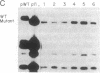
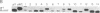Abstract
Using a cotransformation system to identify chloroplast transformants in Chlamydomonas reinhardtii, we converted histidine-195 of the photosystem II reaction center D1 protein to a tyrosine residue. The mutants were characterized by a reduced quantum efficiency for photosynthetic oxygen evolution, which varied in a pH-dependent manner, a reduced capacity to oxidize artificial donors to photosystem II, and P680+ reduction kinetics (microsecond) that were essentially similar to wild type. In addition, a dark-stable radical was detected by ESR in mutant photosystem II particles but not in wild-type particles. This radical was similar in g value and lineshape to chlorophyll or carotenoid cations but could have arisen from a tyrosine-195 cation. The ability of the photosystem II trap (P680+) to oxidize tyrosine residues suggests that the mutant tyrosine residue could be used as a redox-sensitive probe to investigate the environment around the photosystem II trap.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5730–5734. doi: 10.1073/pnas.84.16.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry B. A., Babcock G. T. Tyrosine radicals are involved in the photosynthetic oxygen-evolving system. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7099–7103. doi: 10.1073/pnas.84.20.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blowers A. D., Bogorad L., Shark K. B., Sanford J. C. Studies on Chlamydomonas chloroplast transformation: foreign DNA can be stably maintained in the chromosome. Plant Cell. 1989 Jan;1(1):123–132. doi: 10.1105/tpc.1.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conjeaud H., Mathis P. The effects of pH on the reductions kinetics of P-680 in Tris-treated chloroplasts. Biochim Biophys Acta. 1980 May 9;590(3):353–359. doi: 10.1016/0005-2728(80)90206-6. [DOI] [PubMed] [Google Scholar]
- Debus R. J., Barry B. A., Babcock G. T., McIntosh L. Site-directed mutagenesis identifies a tyrosine radical involved in the photosynthetic oxygen-evolving system. Proc Natl Acad Sci U S A. 1988 Jan;85(2):427–430. doi: 10.1073/pnas.85.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner B. A., Wollman F. A. Isolation of highly active photosystem II particles from a mutant of Chlamydomonas reinhardtii. Eur J Biochem. 1980 Sep;110(2):521–526. doi: 10.1111/j.1432-1033.1980.tb04894.x. [DOI] [PubMed] [Google Scholar]
- Eccles J., Honig B. Charged amino acids as spectroscopic determinants for chlorophyll in vivo. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4959–4962. doi: 10.1073/pnas.80.16.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. M., Rahire M., Rochaix J. D., Mets L. Herbicide resistance and cross-resistance: changes at three distinct sites in the herbicide-binding protein. Science. 1985 Apr 12;228(4696):204–207. doi: 10.1126/science.228.4696.204. [DOI] [PubMed] [Google Scholar]
- Harris E. H., Burkhart B. D., Gillham N. W., Boynton J. E. Antibiotic resistance mutations in the chloroplast 16S and 23S rRNA genes of Chlamydomonas reinhardtii: correlation of genetic and physical maps of the chloroplast genome. Genetics. 1989 Oct;123(2):281–292. doi: 10.1093/genetics/123.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. M., Fromm M., Weissinger A., Tomes D., Schaaf S., Sletten M., Sanford J. C. Transfer of foreign genes into intact maize cells with high-velocity microprojectiles. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4305–4309. doi: 10.1073/pnas.85.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz J. G., Nixon P. J., Rögner M., Brudvig G. W., Diner B. A. Directed alteration of the D1 polypeptide of photosystem II: evidence that tyrosine-161 is the redox component, Z, connecting the oxygen-evolving complex to the primary electron donor, P680. Biochemistry. 1989 Aug 22;28(17):6960–6969. doi: 10.1021/bi00443a028. [DOI] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. M., Boynton J. E., Gillham N. W., Randolph-Anderson B. L., Johnson A. M., Harris E. H. Transformation of chloroplast ribosomal RNA genes in Chlamydomonas: molecular and genetic characterization of integration events. Genetics. 1990 Dec;126(4):875–888. doi: 10.1093/genetics/126.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W., Chu Z. T., Warshel A. Electrostatic control of charge separation in bacterial photosynthesis. Biochim Biophys Acta. 1990 Jun 26;1017(3):251–272. doi: 10.1016/0005-2728(90)90192-7. [DOI] [PubMed] [Google Scholar]
- Petersen J., Dekker J. P., Bowlby N. R., Ghanotakis D. F., Yocum C. F., Babcock G. T. EPR characterization of the CP47-D1-D2-cytochrome b-559 complex of photosystem II. Biochemistry. 1990 Apr 3;29(13):3226–3231. doi: 10.1021/bi00465a012. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sayre R. T., Andersson B., Bogorad L. The topology of a membrane protein: the orientation of the 32 kd Qb-binding chloroplast thylakoid membrane protein. Cell. 1986 Nov 21;47(4):601–608. doi: 10.1016/0092-8674(86)90624-0. [DOI] [PubMed] [Google Scholar]
- Tollin G., Meyer T. E., Cusanovich M. A. Elucidation of the factors which determine reaction-rate constants and biological specificity for electron-transfer proteins. Biochim Biophys Acta. 1986 Nov 4;853(1):29–41. doi: 10.1016/0304-4173(86)90003-0. [DOI] [PubMed] [Google Scholar]
- Vermass W. F., Rutherford A. W., Hansson O. Site-directed mutagenesis in photosystem II of the cyanobacterium Synechocystis sp. PCC 6803: Donor D is a tyrosine residue in the D2 protein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8477–8481. doi: 10.1073/pnas.85.22.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]