Abstract
Background and Purpose—
The NOR-SASS (Norwegian Sonothrombolysis in Acute Stroke Study) aimed to assess effect and safety of contrast-enhanced ultrasound treatment in an unselected acute ischemic stroke population.
Methods—
Patients treated with intravenous thrombolysis within 4.5 hours after symptom onset were randomized 1:1 to either contrast-enhanced sonothrombolysis (CEST) or sham CEST. A visible arterial occlusion on baseline computed tomography angiography was not a prerequisite for inclusion. Pulse-wave 2 MHz ultrasound was given for 1 hour and contrast (SonoVue) as an infusion for ≈30 minutes. Magnetic resonance imaging and angiography were performed after 24 to 36 hours. Primary study end points were neurological improvement at 24 hours defined as National Institutes of Health Stroke Scale score 0 or reduction of ≥4 National Institutes of Health Stroke Scale points compared with baseline National Institutes of Health Stroke Scale and favorable functional outcome at 90 days defined as modified Rankin scale score 0 to 1.
Results—
A total of 183 patients were randomly assigned to either CEST (93 patient) or sham CEST (90 patients). The rates of symptomatic intracerebral hemorrhage, asymptomatic intracerebral hemorrhage, or mortality were not increased in the CEST group. Neurological improvement at 24 hours and functional outcome at 90 days was similar in the 2 groups both in the intention-to-treat analysis and in the per-protocol analysis.
Conclusions—
CEST is safe among unselected ischemic stroke patients with or without a visible occlusion on computed tomography angiography and with varying grades of clinical severity. There was, however, statistically no significant clinical effect of sonothrombolysis in this prematurely stopped trial.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01949961.
Keywords: angiography, brain, randomized controlled trial, safety, stroke
The only proven effective acute ischemic stroke treatment to achieve recanalization, reduce stroke severity, and improve outcome is intravenous thrombolysis with recombinant tPA (tissue-type plasminogen activator) given within 4½ hours from symptom onset.1
Partial-to-complete recanalization of larger occluded vessels is achieved in only 1 out of 3 patients who undergo thrombolysis. Many stroke patients, therefore, face severe brain damage, disability, and death.2 An improved acute treatment is urgently needed. Contrast-enhanced sonothrombolysis (CEST), encompassing a thrombolytic drug, ultrasound, and microbubbles, may be the next step in acute ischemic stroke treatment. Transcranial ultrasound weakens fibrin cross-links; promotes motion of blood around the clot; increases uptake, penetration, and concentration of tPA within the thrombus; and thus augments clot lysis.3–6 Intravenous alteplase therapy with transcranial ultrasound is safe and may induce higher rates of arterial recanalization, as compared with placebo.7,8 Adding intravenous contrast (microbubbles) induces further acceleration of ultrasound-enhanced thrombolysis, leading to a more complete recanalization and to a trend toward better short- and long-term outcome.9
Previous sonothrombolysis studies, with or without microbubbles, were performed in patients with visible occlusions on computed tomography angiography (CTA).4,7,10,11 Patients with visible occlusions constitute 10% to 28% of unselected acute ischemic stroke patients.12,13 There are no data on the effect of sonothrombolysis in the absence of a visible occlusion. However, sonothrombolysis might also have recanalizing effects in minor arteries, arterioles, or even microcirculation, that is, in mild strokes with small invisible thrombi.
In NOR-SASS (Norwegian Sonothrombolysis in Acute Stroke Study), we hypothesize that in acute stroke patients, eligible for intravenous thrombolysis, the clinical effect of additional CEST, given within 4½ hours from symptom onset, is superior to that of intravenous thrombolysis alone. The aims of this phase III study were to assess the effect and safety of contrast-enhanced ultrasound treatment in a cohort of unselected acute ischemic stroke patients.
Methods
Study Design and Participants
NOR-SASS was designed as a multicenter, prospective, PROBE trial (phase III, randomized, open-label, blinded end point), with the aim to demonstrate superiority of contrast-enhanced ultrasound treatment (sonothrombolysis) versus sham ultrasound treatment in consecutively admitted patients with acute ischemic stroke within 4½ hours after stroke onset. All patients were pretreated with intravenous thrombolysis, either alteplase or tenecteplase in a 1:1 random fashion. NOR-SASS was superimposed on The NOR-TEST (Norwegian Tenecteplase Stroke Trial), which randomizes patients with acute ischemic stroke to either tenecteplase or alteplase.14 The NOR-SASS study protocol has been published.12 The study is approved by the Regional Ethics Committee and the Norwegian Medicines Agency and is registered with EudraCT No 201200032341, and the subjects gave informed consent. The trial is conducted according to Good Clinical Practice: Consolidated guideline (CPMP/ICH/135/95).
The study was conducted at Center for Neurovascular Diseases, Department of Neurology, Haukeland University Hospital, Bergen, Norway. Patients ≥18 years of age with acute ischemic stroke symptoms admitted within 4½ hours of symptom onset were included in the study.
Clinical severity was assessed with the National Institutes of Health Stroke Scale (NIHSS), and function was assessed with the modified Rankin scale (mRS) and Barthel index. All patients underwent CTA on admission. Intracranial arterial occlusion on pretreatment CTA was not a prerequisite for inclusion because this study also includes sonothrombolysis of distal arteries and central perforating arteries. Magnetic resonance imaging (MRI) and magnetic resonance angiography or CT/CTA were performed after 22 to 36 hours to verify the infarct, assess intracranial recanalization, and detect hemorrhagic transformation. All NIHSS and mRS scores at 90 days were assessed by a neurologist or research nurses blinded to the given treatment. All CT, CTA, and MRI were interpreted by 2 experienced neuroradiologists blinded to the given treatment.
Randomization and Blinding
NOR-SASS randomized eligible patients to ultrasound, microbubbles (SonoVue) plus alteplase/tenecteplase versus sham ultrasound, sham microbubbles (NaCl 0.9%) plus alteplase/tenecteplase in a 1:1 randomization using sealed envelopes (block randomization). Patients, treating physicians, radiologist, and nurses were blinded to the patients’ study group assignment. Statistically analyzed outcomes were assessed after blinding was broken, on the basis of a statistical analysis plan finalised before blinding was broken.
Procedures
The use of either tenecteplase or alteplase was specifically allowed in this study. Intravenous thrombolysis was initiated within 4½ hours after the onset of ischemic stroke symptoms. Tenecteplase was given as a bolus in a dose of 0.4 mg/kg, maximum dose 40 mg. Alteplase was given in a dose of 0.9 mg/kg (10% bolus+90% infusion during 1 hour), maximum dose 90 mg. Sonothrombolysis was performed in parallel with intravenous thrombolytic treatment.
Before sonothrombolysis, a brief transcranial color-coded duplex sonography was performed by an experienced ultrasonographer working independently from the treating physician. Sonothrombolysis was performed with 2 MHz pulse-wave transcranial Doppler (TCD) (Nicolet, SONARA; Natus Neurology Incorporate) monitoring for 60 minutes, using a hand-held TCD probe for the foraminal window or a probe in a fixation head-band secured at a stable angle for the temporal window. TCD-emitted output power was set at the maximal achievable level with a mechanical index <1.0. Intravenous microbubbles (10 mL SonoVue; BRACCO, Switzerland) were given as an infusion of 0.3 mL/min for ≈30 minutes, using an infusion pump. In patients with a defined occlusion on CTA, ultrasound was applied to the region of the occlusion. In patients without a CTA-defined clot/occlusion, the investigator judged the clinical picture and applied ultrasound to the region in which she/he thought the lesion/clot was located, through either the temporal or foraminal window, as appropriate.
In patients with occlusion of perforating arteries, the sample volume was set at the depth of the proximal middle cerebral artery (MCA)1 segment, that is, at 50 to 55 mm. In distal MCA2 occlusions, the sample volume was set at 30 to 40 mm depth. In anterior cerebral artery/posterior cerebral artery symptoms, the sample volume was set at 60 to 80 mm. A similar algorithm was applied for the foraminal window. For proximal basilar artery, the sample volume was set at 80 mm and at 100 mm for distal basilar artery. The depth setting was chosen for monitoring purposes only because a therapeutic effect is related to the ultrasound field as such.
In patients without a defined clot on CTA, insonation of the presumed region of interest was performed with continuous slight fanning of the ultrasound beam. Sham sonothrombolysis was performed with the 2 MHz TCD probe plugged into an inactive channel, and sham ultrasound was given for 60 minutes after baseline transcranial color-coded duplex sonography examination. An intravenous infusion of sham intravenous contrast (NaCl 0.9%) 0.3 mL/min for ≈30 minutes was given using an infusion pump. The sound of TCD both in sonothrombolysis and in sham sonothrombolysis was muted, and the visual display was turned away from the patient, treating physicians, and nurses to keep them blinded to the patients’ study group assignment.
Outcomes
Primary study end points were neurological improvement at 24 hours and functional handicap at 90 days. Main secondary study safety end points included hemorrhagic transformation (hemorrhagic infarct/hematoma), symptomatic intracerebral hemorrhage (sICH), and death.
Early (24 hours) clinical outcome and long-term (day 90) functional outcome were assessed in a blinded fashion by a trained stroke nurse or neurologist. Early clinical outcome at 24 hours was defined by NIHSS score as (1) neurological improvement (NIHSS24=0 or reduction of ≥4 NIHSS points compared with baseline NIHSS0) and as (2) absolute reduction in NIHSS score (NIHSS24=NIHSS0−NIHSS24). Long-term outcome at 90 days was defined by mRS score using (1) single mRS group comparison; (2) fixed dichotomy (excellent outcome=mRS score 0–1 versus unfavorable outcome=mRS score 2–6 and good outcome=mRS score 0–2 versus bad outcome=mRS score 3–6); (3) sliding dichotomy/responder analysis (excellent outcome=mRS score 0 with baseline NIHSS score ≤7; mRS score 0–1 with baseline NIHSS score 8–14; and mRS score 0–2 with baseline NIHSS score ≥15); and (4) ordinal logistic regression analysis.
Patients were monitored in the stroke unit with NIHSS scoring at close intervals for 24 to 36 hours. Safety was determined by the incidence of hemorrhagic transformation within 22 to 36 hours of treatment on MRI (or CT when MRI was not possible) by 2 neuroradiologists blinded to all clinical data. ECASS criteria (European Cooperative Acute Stroke Study) were used for classification of hemorrhagic transformation.15 sICH was defined as local or remote parenchymal hemorrhage type 2 on the 22 to 36 hours post-treatment imaging scan, combined with a neurological deterioration of ≥4 points on the NIHSS from baseline or from the lowest NIHSS value between baseline and 24 hours, or leading to death (SITS-MOST criteria [Safe Implementation of Thrombolysis in Stroke-Monitoring Study]).16 Details are published.12
Statistical Analysis
A statistical analysis plan was written in co-operation with the Center for Clinical Research, Haukeland University Hospital, before closing the database and analyzing the data set. Our NORSTROKE database showed that patients treated with thrombolysis (n=223) had a 37% improvement of mean NIHSS score from 8.7 to 5.5 (3.2; SD=5.9) from admission to day 7. Also, based on previous meta-analysis of sonothrombolysis clinical studies7,8,11 and clinical studies on thrombolysis in mild stroke,17,18 NOR-SASS aimed at detecting a 15% higher reduction of mean NIHSS among patients receiving CEST versus sham CEST (P1=0.37; P2= 0.52; power 0.8) and intended to include 276 patients over 3 to 4 years. Because of the lack of funding, NOR-SASS had to be stopped prematurely.
Descriptive statistics are reported as mean values with SD, median and interquartile range, or proportions as appropriate. The χ2 test was used to analyze equality of proportions. Continuous data were analyzed by Student t test or Mann–Whitney U test as appropriate. The Fisher exact test was used to analyze categorical data with possibly few patients in some cells. The change in NIHSS score between baseline and at 24 hours was tested in a mixed linear regression analyses with repeated measures. The effect of treatment on long-term outcome was tested by ordinal logistic regression with mRS score as the dependent variable and treatment as an independent variable. We tested that our ordinal logistic regression model did not violate the proportional odds assumption.
The primary analyses were based on the intention-to-treat principle. Additional per-protocol analyses were also performed. The level of significance was set at 0.05, and 2-sided tests were used for all analyses. Stata 14.1 (StataCorp, College Station, TX) was used for all analyses.
Results
Between September 2012 and June 2015, a total of 183 patients (98 men and 85 women) treated with intravenous tPA were enrolled in NOR-SASS and randomized to either CEST (n=93) or sham CEST (n=90) (Figure). Table 1 shows the baseline characteristics of the patients. The mean age for all patients was 68.8 years (SD±16.2), median age 71 years (interquartile range, 56–82); range 24 to 100 years. The time between symptoms onset and CEST treatment was median 160 minutes and mean 170 (SD ±69) minutes. Vascular risk factors, history of cardiovascular disorders, stroke subtype, and demographics were similar in both groups (Table 2). The intention-to-treat analysis included 43 stroke mimics (23.5%), 1 patient (0.5%) who underwent embolectomy, 1 patient (0.5%) who was included >4½ hours after symptoms onset, 1 patient (0.5%) who had contraindication to microbubbles and received only ultrasound, and 24 patients (13.1%) with premorbid mRS score ≥3 points. Seventeen percent of patients had posterior circulation events. The number of stroke mimics was balanced between the groups. No serious adverse events occurred during the CEST or Sham CEST treatment.
Table 1.
Characteristics of the Patients at Baseline
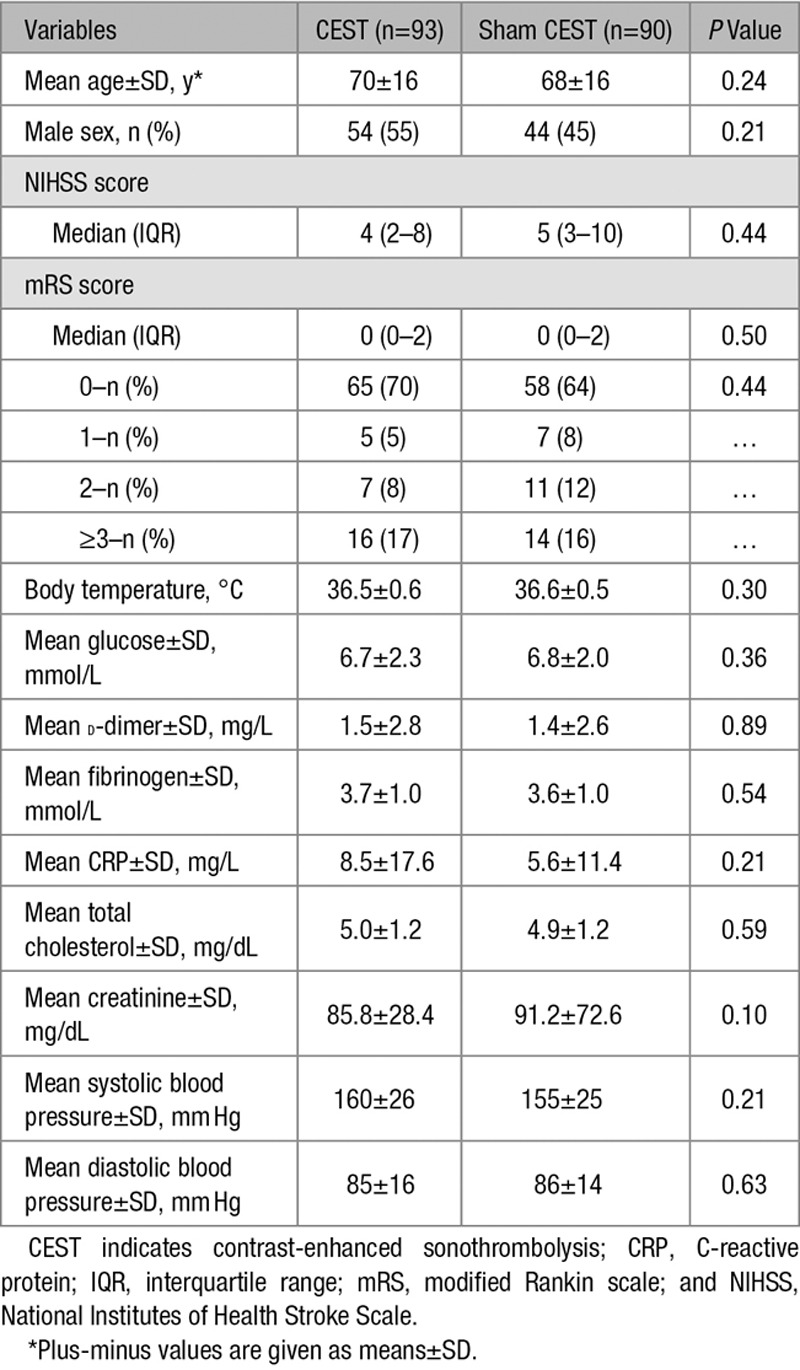
Table 2.
Vascular Risk Factors, History of Cardiovascular Disease, and Stroke Subtypes
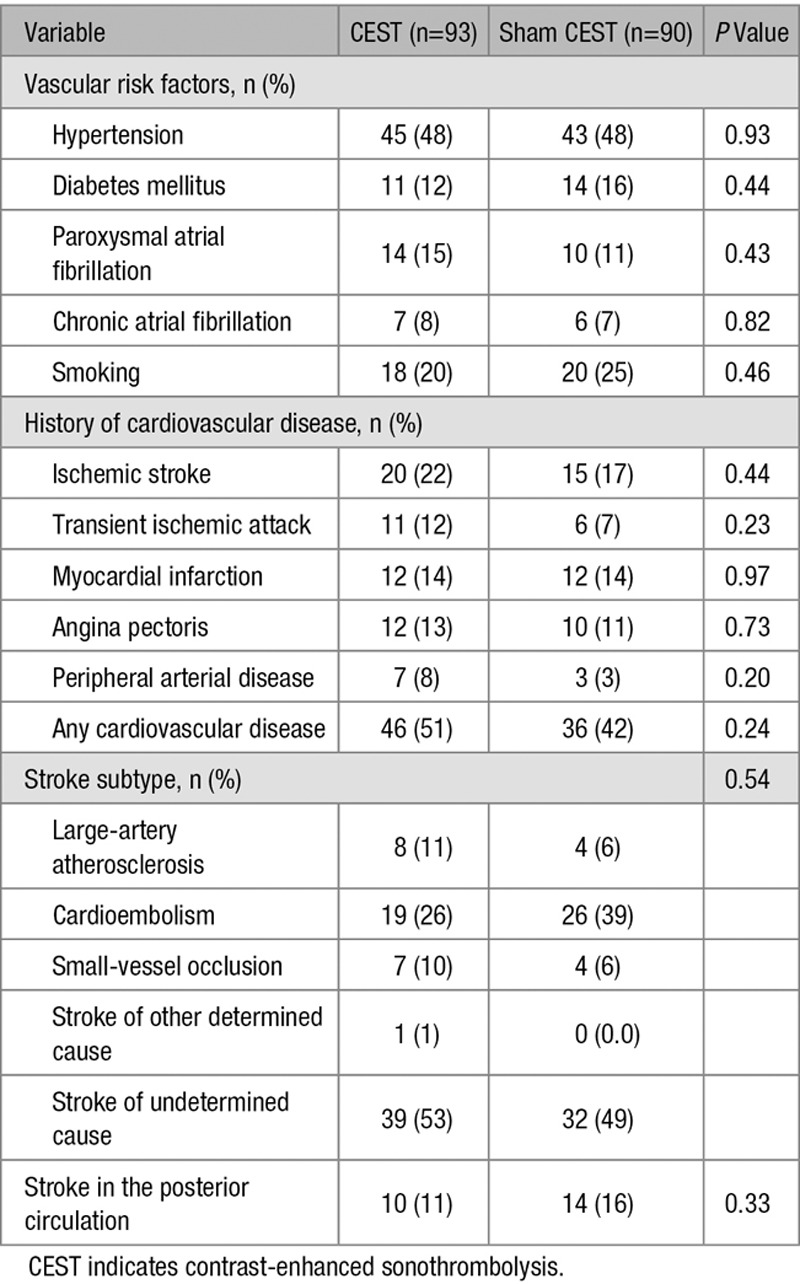
Figure.
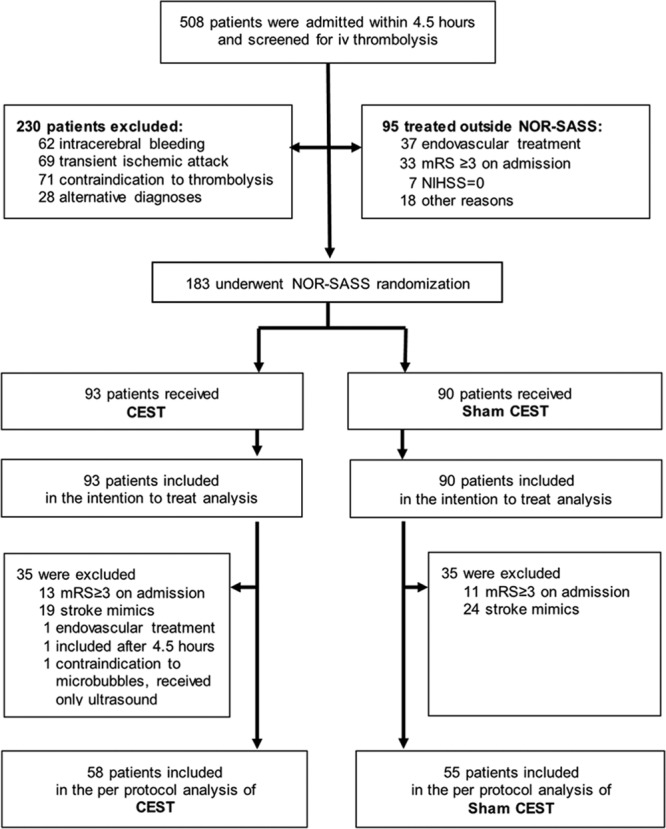
Trial profile. CEST indicates contrast-enhanced sonothrombolysis; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; and NOR-SASS, Norwegian Sonothrombolysis in Acute Stroke Study.
Admission NIHSS and 24-hour NIHSS scores were available for all 183 patients, of which 133 patients (72.7%) were improved, 27 patients (14.8%) were deteriorated, and 23 patients (12.6%) remained stable. All patients were followed for 90 days after study entry. Table 3 shows the primary study end points and secondary safety end points.
Table 3.
Primary and Secondary Clinical and Safety Study End Points
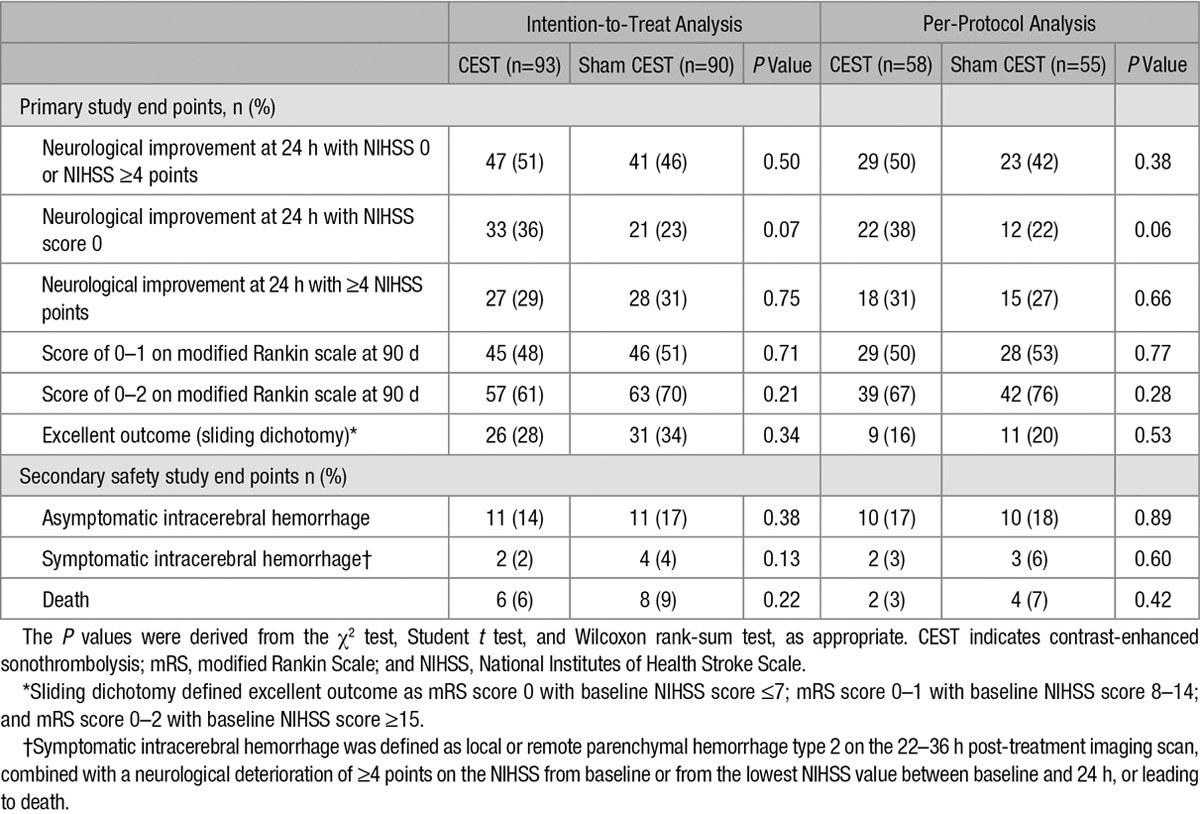
The primary study end point, early neurological improvement at 24 hours with NIHSS score 0 or with NIHSS score improvement of ≥4, was found in 51% of the patients in the CEST group compared with 46% in the sham CEST group (P=0.50) in the intention-to-treat analysis. In the per-protocol analysis, this end point was found in 50% of the patients in the CEST group compared with 42% of the patients in the sham CEST group (P=0.38). The single end point NIHSS score 0 was found in 36% versus 23% of the patients (P=0.07) in the intention-to-treat analysis and in 38% versus 22% of the patients (P=0.06) in the per-protocol analysis. The single end point NIHSS score improvement of ≥4 was found in 29% versus 31% of the patients (P=0.76) in the intention-to-treat analysis and in 31% versus 27% of the patients (P=0.66) in the per-protocol analysis. The data suggest no differences between CEST and sham CEST treatment groups in functional outcome at 90 days (excellent mRS 0–1: 50% versus 53%; P=0.77; Table 3).
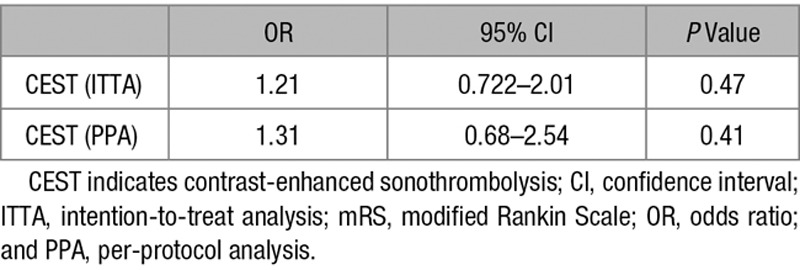
On admission, CT and CTA scans were performed in all patients. After 22 to 36 hours, control MRI was performed in 89% and control CT in 10%. sICH occurred in 2 patients in the CEST group and 4 in the sham CEST group (2% versus 4%; P=0.38). Asymptomatic intracranial hemorrhage on control MRI/CT occurred in 11 patients in each treatment groups (14.0% versus 17%; P=0.93). NOR-SASS did not have any cases of sICH among stroke mimics, neither in the CEST nor in the sham CEST groups. There were 6% deaths in CEST group compared with 9% deaths in sham CEST group (P=0.53) in the intention-to-treat analysis and 3% deaths in CEST group compared with 7% deaths in sham CEST group (P=0.60) in the per-protocol analysis. Early death caused by sICH occurred in 2 patients (1%), one in each treatment group. No statistically significant differences were noted for any primary and secondary safety study end points. Table 4 shows ordinal logistic regression with mRS as outcome variable and CEST treatment as the explanatory variable.
Table 4.
Ordinal Logistic Regression With mRS as Outcome Variable and CEST Treatment as the Explanatory Variable
Twenty-six stroke patients (19%) had an arterial occlusion on CTA. Sixteen patients (11.5%) had total MCA1 or MCA2 occlusion on admission. After 24 hours, 8 patients had no signs of recanalization (37.5% CEST versus 62.5% sham CEST), and 8 patients had partial to full recanalization (62.5% CEST versus 37.5% sham CEST; P=0.18).
NOR-SASS included 7 patients with confirmed acute ischemic stroke diagnosis who were randomized to CEST and received inappropriate insonation because of incorrect localization of the potential intracranial occlusion. Six patients had a posterior circulation stroke and were insonated via the transtemporal window; 1 patient had MCA stroke and was insonated via the foramen magnum window. These patients were not excluded from the per-protocol analysis because the protocol did not foresee this possibility as an exclusion criteria.
Discussion
NOR-SASS shows that in an unselected acute ischemic stroke population CEST given within 4½ hours after stroke onset is safe. Neither bleeding nor death was increased. The safety of CEST was not modified by stroke severity. Our findings, therefore, suggest that CEST using 2 MHz pulse-wave TCD monitoring for 60 minutes is safe in patients with varying grades of clinical severity, with or without a visible occlusion on CTA and thus with large or small thrombi.
There have been concerns about safety of CEST in acute ischemic stroke patients.10,19,20 NOR-SASS does not support this concern. In NOR-SASS, sICH occurred in 3% and asymptomatic intracerebral hemorrhage in 12% of all patients included. This is comparable to previous CEST studies in patients with MCA occlusion, reporting sICH in 0% to 27% and asymptomatic intracerebral hemorrhage in 23% to 75% of patients.9,10,19 Early death caused by sICH was 1% in NOR-SASS, compared with ≈3% in patients treated only with tPA.21 A higher number of microbleedings are detected with MRI compared with CT.20 Use of MRI as a 22- to 36-hour imaging modality in the NOR-SASS study may, therefore, account for the overall high rate of cerebral bleeding complications even among mild stroke patients.
NOR-SASS is a neutral study as to clinical effect. There was an overall trend toward a higher percentage of patients reaching the primary end points in the CEST group, but the differences did not reach statistical significance.
NOR-SASS aimed to include 276 patients. Because of lack of funding, NOR-SASS study had to be stopped prematurely after including only 183 patients. This low number may have contributed to the overall neutral results of the study.
NOR-SASS is the first randomized study of CEST in an unselected acute ischemic stroke population, mostly without a visible clot on the baseline CT. Patients treated may, therefore, have had small clots in arterial branches or disseminated occlusions in the microcirculation or both. Clinical data on the effect of CEST treatment on the cerebral microcirculation are lacking. In vivo and in vitro studies have, however, demonstrated beneficial effects of CEST on occlusion of small vessels down to the capillary level and reperfusion of the microvasculature.22–24 An in vitro flow model of partially occluded microcirculation demonstrated more rapid and complete clot lysis after CEST.22 A recent in vitro study examined microscale evolution of the erosion front of blood clots exposed to ultrasound stimulated microbubbles.25 The development of a complex zonal erosion pattern was observed. The fibrin zone architecture was dependent on exposure conditions forming large-scale tunnels.25 An in vivo study with a reperfusion model in rats showed beneficial effects of CEST on the level of the microvasculature, resulting in complete reversal of flow obstruction, decreased ischemic lesion volume, and decreased edema formation.23,24 Comparing published clinical trials of CEST treatment with NOR-SASS is difficult because of the wide range of treatment protocols, different end points, and limited number of patients. The NOR-SASS population differs substantially from those in previous CEST trials. Previous trials were conducted in selected stroke populations with visible thrombi in major intracranial arteries on CT/CTA, mostly MCA occlusions.9,19,26,27 To our knowledge, NOR-SASS is the first clinical trial that studied the effect and safety of CEST in an unselected stroke population, predominantly with mild stroke without visible thrombi on admission CTA. This group includes 70% to 80% of all acute ischemic stroke patients.12,13 Mild stroke with small thrombi have a higher recanalization rate after intravenous thrombolysis.28 It is, therefore, conceivable that also ultrasound may have a better effect on small arterial thrombi and on microcirculation.23 NOR-SASS indicates that CEST may be a therapeutic strategy for patients with persistent hypoperfusion (no reflow phenomenon) of the microvasculature, for which there is currently no direct therapy. We could, however, not show a significant clinical effect of CEST in these patients. The NIHSS score of median 4 to 5 point on admission suggests mild strokes, in which it may be difficult to achieve a positive result because of a ceiling effect. There was a contradictory trend between improvements in NIHSS score at 24 hours in favor of CEST, which was not seen in mRS at 3 months. Reasons for this may be that mRS outcome measures are insensitive to minor neurological improvement at 3 months or even an insufficient effect of CEST. A larger number of patients and more sensitive outcome measures should be applied in future studies with adequate power. NOR-SASS was superimposed in NOR-TEST trial. Using 2 different thrombolytic agents (tenecteplase and alteplase) in well-balanced NOR-SASS randomization (1:1) has not affected the study results as such. After NOR-TEST trial is finished, the NOR-SASS results on CEST with tenecteplase versus alteplase will be published.
In previous sonothrombolysis study patients with NIHSS ≥10 points has been shown to have a high positive predictive value (>95%) for proximal intracranial occlusion both in the anterior and posterior circulation.29 Moreover, in a subgroup analysis of CLOTBUST (Combined Lysis of Thrombus in Brain Ischemia With Transcranial Ultrasound and Systemic tPA), sonothrombolysis seemed to have greater efficacy in terms of 3-month functional outcome in this specific stroke subgroup.30 In NOR-SASS, the patients with NIHSS ≥10 points were thus too few to be analyzed and came with some conclusions.
Strengths of NOR-SASS, albeit with a low number of patients, are it being the first pragmatic randomized controlled trial on CEST in an unselected stroke population, being the biggest CEST study till now, and having well-balanced treatment groups and complete follow-up data at 90 days. Nevertheless, NOR-SASS showed new results in a specific population of acute stroke patients, who have not yet been investigated. The high rate of MRI controls renders data on infarcts and bleedings (symptomatic and asymptomatic) reliable. NOR-SASS, therefore, was performed in a clinically highly relevant setting.
As expected, the pragmatic decision to include mild strokes and use CT as baseline imaging modality increased the number of stroke mimics and reduced the chance of detecting a significant difference between the treatment groups. Using MRI at admission may decrease the number of stroke mimics but will increase the time to treatment. Also, not every hospital has access to acute MRI. The protocol is, therefore, suitable also for smaller hospitals. A weakness of NOR-SASS is the accidental inclusion of a high number of presumably functional independent patients, who after investigations turned out to have pretreatment mRS ≥3 points and thus had to be excluded from the per-protocol analysis.
In conclusion, we found that CEST is safe when given within 4½ hours after stroke onset to acute ischemic stroke patients with varying grades of clinical severity, with or without a visible occlusion on CTA and thus with larger or smaller thrombi. An overall clinical effect could not be shown in the small number of patients included. NOR-SASS demonstrates, however, the potential of CEST treatment as a pragmatic adjuvant therapy for acute ischemic stroke patients.
Acknowledgments
We thank research nurses Linn Elin Rødal, Leila Marie Frid, and Maren Inselseth for their excellent work and dedication and the participating patients in NOR-SASS. L. Thomassen was the Study Director, designed and managed the study, and edited the report. A. Nacu was the Principal Investigator, recruited patients, and wrote the first draft and subsequent versions with input and key revisions by all coauthors. C.E. Kvistad was the Study Chair, recruited patients, and edited the report. J. Assmus, H. Naess, and H. Øygarden calculated the sample size, developed the statistical analysis plan, and did the statistical analysis. U. Waje-Andreassen, H. Naess, and N. Logallo were part of the executive working group, contributed to data analysis, and reviewed the report. K.D. Kurz and G. Neckelmann analyzed all medical images. All authors reviewed and approved the final report.
Disclosures
None.
Supplementary Material
References
- 1.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 2.van der Worp HB, van Gijn J. Clinical practice. Acute ischemic stroke. N Engl J Med. 2007;357:572–579. doi: 10.1056/NEJMcp072057. doi: 10.1056/NEJMcp072057. [DOI] [PubMed] [Google Scholar]
- 3.Xie F, Tsutsui JM, Lof J, Unger EC, Johanning J, Culp WC, et al. Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound Med Biol. 2005;31:979–985. doi: 10.1016/j.ultrasmedbio.2005.03.008. doi: 10.1016/j.ultrasmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. CLOTBUST Investigators. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 5.Behrens S, Spengos K, Daffertshofer M, Wirth S, Hennerici M. Potential use of therapeutic ultrasound in ischemic stroke treatment. Echocardiography. 2001;18:259–263. doi: 10.1046/j.1540-8175.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- 6.Spengos K, Behrens S, Daffertshofer M, Dempfle CE, Hennerici M. Acceleration of thrombolysis with ultrasound through the cranium in a flow model. Ultrasound Med Biol. 2000;26:889–895. doi: 10.1016/s0301-5629(00)00211-8. [DOI] [PubMed] [Google Scholar]
- 7.Tsivgoulis G, Eggers J, Ribo M, Perren F, Saqqur M, Rubiera M, et al. Safety and efficacy of ultrasound-enhanced thrombolysis: a comprehensive review and meta-analysis of randomized and nonrandomized studies. Stroke. 2010;41:280–287. doi: 10.1161/STROKEAHA.109.563304. doi: 10.1161/STROKEAHA.109.563304. [DOI] [PubMed] [Google Scholar]
- 8.Ricci S, Dinia L, Del Sette M, Anzola P, Mazzoli T, Cenciarelli S, et al. Sonothrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2012;10:CD008348. doi: 10.1002/14651858.CD008348.pub3. doi: 10.1002/14651858.CD008348.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–429. doi: 10.1161/01.STR.0000199064.94588.39. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 10.Molina CA, Barreto AD, Tsivgoulis G, Sierzenski P, Malkoff MD, Rubiera M, et al. Transcranial ultrasound in clinical sonothrombolysis (TUCSON) trial. Ann Neurol. 2009;66:28–38. doi: 10.1002/ana.21723. doi: 10.1002/ana.21723. [DOI] [PubMed] [Google Scholar]
- 11.Saqqur M, Tsivgoulis G, Nicoli F, Skoloudik D, Sharma VK, Larrue V, et al. The role of sonolysis and sonothrombolysis in acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials and case-control studies. J Neuroimaging. 2014;24:209–220. doi: 10.1111/jon.12026. doi: 10.1111/jon.12026. [DOI] [PubMed] [Google Scholar]
- 12.Nacu A, Kvistad CE, Logallo N, Naess H, Waje-Andreassen U, Aamodt AH, et al. A pragmatic approach to sonothrombolysis in acute ischaemic stroke: the Norwegian randomised controlled sonothrombolysis in acute stroke study (NOR-SASS). BMC Neurol. 2015;15:110. doi: 10.1186/s12883-015-0359-4. doi: 10.1186/s12883-015-0359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen CK, Christensen A, Ovesen C, Havsteen I, Christensen H. Stroke severity and incidence of acute large vessel occlusions in patients with hyper-acute cerebral ischemia: results from a prospective cohort study based on CT-angiography (CTA). Int J Stroke. 2015;10:336–342. doi: 10.1111/ijs.12383. doi: 10.1111/ijs.12383. [DOI] [PubMed] [Google Scholar]
- 14.Logallo N, Kvistad CE, Nacu A, Naess H, Waje-Andreassen U, Asmuss J, et al. The Norwegian tenecteplase stroke trial (NOR-TEST): randomised controlled trial of tenecteplase vs. alteplase in acute ischaemic stroke. BMC Neurol. 2014;14:106. doi: 10.1186/1471-2377-14-106. doi: 10.1186/1471-2377-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Los Ríos la Rosa F, Khoury J, Kissela BM, Flaherty ML, Alwell K, Moomaw CJ, et al. Eligibility for intravenous eecombinant tissue- type plasminogen activator within a population: the effect of the European Cooperative Acute Stroke Study (ECASS) III trial. Stroke. 2012;43:1591–1595. doi: 10.1161/STROKEAHA.111.645986. doi: 10.1161/STROKEAHA.111.645986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 17.Logallo N, Kvistad CE, Naess H, Waje-Andreassen U, Thomassen L. Mild stroke: safety and outcome in patients receiving thrombolysis. Acta Neurol Scand Suppl. 2014;198:37–40. doi: 10.1111/ane.12235. [DOI] [PubMed] [Google Scholar]
- 18.Laurencin C, Philippeau F, Blanc-Lasserre K, Vallet AE, Cakmak S, Mechtouff L, et al. Thrombolysis for acute minor stroke: outcome and barriers to management. Results from the RESUVAL Stroke Network. Cerebrovasc Dis. 2015;40:3–9. doi: 10.1159/000381866. doi: 10.1159/000381866. [DOI] [PubMed] [Google Scholar]
- 19.Viguier A, Petit R, Rigal M, Cintas P, Larrue V. Continuous monitoring of middle cerebral artery recanalization with transcranial color-coded sonography and levovist. J Thromb Thrombolysis. 2005;19:55–59. doi: 10.1007/s11239-005-0940-6. doi: 10.1007/s11239-005-0940-6. [DOI] [PubMed] [Google Scholar]
- 20.Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36:1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- 21.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leeman JE, Kim JS, Yu FT, Chen X, Kim K, Wang J, et al. Effect of acoustic conditions on microbubble-mediated microvascular sonothrombolysis. Ultrasound Med Biol. 2012;38:1589–1598. doi: 10.1016/j.ultrasmedbio.2012.05.020. doi: 10.1016/j.ultrasmedbio.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Nedelmann M, Ritschel N, Doenges S, Langheinrich AC, Acker T, Reuter P, et al. Combined contrast-enhanced ultrasound and rt-PA treatment is safe and improves impaired microcirculation after reperfusion of middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2010;30:1712–1720. doi: 10.1038/jcbfm.2010.82. doi: 10.1038/jcbfm.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schleicher N, Tomkins AJ, Kampschulte M, Hyvelin JM, Botteron C, Juenemann M, et al. Sonothrombolysis with BR38 microbubbles improves microvascular patency in a rat model of stroke. PLoS One. 2016;11:e0152898. doi: 10.1371/journal.pone.0152898. doi: 10.1371/journal.pone.0152898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acconcia CN, Leung BY, Goertz DE. The microscale evolution of the erosion front of blood clots exposed to ultrasound stimulated microbubbles. J Acoust Soc Am. 2016;139:EL135. doi: 10.1121/1.4946045. doi: 10.1121/1.4946045. [DOI] [PubMed] [Google Scholar]
- 26.Alexandrov AV, Mikulik R, Ribo M, Sharma VK, Lao AY, Tsivgoulis G, et al. A pilot randomized clinical safety study of sonothrombolysis augmentation with ultrasound-activated perflutren-lipid microspheres for acute ischemic stroke. Stroke. 2008;39:1464–1469. doi: 10.1161/STROKEAHA.107.505727. doi: 10.1161/STROKEAHA.107.505727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubiera M, Ribo M, Delgado-Mederos R, Santamarina E, Maisterra O, Delgado P, et al. Do bubble characteristics affect recanalization in stroke patients treated with microbubble-enhanced sonothrombolysis? Ultrasound Med Biol. 2008;34:1573–1577. doi: 10.1016/j.ultrasmedbio.2008.02.011. doi: 10.1016/j.ultrasmedbio.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 29.Fischer U, Arnold M, Nedeltchev K, Brekenfeld C, Ballinari P, Remonda L, et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36:2121–2125. doi: 10.1161/01.STR.0000182099.04994.fc. doi: 10.1161/01.STR.0000182099.04994.fc. [DOI] [PubMed] [Google Scholar]
- 30.Barlinn K, Tsivgoulis G, Barreto AD, Alleman J, Molina CA, Mikulik R, et al. Outcomes following sonothrombolysis in severe acute ischemic stroke: subgroup analysis of the CLOTBUST trial. Int J Stroke. 2014;9:1006–1010. doi: 10.1111/ijs.12340. doi: 10.1111/ijs.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]


