:508–16. [DOI] [PubMed] [Google Scholar]
- [87].Tugues S, Honjo S, Konig C, et al. Genetic deficiency in plasma protein HRG enhances tumor growth and metastasis by exacerbating immune escape and vessel abnormalization. Cancer Res 2012;72:1953–63. [DOI] [PubMed] [Google Scholar]
- [88].Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 2004;114:623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Liu CY, Xu JY, Shi XY, et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest 2013;93:844–54. [DOI] [PubMed] [Google Scholar]
- [90].Kurahara H, Takao S, Maemura K, et al. M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas 2013;42:155–9. [DOI] [PubMed] [Google Scholar]
- [91].Ginhoux F, Schultze JL, Murray PJ, et al. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol 2016;17:34–40. [DOI] [PubMed] [Google Scholar]
- [92].Dineen SP, Lynn KD, Holloway SE, et al. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res 2008;68:4340–6. [DOI] [PubMed] [Google Scholar]
- [93].Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549–55. [DOI] [PubMed] [Google Scholar]
- [94].Kimsey TF, Campbell AS, Albo D, et al. Co-localization of macrophage inflammatory protein-3alpha (Mip-3alpha) and its receptor, CCR6, promotes pancreatic cancer cell invasion. Cancer J 2004;10:374–80. [DOI] [PubMed] [Google Scholar]
- [95].Campbell AS, Albo D, Kimsey TF, et al. Macrophage inflammatory protein-3alpha promotes pancreatic cancer cell invasion. J Surg Res 2005;123:96–101. [DOI] [PubMed] [Google Scholar]
- [96].Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 2014;74:5057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Tsuji S, Hosotani R, Yonehara S, et al. Endogenous decoy receptor 3 blocks the growth inhibition signals mediated by Fas ligand in human pancreatic adenocarcinoma. Int J Cancer 2003;106:17–25. [DOI] [PubMed] [Google Scholar]
- [98].Chang YC, Chen TC, Lee CT, et al. Epigenetic control of MHC class II expression in tumor-associated macrophages by decoy receptor 3. Blood 2008;111:5054–63. [DOI] [PubMed] [Google Scholar]
- [99].Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006;66:1123–31. [DOI] [PubMed] [Google Scholar]
- [100].Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 2010;59:1593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 2001;166:678–89. [DOI] [PubMed] [Google Scholar]
- [102].Bayne LJ, Beatty GL, Jhala N, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 2012;21:822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Liu C, Yu S, Kappes J, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood 2007;109:4336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sinha P, Clements VK, Bunt SK, et al. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 2007;179:977–83. [DOI] [PubMed] [Google Scholar]
- [105].Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007;109:1568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lu T, Ramakrishnan R, Altiok S, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 2011;121:4015–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hanson EM, Clements VK, Sinha P, et al. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol 2009;183:937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Srivastava MK, Sinha P, Clements VK, et al. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res 2010;70:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kusmartsev S, Nefedova Y, Yoder D, et al. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 2004;172:989–99. [DOI] [PubMed] [Google Scholar]
- [110].Diaz-Montero CM, Salem ML, Nishimura MI, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 2009;58:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Stromnes IM, Brockenbrough JS, Izeradjene K, et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 2014;63:1769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Emmrich J, Weber I, Nausch M, et al. Immunohistochemical characterization of the pancreatic cellular infiltrate in normal pancreas, chronic pancreatitis and pancreatic carcinoma. Digestion 1998;59:192–8. [DOI] [PubMed] [Google Scholar]
- [113].Wenger FA, Jacobi CA, Zieren J, et al. Tumor size and lymph-node status in pancreatic carcinoma—is there a correlation to the preoperative immune function? Langenbecks Arch Surg 1999;384:473–8. [DOI] [PubMed] [Google Scholar]
- [114].Helm O, Mennrich R, Petrick D, et al. Comparative characterization of stroma cells and ductal epithelium in chronic pancreatitis and pancreatic ductal adenocarcinoma. PLoS One 2014;9:e94357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Fukunaga A, Miyamoto M, Cho Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004;28:e26–31. [DOI] [PubMed] [Google Scholar]
- [116].Fogar P, Basso D, Fadi E, et al. Pancreatic cancer alters human CD4+ T lymphocyte function: a piece in the immune evasion puzzle. Pancreas 2011;40:1131–7. [DOI] [PubMed] [Google Scholar]
- [117].Tassi E, Gavazzi F, Albarello L, et al. Carcinoembryonic antigen-specific but not antiviral CD4+ T cell immunity is impaired in pancreatic carcinoma patients. J Immunol 2008;181:6595–603. [DOI] [PubMed] [Google Scholar]
- [118].Tassi E, Braga M, Longhi R, et al. Non-redundant role for IL-12 and IL-27 in modulating Th2 polarization of carcinoembryonic antigen specific CD4 T cells from pancreatic cancer patients. PLoS One 2009;4:e7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Xu YF, Lu Y, Cheng H, et al. Abnormal distribution of peripheral lymphocyte subsets induced by PDAC modulates overall survival. Pancreatology 2014;14:295–301. [DOI] [PubMed] [Google Scholar]
- [120].Trapani JA. The dual adverse effects of TGF-beta secretion on tumor progression. Cancer Cell 2005;8:349–50. [DOI] [PubMed] [Google Scholar]
- [121].Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005;8:369–80. [DOI] [PubMed] [Google Scholar]
- [122].Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- [123].Peggs KS, Quezada SA, Chambers CA, et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med 2009;206:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Soares KC, Rucki AA, Wu AA, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother 2015;38:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ellermeier J, Wei J, Duewell P, et al. Therapeutic efficacy of bifunctional siRNA combining TGF-beta1 silencing with RIG-I activation in pancreatic cancer. Cancer Res 2013;73:1709–20. [DOI] [PubMed] [Google Scholar]
- [126].Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 1970;18:723–37. [PMC free article] [PubMed] [Google Scholar]
- [127].Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151–64. [PubMed] [Google Scholar]
- [128].Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330–6. [DOI] [PubMed] [Google Scholar]
- [129].Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–61. [DOI] [PubMed] [Google Scholar]
- [130].Azuma T, Takahashi T, Kunisato A, et al. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res 2003;63:4516–20. [PubMed] [Google Scholar]
- [131].Liang B, Workman C, Lee J, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol 2008;180:5916–26. [DOI] [PubMed] [Google Scholar]
- [132].Kobayashi N, Kubota K, Kato S, et al. FOXP3+ regulatory T cells and tumoral indoleamine 2,3-dioxygenase expression predicts the carcinogenesis of intraductal papillary mucinous neoplasms of the pancreas. Pancreatology 2010;10:631–40. [DOI] [PubMed] [Google Scholar]
- [133].Tang Y, Xu X, Guo S, et al. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS One 2014;9:e91551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Amedei A, Niccolai E, Benagiano M, et al. Ex vivo analysis of pancreatic cancer-infiltrating T lymphocytes reveals that ENO-specific Tregs accumulate in tumor tissue and inhibit Th1/Th17 effector cell functions. Cancer Immunol Immunother 2013;62:1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Tan MC, Goedegebuure PS, Belt BA, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol 2009;182:1746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Liyanage UK, Goedegebuure PS, Moore TT, et al. Increased prevalence of regulatory T cells (Treg) is induced by pancreas adenocarcinoma. J Immunother 2006;29:416–24. [DOI] [PubMed] [Google Scholar]
- [137].Viehl CT, Moore TT, Liyanage UK, et al. Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann Surg Oncol 2006;13:1252–8. [DOI] [PubMed] [Google Scholar]
- [138].Waters CA, Schimke PA, Snider CE, et al. Interleukin 2 receptor-targeted cytotoxicity. Receptor binding requirements for entry of a diphtheria toxin-related interleukin 2 fusion protein into cells. Eur J Immunol 1990;20:785–91. [DOI] [PubMed] [Google Scholar]
- [139].Morse MA, Hobeika AC, Osada T, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood 2008;112:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Plate J. Clinical trials of vaccines for immunotherapy in pancreatic cancer. Expert Rev Vaccines 2011;10:825–36. [DOI] [PubMed] [Google Scholar]
- [141].Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother 2009;58:1535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother 2003;52:715–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Coronella JA, Telleman P, Kingsbury GA, et al. Evidence for an antigen-driven humoral immune response in medullary ductal breast cancer. Cancer Res 2001;61:7889–99. [PubMed] [Google Scholar]
- [144].Wang Y, Ylera F, Boston M, et al. Focused antibody response in plasma cell-infiltrated non-medullary (NOS) breast cancers. Breast Cancer Res Treat 2007;104:129–44. [DOI] [PubMed] [Google Scholar]
- [145].Shah S, Divekar AA, Hilchey SP, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer 2005;117:574–86. [DOI] [PubMed] [Google Scholar]
- [146].Kobayashi T, Hamaguchi Y, Hasegawa M, et al. B cells promote tumor immunity against B16F10 melanoma. Am J Pathol 2014;184:3120–9. [DOI] [PubMed] [Google Scholar]
- [147].Gunderson AJ, Kaneda MM, Tsujikawa T, et al. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov 2016;6:270–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Koizumi M, Hiasa Y, Kumagi T, et al. Increased B cell-activating factor promotes tumor invasion and metastasis in human pancreatic cancer. PLoS One 2013;8:e71367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Le HK, Graham L, Cha E, et al. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol 2009;9:900–9. [DOI] [PubMed] [Google Scholar]
- [151].Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res 2008;14:3536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Khallouf H, Marten A, Serba S, et al. 5-Fluorouracil and interferon-alpha immunochemotherapy enhances immunogenicity of murine pancreatic cancer through upregulation of NKG2D ligands and MHC class I. J Immunother 2012;35:245–53. [DOI] [PubMed] [Google Scholar]
- [153].Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010;70:3052–61. [DOI] [PubMed] [Google Scholar]
- [154].Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Zhang Y, Choi M. Immune therapy in pancreatic cancer: now and the future? Rev Recent Clin Trials 2015;10:317–25. [DOI] [PubMed] [Google Scholar]
- [157].Amedei A, Niccolai E, Prisco D. Pancreatic cancer: role of the immune system in cancer progression and vaccine-based immunotherapy. Hum Vaccin Immunother 2014;10:3354–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 2011;12:256–62. [DOI] [PubMed] [Google Scholar]
- [159].Middleton G, Silcocks P, Cox T, et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol 2014;15:829–40. [DOI] [PubMed] [Google Scholar]
- [160].Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015;33:1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2014;2:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Abate-Daga D, Lagisetty KH, Tran E, et al. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther 2014;25:1003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Kawaoka T, Oka M, Takashima M, et al. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol Rep 2008;20:155–63. [PubMed] [Google Scholar]


